Application of Indocyanine Green Fluorescence Imaging in Assisting Biopsy of Musculoskeletal Tumors
Abstract
Simple Summary
Abstract
1. Introduction
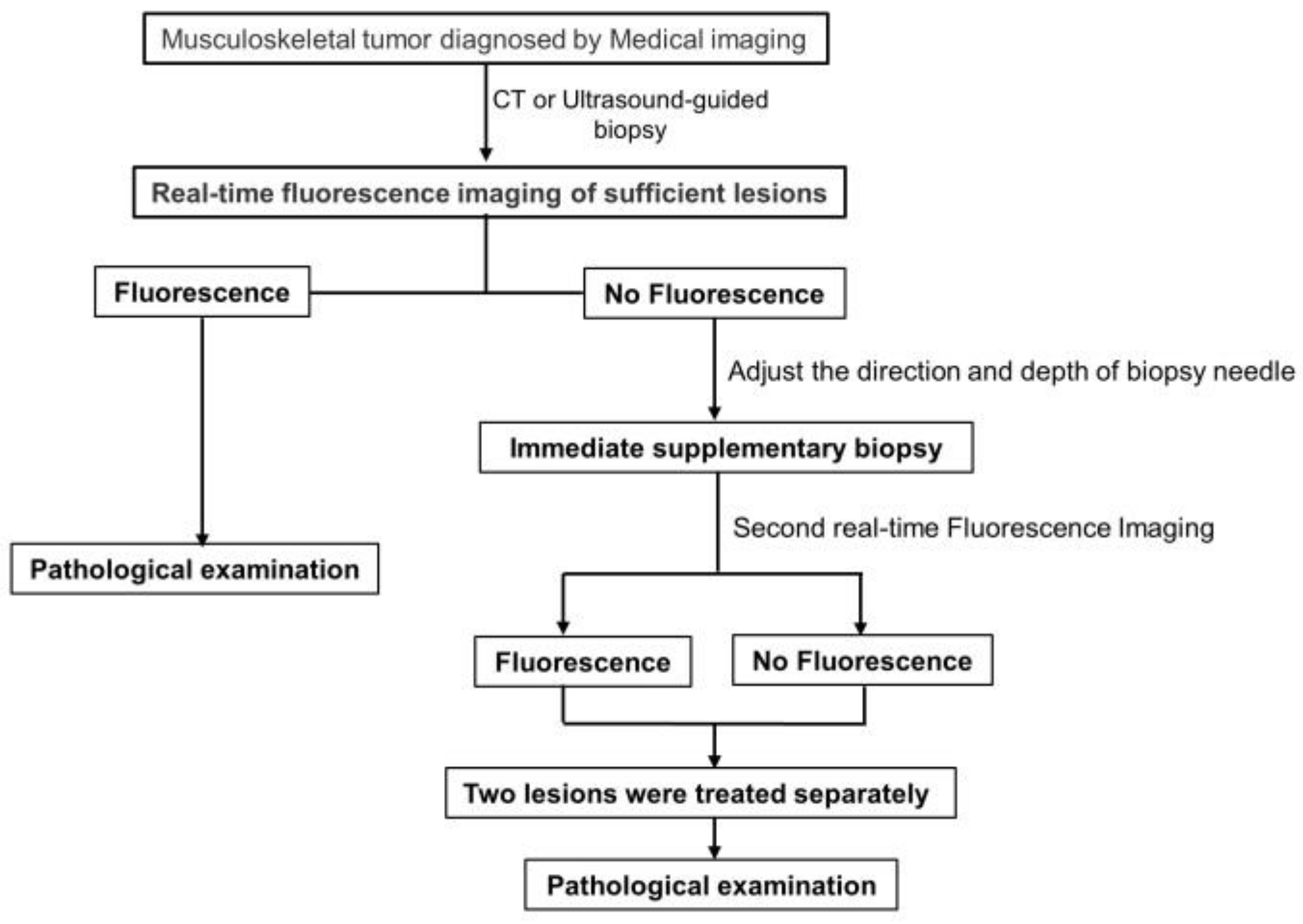
2. Materials and Methods
2.1. Patients and Groups
2.2. Core Biopsy Guided by Imaging Equipment
2.3. Research Design
2.3.1. Control Group
2.3.2. Test Group
2.4. Investigational Drug
2.5. Experimental Equipment
2.5.1. Photo Device
2.5.2. Biopsy Device
2.6. Effectiveness of Fluorescence-Assisted Biopsy
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, W.J.; Doyle, L.A. Updates from the 2020 World Health Organization Classification of Soft Tissue and Bone Tumours. Histopathology 2021, 78, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv. Anat. Pathol. 2021, 28, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv51–iv67. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A. Bone and Soft Tissue Sarcoma. Cancers 2020, 12, 2609. [Google Scholar] [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- Traina, F.; Errani, C.; Toscano, A.; Pungetti, C.; Fabbri, D.; Mazzotti, A.; Donati, D.; Faldini, C. Current concepts in the biopsy of musculoskeletal tumors: AAOS exhibit selection. J. Bone Jt. Surg. 2015, 97, e7. [Google Scholar] [CrossRef]
- Meek, R.D.; Mills, M.K.; Hanrahan, C.J.; Beckett, B.R.; Leake, R.L.; Allen, H.; Williams, D.D.; Tommack, M.; Schmahmann, S.; Hansford, B.G. Pearls and Pitfalls for Soft-Tissue and Bone Biopsies: A Cross-Institutional Review. RadioGraphics 2020, 40, 266–290. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Rijs, Z.; Jeremiasse, B.; Shifai, N.; Gelderblom, H.; Sier, C.F.M.; Vahrmeijer, A.L.; van Leeuwen, F.W.B.; van der Steeg, A.F.W.; van de Sande, M.A.J. Introducing Fluorescence-Guided Surgery for Pediatric Ewing, Osteo-, and Rhabdomyosarcomas: A Literature Review. Biomedicines 2021, 9, 1388. [Google Scholar] [CrossRef]
- Conversano, A.; Abbaci, M.; Karimi, M.; Mathieu, M.C.; de Leeuw, F.; Michiels, S.; Laplace-Builhe, C.; Mazouni, C. Axillary reverse mapping using near-infrared fluorescence imaging in invasive breast cancer (ARMONIC study). Eur. J. Surg. Oncol. 2022, 48, 2393–2400. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Xie, J.W.; Zhong, Q.; Wang, J.B.; Lin, J.X.; Lu, J.; Cao, L.L.; Lin, M.; Tu, R.H.; Huang, Z.N.; et al. Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients with Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 300–311. [Google Scholar] [CrossRef]
- Predina, J.D.; Newton, A.D.; Corbett, C.; Shin, M.; Sulfyok, L.F.; Okusanya, O.T.; Delikatny, E.J.; Nie, S.; Gaughan, C.; Jarrar, D.; et al. Near-infrared intraoperative imaging for minimally invasive pulmonary metastasectomy for sarcomas. J. Thorac. Cardiovasc. Surg. 2019, 157, 2061–2069. [Google Scholar] [CrossRef]
- Terasawa, M.; Ishizawa, T.; Mise, Y.; Inoue, Y.; Ito, H.; Takahashi, Y.; Saiura, A. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg. Endosc. 2017, 31, 5111–5118. [Google Scholar] [CrossRef]
- Abbaci, M.; De Leeuw, F.; Breuskin, I.; Casiraghi, O.; Lakhdar, A.B.; Ghanem, W.; Laplace-Builhe, C.; Hartl, D. Parathyroid gland management using optical technologies during thyroidectomy or parathyroidectomy: A systematic review. Oral. Oncol. 2018, 87, 186–196. [Google Scholar] [CrossRef]
- Predina, J.D.; Keating, J.; Newton, A.; Corbett, C.; Xia, L.; Shin, M.; Frenzel Sulyok, L.; Deshpande, C.; Litzky, L.; Nie, S.; et al. A clinical trial of intraoperative near-infrared imaging to assess tumor extent and identify residual disease during anterior mediastinal tumor resection. Cancer 2018, 125, 807–817. [Google Scholar] [CrossRef]
- Brookes, M.J.; Chan, C.D.; Nicoli, F.; Crowley, T.P.; Ghosh, K.M.; Beckingsale, T.; Saleh, D.; Dildey, P.; Gupta, S.; Ragbir, M.; et al. Intraoperative Near-Infrared Fluorescence Guided Surgery Using Indocyanine Green (ICG) for the Resection of Sarcomas May Reduce the Positive Margin Rate: An Extended Case Series. Cancers 2021, 13, 6284. [Google Scholar] [CrossRef]
- Nicoli, F.; Saleh, D.B.; Baljer, B.; Chan, C.D.; Beckingsale, T.; Ghosh, K.M.; Ragbir, M.; Rankin, K.S. Intraoperative Near-infrared Fluorescence (NIR) Imaging with Indocyanine Green (ICG) Can Identify Bone and Soft Tissue Sarcomas Which May Provide Guidance for Oncological Resection. Ann. Surg. 2021, 273, e63–e68. [Google Scholar] [CrossRef]
- Wu, J.S.; Goldsmith, J.D.; Horwich, P.J.; Shetty, S.K.; Hochman, M.G. Bone and soft-tissue lesions: What factors affect diagnostic yield of image-guided core-needle biopsy? Radiology 2008, 248, 962–970. [Google Scholar] [CrossRef]
- Datir, A.; Pechon, P.; Saifuddin, A. Imaging-guided percutaneous biopsy of pathologic fractures: A retrospective analysis of 129 cases. Am. J. Roentgenol. 2009, 193, 504–508. [Google Scholar] [CrossRef]
- Ilivitzki, A.; Abugazala, M.; Arkovitz, M.; Benbarak, A.; Postovsky, S.; Arad-Cohen, N.; Ben-Arush, M. Ultrasound-guided core biopsy as the primary tool for tissue diagnosis in pediatric oncology. J. Pediatr. Hematol. Oncol. 2014, 36, 333–336. [Google Scholar] [CrossRef]
- Rehm, J.; Veith, S.; Akbar, M.; Kauczor, H.U.; Weber, M.A. CT-Guided Percutaneous Spine Biopsy in Suspected Infection or Malignancy: A Study of 214 Patients. Rofo 2016, 188, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Zhao, M.; Hu, T.; Zhang, G. Diagnostic yield of percutaneous core needle biopsy in suspected soft tissue lesions of extremities. J. Int. Med. Res. 2019, 47, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Madajewski, B.; Judy, B.F.; Mouchli, A.; Kapoor, V.; Holt, D.; Wang, M.D.; Nie, S.; Singhal, S. Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin. Cancer Res. 2012, 18, 5741–5751. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug. Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Skubleny, D.; Dang, J.T.; Skulsky, S.; Switzer, N.; Tian, C.; Shi, X.; de Gara, C.; Birch, D.W.; Karmali, S. Diagnostic evaluation of sentinel lymph node biopsy using indocyanine green and infrared or fluorescent imaging in gastric cancer: A systematic review and meta-analysis. Surg. Endosc. 2018, 32, 2620–2631. [Google Scholar] [CrossRef]
- Guo, J.; Yang, H.; Wang, S.; Cao, Y.; Liu, M.; Xie, F.; Liu, P.; Zhou, B.; Tong, F.; Cheng, L.; et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: A prospective cohort study. World J. Surg. Oncol. 2017, 15, 196. [Google Scholar] [CrossRef]
- Wu, M.H.; Xiao, L.F.; Liu, H.W.; Yang, Z.Q.; Liang, X.X.; Chen, Y.; Lei, J.; Deng, Z.M. PET/CT-guided versus CT-guided percutaneous core biopsies in the diagnosis of bone tumors and tumor-like lesions: Which is the better choice? Cancer Imaging 2019, 19, 69. [Google Scholar] [CrossRef]
- Hadjimichael, A.C.; Pergaris, A.; Kaspiris, A.; Foukas, A.F.; Theocharis, S.E. Liquid Biopsy: A New Translational Diagnostic and Monitoring Tool for Musculoskeletal Tumors. Int. J. Mol. Sci. 2021, 22, 11526. [Google Scholar] [CrossRef]
- Villard, A.; Breuskin, I.; Casiraghi, O.; Asmandar, S.; Laplace-Builhe, C.; Abbaci, M.; Moya Plana, A. Confocal laser endomicroscopy and confocal microscopy for head and neck cancer imaging: Recent updates and future perspectives. Oral. Oncol. 2022, 127, 105826. [Google Scholar] [CrossRef]
- Rougraff, B.T.; Aboulafia, A.; Biermann, J.S.; Healey, J. Biopsy of soft tissue masses: Evidence-based medicine for the musculoskeletal tumor society. Clin. Orthop. Relat. Res. 2009, 467, 2783–2791. [Google Scholar] [CrossRef]
- Virayavanich, W.; Ringler, M.D.; Chin, C.T.; Baum, T.; Giaconi, J.C.; O’Donnell, R.J.; Horvai, A.E.; Jones, K.D.; Link, T.M. CT-guided biopsy of bone and soft-tissue lesions: Role of on-site immediate cytologic evaluation. J. Vasc. Interv. Radiol. 2011, 22, 1024–1030. [Google Scholar] [CrossRef]
- Adams, S.C.; Potter, B.K.; Pitcher, D.J.; Temple, H.T. Office-based core needle biopsy of bone and soft tissue malignancies: An accurate alternative to open biopsy with infrequent complications. Clin. Orthop. Relat. Res. 2010, 468, 2774–2780. [Google Scholar] [CrossRef]
- Sung, K.S.; Seo, S.W.; Shon, M.S. The diagnostic value of needle biopsy for musculoskeletal lesions. Int. Orthop. 2009, 33, 1701–1706. [Google Scholar] [CrossRef]
- Errani, C.; Traina, F.; Perna, F.; Calamelli, C.; Faldini, C. Current concepts in the biopsy of musculoskeletal tumors. Sci. World J. 2013, 2013, 538152. [Google Scholar] [CrossRef]
- Bickels, J.; Jelinek, J.S.; Shmookler, B.M.; Neff, R.S.; Malawer, M.M. Biopsy of musculoskeletal tumors. Current concepts. Clin. Orthop. Relat. Res. 1999, 368, 212–219. [Google Scholar] [CrossRef]
- Lok, J.; Tse, K.Y.; Lee, E.Y.P.; Wong, R.W.C.; Cheng, I.S.Y.; Chan, A.N.H.; Leung, C.K.L.; Cheung, A.N.Y.; Ip, P.P.C. Intraoperative Frozen Section Biopsy of Uterine Smooth Muscle Tumors: A Clinicopathologic Analysis of 112 Cases with Emphasis on Potential Diagnostic Pitfalls. Am. J. Surg. Pathol. 2021, 45, 1179–1189. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tada, K.; Higuchi, T.; Yonezawa, H.; Morinaga, S.; Araki, Y.; et al. Diagnostic accuracies of intraoperative frozen section and permanent section examinations for histological grades during open biopsy of bone tumors. Int. J. Clin. Oncol. 2021, 26, 613–619. [Google Scholar] [CrossRef]
- Wallace, M.T.; Lin, P.P.; Bird, J.E.; Moon, B.S.; Satcher, R.L.; Lewis, V.O. The Accuracy and Clinical Utility of Intraoperative Frozen Section Analysis in Open Biopsy of Bone. J. Am. Acad. Orthop. Surg. 2019, 27, 410–417. [Google Scholar] [CrossRef]
- Sezak, M.; Doganavsargil, B.; Kececi, B.; Argin, M.; Sabah, D. Feasibility and clinical utility of intraoperative consultation with frozen section in osseous lesions. Virchows Arch. 2012, 461, 195–204. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tada, K.; Higuchi, T.; Yonezawa, H.; Morinaga, S.; Araki, Y.; et al. Accuracy of histological grades from intraoperative frozen-section diagnoses of soft-tissue tumors. Int. J. Clin. Oncol. 2020, 25, 2158–2165. [Google Scholar] [CrossRef] [PubMed]

| Variable | Test Group n = 59 | Control Group n = 51 | χ2 or t Value | p Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 37 | 28 | 0.690 | 0.406 |
| Female | 22 | 23 | ||
| Age (mean + SD) | 53.61 ± 19.05 | 39.53 ± 17.44 | ||
| Localization of the lesion biopsy | ||||
| Spinal column | ||||
| Thoracic vertebrae | 1 | 1 | 0.011 | 0.917 |
| Lumbar vertebrae | 2 | 1 | 0.016 | 0.898 |
| Sacrum | 2 | 2 | 0.131 | 0.717 |
| Upper extremity | ||||
| Humerus | 3 | 5 | 0.339 | 0.560 |
| Ulna | 1 | 1 | 0.011 | 0.917 |
| Upper soft tissue | 5 | 4 | 0.052 | 0.819 |
| Lower extremity | ||||
| Femur | 12 | 12 | 0.163 | 0.686 |
| Tibia | 2 | 3 | 0.028 | 0.867 |
| Calcaneus | 2 | 1 | 0.016 | 0.898 |
| Lower soft tissue | 13 | 9 | 0.329 | 0.566 |
| Pelvis | 8 | 8 | 0.100 | 0.752 |
| Trunk | 8 | 4 | 0.920 | 0.338 |
| Postsurgical histopathology diagnosis | ||||
| Primary benign tumor | 9 | 10 | 0.363 | 0.547 |
| Primary malignancies | 25 | 26 | 0.815 | 0.367 |
| Metastases | 19 | 12 | 1.017 | 0.313 |
| Tumor-like lesions | 6 | 3 | 0.220 | 0.639 |
| Biopsy guidance method | ||||
| CT guidance | 36 | 34 | 0.377 | 0.539 |
| Ultrasound aguidance | 23 | 17 |
| Postsurgical Histopathology Diagnosis | Test Group n = 59 | Normal Groups n = 51 | χ2 Value | p Value |
|---|---|---|---|---|
| Primary benign tumors | ||||
| Chondroma | 2 | 3 | 0.028 | 0.867 |
| Hemangioma | 1 | 2 | 0.016 | 0.898 |
| Lipoma | 1 | 2 | 0.016 | 0.898 |
| Schwannoma | 3 | 1 | 0.131 | 0.717 |
| Giant-cell tumor | 1 | 1 | 0.011 | 0.917 |
| Myxoma | 1 | 1 | 0.011 | 0.917 |
| Primary malignancies | ||||
| Liposarcoma | 4 | 4 | 0.024 | 0.878 |
| Lymphoma | 4 | 2 | 0.056 | 0.812 |
| Fibrosarcoma | 3 | 3 | 0.056 | 0.812 |
| Rhabdomyosarcoma | 1 | 1 | 0.011 | 0.917 |
| Leiomyosarcoma | 1 | 3 | 0.435 | 0.510 |
| Chondrosarcoma | 1 | 1 | 0.011 | 0.917 |
| Osteosarcoma | 9 | 10 | 0.363 | 0.547 |
| Ewing sarcoma | 1 | 2 | 0.016 | 0.898 |
| Granulosa cell tumor | 1 | 0 | 0.373 | 0.542 |
| Metastases | ||||
| Lung tumor | 6 | 4 | 0.008 | 0.928 |
| Breast tumor | 5 | 3 | 0.024 | 0.878 |
| Kidney tumor | 2 | 1 | 0.016 | 0.898 |
| Prostate tumor | 2 | 1 | 0.016 | 0.898 |
| Stomach tumor | 1 | 0 | 0.373 | 0.542 |
| Uterus tumor | 2 | 3 | 0.028 | 0.867 |
| Skin tumor | 1 | 0 | 0.373 | 0.542 |
| Tumor-like lesions | ||||
| Fibrous dysplasia of bone | 3 | 0 | 1.094 | 0.296 |
| Aneurysmal bone cyst | 1 | 2 | 0.016 | 0.898 |
| Simple cyst | 2 | 1 | 0.016 | 0.898 |
| Biopsy Group | χ2 Value | p Value | ||
|---|---|---|---|---|
| Test Group | Control Group | |||
| Overall diagnostic yield | 94.92% (56/59) | 82.36% (42/51) | 4.442 | 0.035 |
| Diagnostic accuracy | 94.92% (56/59) | 82.36% (42/51) | 4.442 | 0.035 |
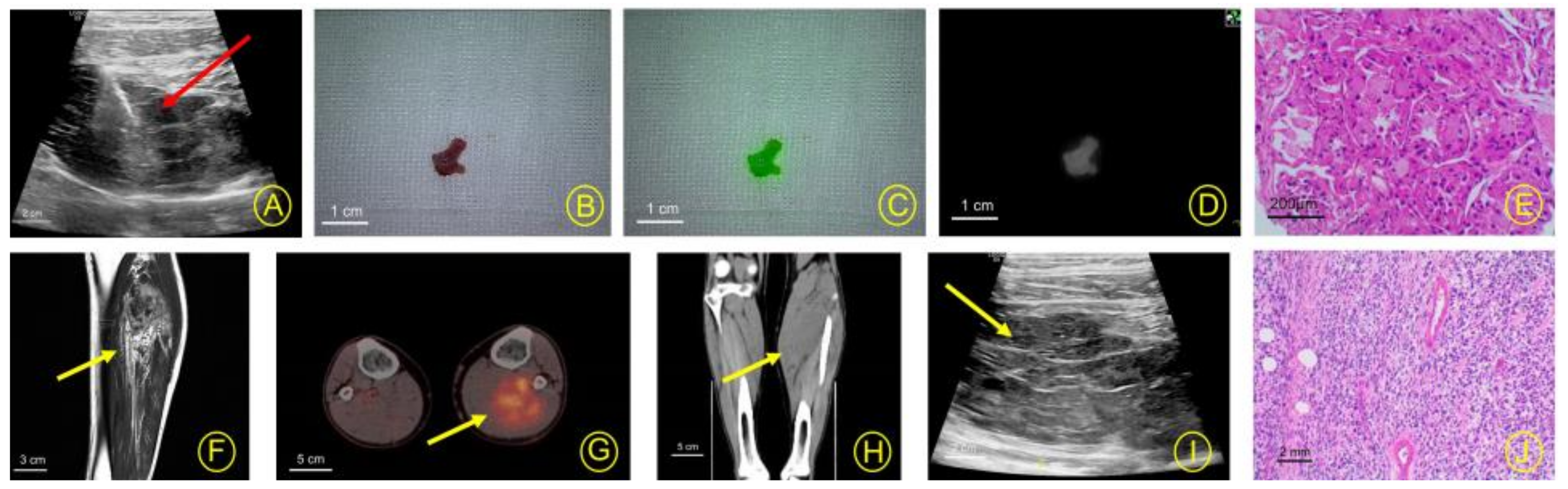
| Number | Age | Sex | Location | Max Dimension (mm) | Operation Time | ICG Dose | Time of Administration | First Fluorescent | Second Fluorescent | Bleeding Volume (mL) | Biopsy Dignosis | Open Surgey Dignosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | M | Femur | 45 | 0.5 h | 0.3 mg/kg | 3 h pre-op | yes | no | 20 | Tumor-like lesions | Tumor-like lesions |
| 2 | 32 | M | Lower soft tissue | 86 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 5 | Malignancies | Lymphoma |
| 3 | 53 | M | Trunk | 156 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | no | Yes | 10 | Malignancies | Granulosa cell tumor |
| 4 | 61 | F | Trunk | 55 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 20 | Malignancies | Osteosarcoma |
| 5 | 69 | F | Femur | 27 | 0.8 h | 0.3 mg/kg | 1 h pre-op | yes | no | 20 | Malignancies | Metastases |
| 6 | 21 | M | Femur | 49 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 40 | Malignancies | Osteosarcoma |
| 7 | 50 | F | Lower soft tissue | 165 | 0.5 h | 0.3 mg/kg | 1 h pre-op | yes | no | 8 | Malignancies | Osteosarcoma |
| 8 | 50 | F | Upper soft tissue | 91 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | no | no | 5 | Non-tumor | Metastases |
| 9 | 68 | M | Ulna | 39 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 21 | Malignancies | Metastases |
| 10 | 52 | F | Lower soft tissue | 125 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | no | no | 10 | Non-tumor | Liposarcoma |
| 11 | 73 | F | Spinal column | 39 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 6 | Malignancies | Metastases |
| 12 | 64 | F | Spinal column | 46 | 1 h | 0.3 mg/kg | 1.5 h pre-op | no | no | 10 | Non-tumor | Metastases |
| 13 | 45 | F | Spinal column | 42 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 5 | Malignancies | Metastases |
| 14 | 65 | M | Femur | 28 | 0.8 h | 0.3 mg/kg | 1.5 h pre-op | no | no | 12 | Tumor-like lesions | Tumor-like lesions |
| 15 | 45 | M | Tibia | 54 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 15 | Malignancies | Osteosarcoma |
| 16 | 64 | M | Upper soft tissue | 48 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 15 | Malignancies | Osteosarcoma |
| 17 | 68 | M | Calcaneus | 31 | 0.5 h | 0.3 mg/kg | 1 h pre-op | yes | no | 15 | Malignancies | Osteosarcoma |
| 18 | 80 | M | Upper soft tissue | 48 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 5 | Malignancies | Osteosarcoma |
| 19 | 18 | M | Femur | 54 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 15 | Malignancies | Osteosarcoma |
| 20 | 62 | F | Pelvis | 26 | 1 h | 0.3 mg/kg | 1.5 h pre-op | no | no | 10 | Tumor-like lesions | Tumor-like lesions |
| 21 | 67 | M | Pelvis | 100 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 20 | Malignancies | Metastases |
| 22 | 71 | M | Pelvis | 14 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 16 | Malignancies | Metastases |
| 23 | 52 | M | Humerus | 26 | 0.8 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 5 | Benign tumor | Chondroma |
| 24 | 66 | F | Spinal column | 36 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 5 | Benign tumor | Bone hemangioma |
| 25 | 23 | M | Calcaneus | 49 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 16 | Tumor-like lesions | Tumor-like lesions |
| 26 | 73 | F | Trunk | 84 | 0.5 h | 0.3 mg/kg | 2 h pre-op | yes | no | 5 | Malignancies | Lymphoma |
| 27 | 60 | M | Pelvis | 32 | 1 h | 0.3 mg/kg | 2 h pre-op | yes | no | 20 | Malignancies | Metastases |
| 28 | 51 | M | Femur | 86 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 15 | Malignancies | Metastases |
| 29 | 58 | F | Pelvis | 36 | 1 h | 0.3 mg/kg | 1.5 h pre-op | no | Yes | 10 | Malignancies | Metastases |
| 30 | 60 | M | Femur | 45 | 2 h | 0.3 mg/kg | 2 h pre-op | yes | no | 15 | Malignancies | Metastases |
| 31 | 50 | F | Tibia | 36 | 1 h | 0.3 mg/kg | 1.5 h pre-op | no | no | 10 | Tumor-like lesions | Tumor-like lesions |
| 32 | 35 | M | Pelvis | 186 | 1 h | 0.3 mg/kg | 2 h pre-op | no | Yes | 10 | Malignancies | Bone fibrosarcoma |
| 33 | 60 | M | Femur | 48 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 10 | Malignancies | Metastases |
| 34 | 75 | F | Lower soft tissue | 66 | 1 h | 0.3 mg/kg | 2 h pre-op | no | Yes | 10 | Malignancies | Rhabdomyosarcoma |
| 35 | 59 | F | Upper soft tissue | 78 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 5 | Benign tumor | Lipoma |
| 36 | 77 | M | Femur | 38 | 1 h | 0.3 mg/kg | 2 h pre-op | yes | no | 15 | Malignancies | Metastases |
| 37 | 78 | F | Lower soft tissue | 128 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 5 | Malignancies | Lymphoma |
| 38 | 73 | F | Trunk | 206 | 0.5 h | 0.3 mg/kg | 2 h pre-op | yes | no | 3 | Benign tumor | Schwannoma |
| 39 | 17 | M | Lower soft tissue | 65 | 0.5 h | 0.3 mg/kg | 2 h pre-op | yes | no | 5 | Malignancies | Lymphoma |
| 40 | 17 | F | Humerus | 46 | 0.5 h | 0.3 mg/kg | 2 h pre-op | yes | no | 10 | Benign tumor | Bone giant-cell tumor |
| 41 | 60 | M | Lower soft tissue | 102 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | no | Yes | 5 | Malignancies | Leiomyosarcoma |
| 42 | 61 | M | Lower soft tissue | 34 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 5 | Malignancies | Metastases |
| 43 | 41 | M | Femur | 55 | 1 h | 0.3 mg/kg | 2 h pre-op | yes | no | 10 | Malignancies | Chondrosarcoma |
| 44 | 81 | F | Pelvis | 61 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 5 | Tumor-like lesions | Tumor-like lesions |
| 45 | 47 | M | Trunk | 115 | 0.5 h | 0.3 mg/kg | 2 h pre-op | yes | no | 3 | Malignancies | Fibrosarcoma |
| 46 | 77 | M | Pelvis | 18 | 1 h | 0.3 mg/kg | 2 h pre-op | no | Yes | 10 | Malignancies | Fibrosarcoma |
| 47 | 24 | M | Femur | 105 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 10 | Benign tumor | Chondroma |
| 48 | 32 | M | Trunk | 71 | 1 h | 0.3 mg/kg | 2 h pre-op | yes | no | 5 | Malignancies | Ewing sarcoma |
| 49 | 66 | F | Trunk | 25 | 1 h | 0.3 mg/kg | 1.5 h pre-op | yes | no | 5 | Malignancies | Metastases |
| 50 | 61 | M | Lower soft tissue | 59 | 0.5 h | 0.3 mg/kg | 1.5 h pre-op | no | no | 5 | Benign tumor | Myxoma |
| 51 | 11 | F | Humerus | 42 | 1 h | 0.3 mg/kg | 1.5 h pre-op | no | Yes | 20 | Malignancies | Osteosarcoma |
| 52 | 47 | M | Upper soft tissue | 26 | 0.5 h | 0.3 mg/kg | 2 h pre-op | yes | no | 5 | Malignancies | Metastases |
| 53 | 61 | M | Lower soft tissue | 105 | 1.5 h | 0.3 mg/kg | 0.5 h pre-op | yes | no | 5 | Malignancies | Liposarcoma |
| 54 | 71 | F | Femur | 54 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 10 | Malignancies | Metastases |
| 55 | 51 | M | Lower soft tissue | 65 | 1 h | 0.3 mg/kg | 0.5 h pre-op | yes | no | 5 | Benign tumor | Schwannoma |
| 56 | 15 | M | Lower soft tissue | 57 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 5 | Malignancies | Liposarcoma |
| 57 | 22 | M | Lower soft tissue | 61 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 10 | Malignancies | Liposarcoma |
| 58 | 81 | M | Trunk | 66 | 1 h | 0.3 mg/kg | 0.5 h pre-op | yes | no | 5 | Malignancies | Metastases |
| 59 | 48 | M | Spinal column | 51 | 1 h | 0.3 mg/kg | 1 h pre-op | yes | no | 8 | Benign tumor | Schwannoma |
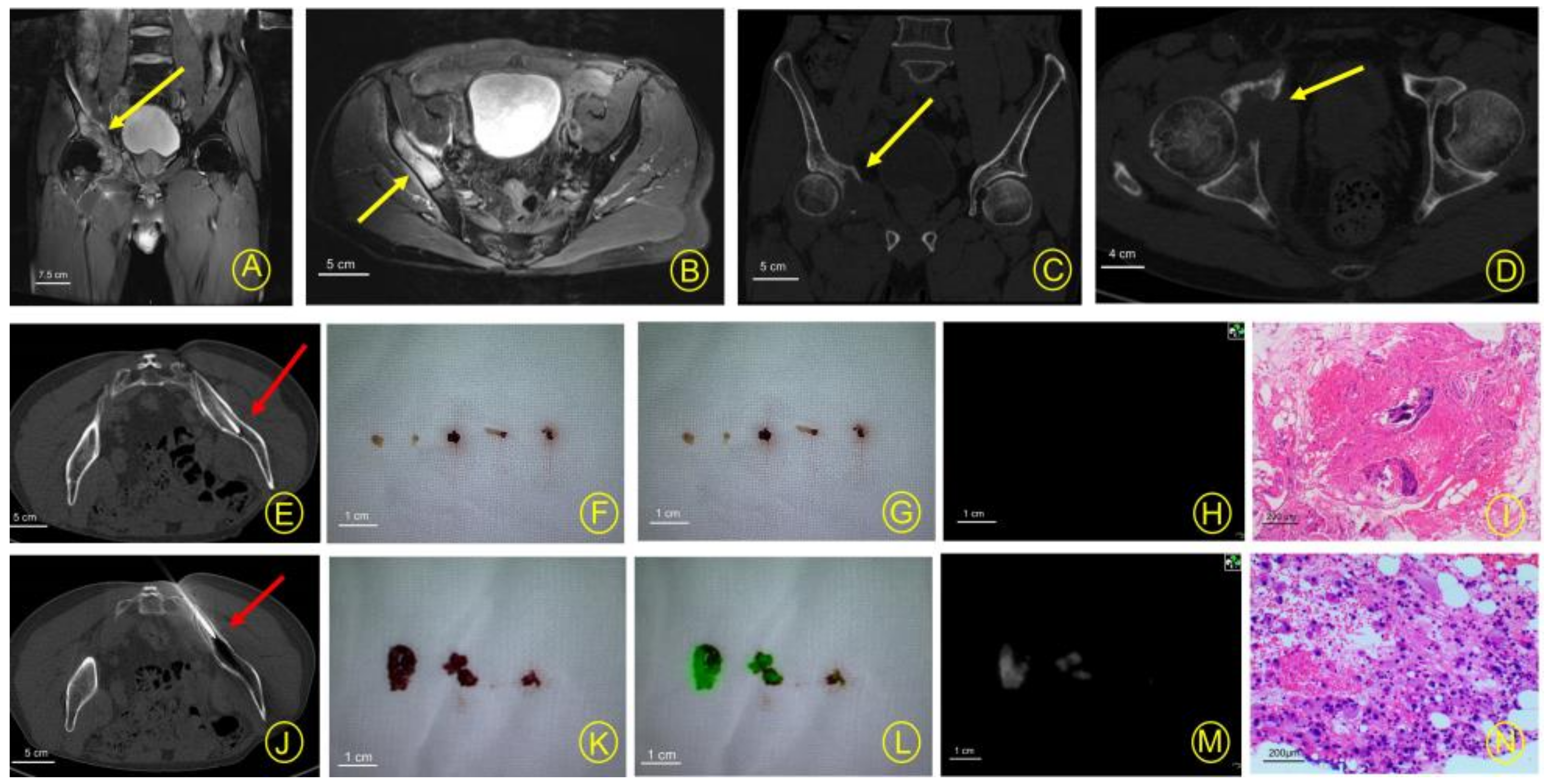
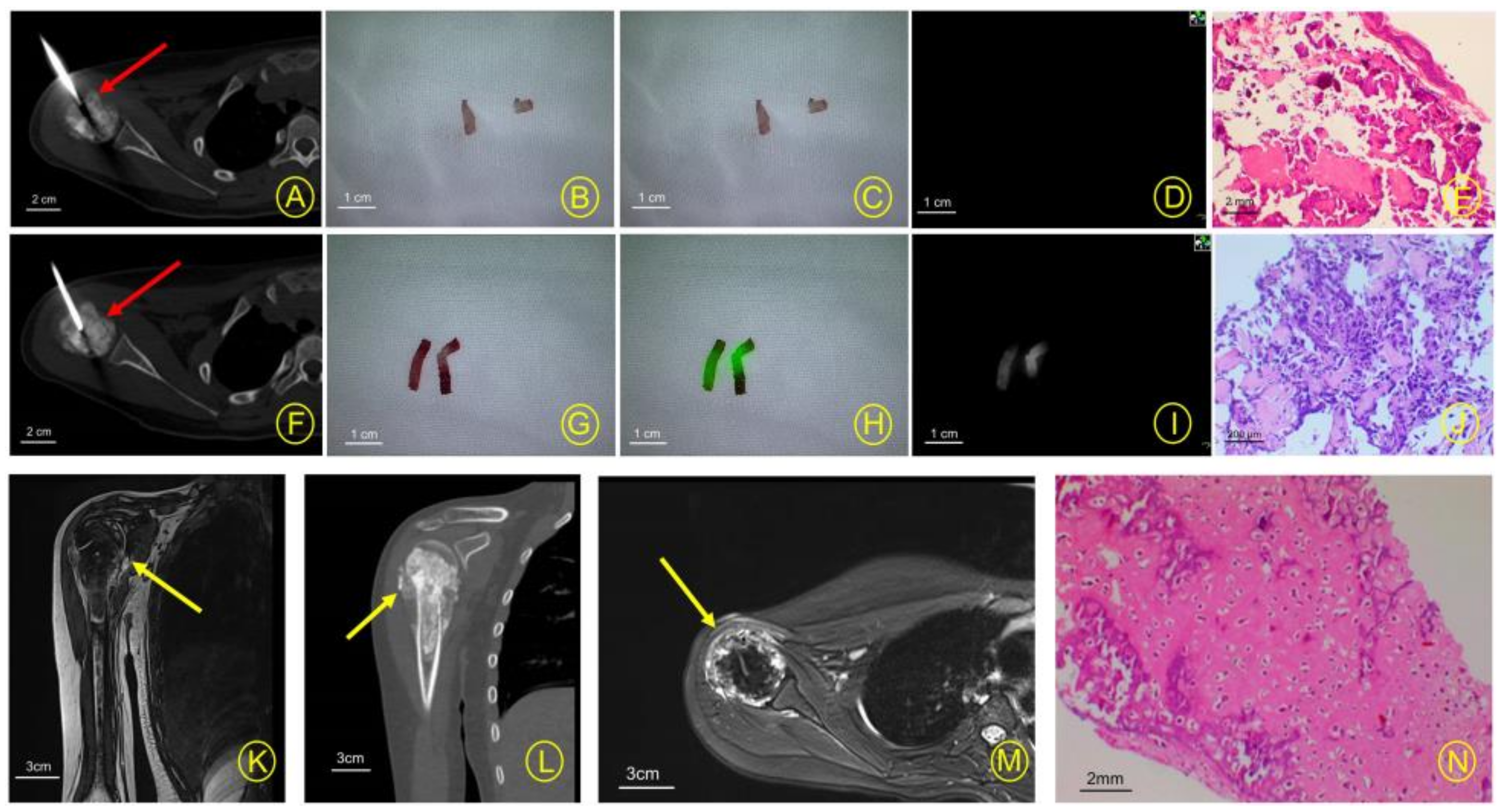
| Number | Location | First Real-Time Fluorescence | Second Real-Time Fluorescence | First Biopsy Histopathology Diagnosis | Supplementary Biopsy Histopathology Diagnosis | After Surgery Resection Histopathology Diagnosis |
|---|---|---|---|---|---|---|
| 1 | Trunk | No | Yes | Non-tumor | Granulosa cell tumor | Granulosa cell tumor |
| 2 | Pelvis | No | Yes | Non-tumor | Metastatic adenocarcinoma | Metastatic adenocarcinoma |
| 3 | Pelvis | No | Yes | Non-tumor | Fibrosarcoma | Fibrosarcoma |
| 4 | Lower extremity | No | Yes | Non-tumor | Rhabdomyosarcoma | Rhabdomyosarcoma |
| 5 | Lower extremity | No | Yes | Non-tumor | Leiomyosarcoma | Leiomyosarcoma |
| 6 | Pelvis | No | Yes | Non-tumor | Fibrosarcoma | Fibrosarcoma |
| 7 | Humerus | No | Yes | Non-tumor | Osteosarcoma | Osteosarcoma |
| 8 | Trunk | No | No | Non-tumor | Non-tumor | Metastatic squamous cell carcinoma |
| 9 | Lower extremity | No | No | Non-tumor | Non-tumor | Liposarcoma |
| 10 | Spine | No | No | Non-tumor | Non-tumor | Metastatic adenocarcinoma |
| 11 | Femur | No | No | Tumor-like lesions | Tumor-like lesions | Tumor-like lesions |
| 12 | Pelvis | No | No | Tumor-like lesions | Tumor-like lesions | Tumor-like lesions |
| 13 | Tibia | No | No | Tumor-like lesions | Tumor-like lesions | Tumor-like lesions |
| 14 | Lower extremity | No | No | Tumor-like lesions | Tumor-like lesions | Tumor-like lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Zhong, A.; Lei, J.; Deng, Z.; Zhu, X.; Wei, R.; Huang, H.; Chen, Z.; Cai, L.; Xie, Y. Application of Indocyanine Green Fluorescence Imaging in Assisting Biopsy of Musculoskeletal Tumors. Cancers 2023, 15, 2402. https://doi.org/10.3390/cancers15082402
He S, Zhong A, Lei J, Deng Z, Zhu X, Wei R, Huang H, Chen Z, Cai L, Xie Y. Application of Indocyanine Green Fluorescence Imaging in Assisting Biopsy of Musculoskeletal Tumors. Cancers. 2023; 15(8):2402. https://doi.org/10.3390/cancers15082402
Chicago/Turabian StyleHe, Siyuan, Ang Zhong, Jun Lei, Zhouming Deng, Xiaobin Zhu, Renxiong Wei, Huayi Huang, Zhenyi Chen, Lin Cai, and Yuanlong Xie. 2023. "Application of Indocyanine Green Fluorescence Imaging in Assisting Biopsy of Musculoskeletal Tumors" Cancers 15, no. 8: 2402. https://doi.org/10.3390/cancers15082402
APA StyleHe, S., Zhong, A., Lei, J., Deng, Z., Zhu, X., Wei, R., Huang, H., Chen, Z., Cai, L., & Xie, Y. (2023). Application of Indocyanine Green Fluorescence Imaging in Assisting Biopsy of Musculoskeletal Tumors. Cancers, 15(8), 2402. https://doi.org/10.3390/cancers15082402





