Pterostilbene and Probiotic Complex in Chemoprevention of Putative Precursor Lesions for Colorectal Cancer in an Experimental Model of Intestinal Carcinogenesis with 1,2-Dimethylhydrazine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. PRO and PS Preparation
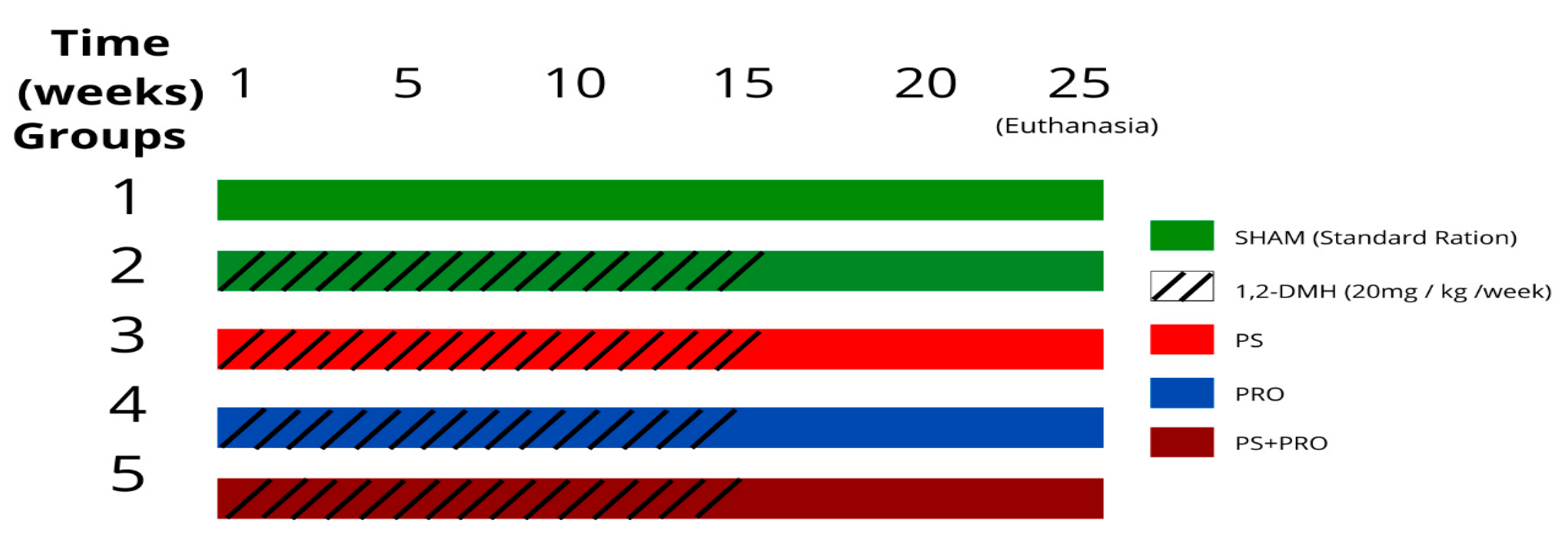
2.3. Experimental Design
2.4. Surgical Procedure and Sample Preparation
2.5. Analyzed Variables
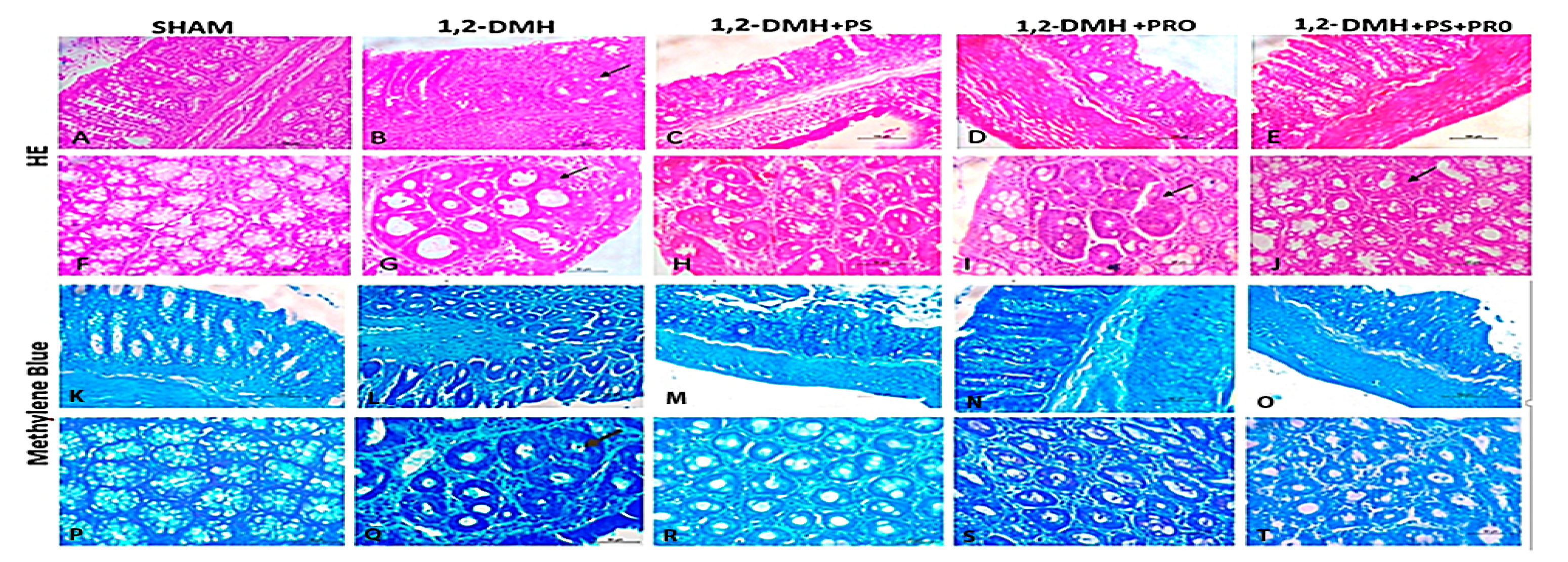
2.5.1. Microscopy
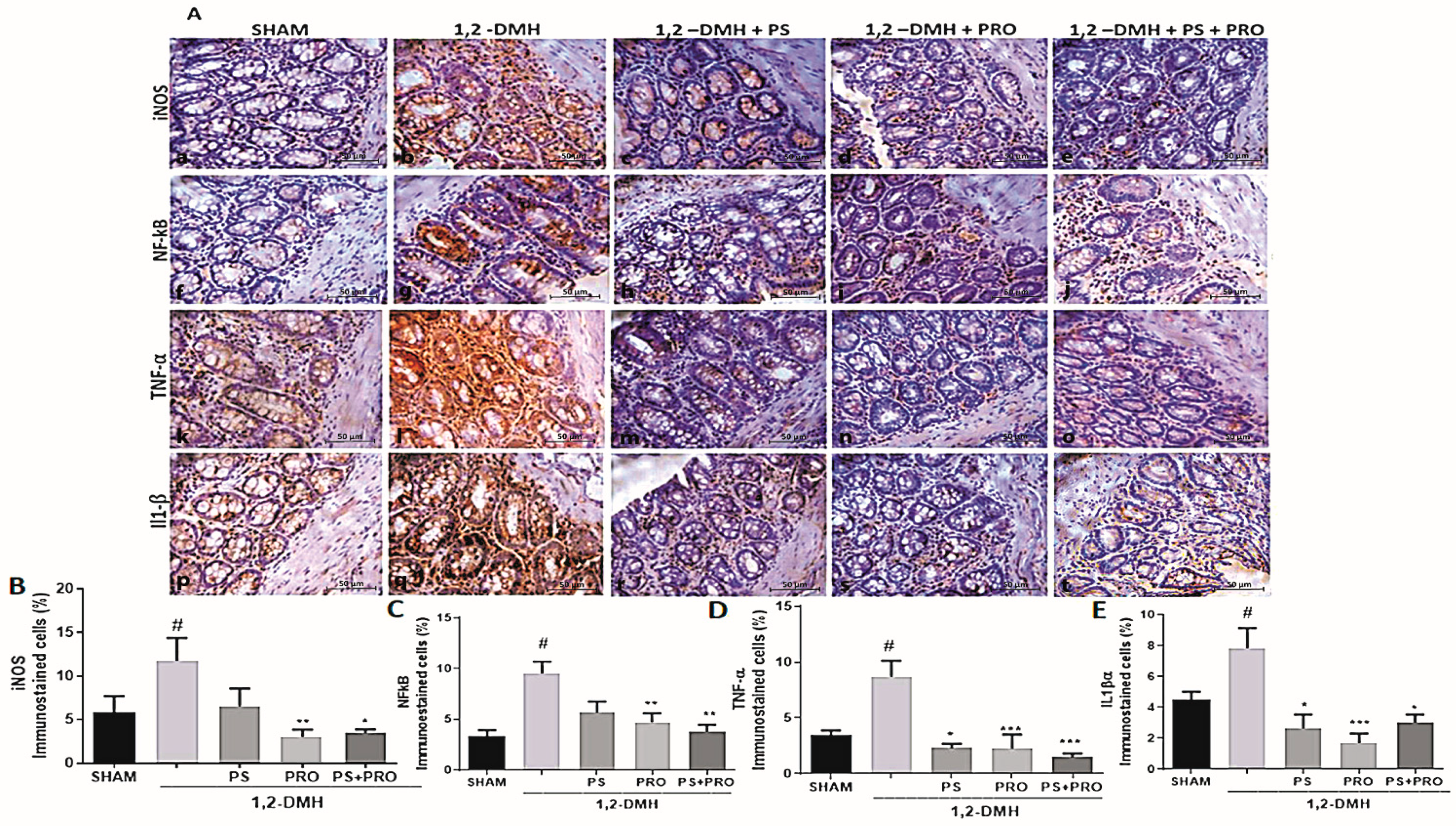
2.5.2. Immunohistochemistry by the Tissue Microarray Technique
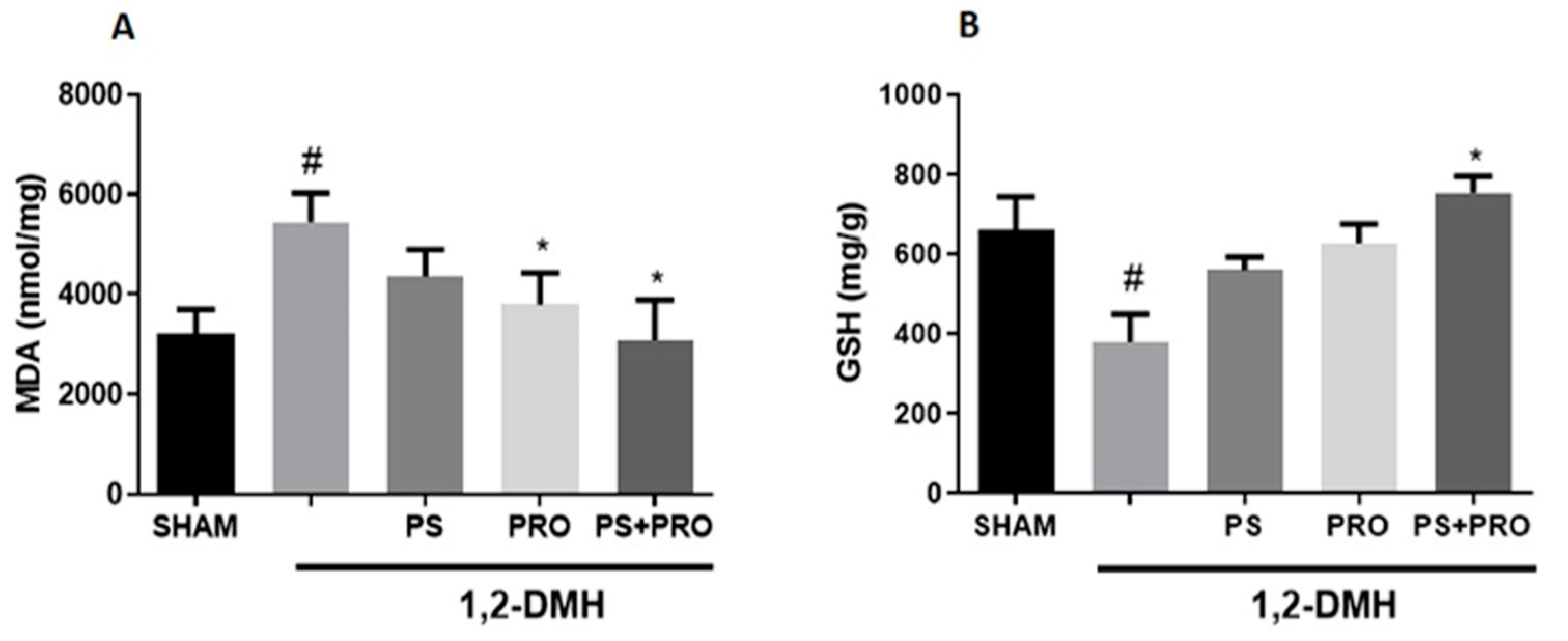
2.5.3. Oxidative Stress Markers
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Vasconcelos, S.M.L.; Goulart, M.O.F.; Moura, J.B.F.; Manfredini, V.; Benfato, M.S.; Kubota, L.T. Reactive oxygen and nitrogen species, antioxidants and markers of oxidative damage in human blood: Main analytical methods for their determination. Quím. Nova 2007, 30, 1323–1338. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Araújo, J.R.; Gonçalves, P.; Martel, F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr. Res. 2011, 31, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Crosara Teixeira, M.; Braghiroli, M.I.; Sabbaga, J.; Hoff, P.M. Primary prevention of colorectal cancer: Myth or reality. World J. Gastroenterol. 2014, 20, 15060–15069. [Google Scholar] [CrossRef] [PubMed]
- Hen, C.C.; Lin, W.C.; Kong, M.S.; Shi, H.N.; Walker, W.A.; Lin, C.Y.; Huang, C.T.; Lin, Y.C.; Jung, S.M.; Lin, T.Y. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef]
- Mahmod, A.I.; Haif, S.K.; Kamal, A.; Al-Ataby, I.A.; Talib, W.H. Chemoprevention effect of the Mediterranean diet on colorectal cancer: Current studies and future prospects. Front. Nutr. 2022, 9, 924192. [Google Scholar] [CrossRef]
- Ganesan, K.; Jayachandran, M.; Xu, B. Diet-Derived Phytochemicals Targeting Colon Cancer Stem Cells and Microbiota in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 3976. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yáñez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, anti-inflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Fernández, M.; Picó, Y.; Mañes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses. Oncol. Rep. 2016, 35, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; La Spina, M.; Mattarei, A.; Paradisi, C.; Zoratti, M.; Biasutto, L. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol. Nutr. Food Res. 2014, 58, 2122–2132. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Sun, C.; Chen, X.; Han, L.; Wang, T.; Liu, J.; Chen, X.; Zhao, D. Effect of Pterostilbene, a Natural Derivative of Resveratrol, in the Treatment of Colorectal Cancer through Top1/Tdp1-Mediated DNA Repair Pathway. Cancers 2021, 13, 4002. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Ji, S.; Jia, P.; Chen, Y.; Li, Y.; Wang, T. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free Radic. Biol. Med. 2021, 177, 1–14. [Google Scholar] [CrossRef]
- Panebianco, C.; Latiano, T.; Pazienza, V. Microbiota Manipulation by Probiotics Administration as Emerging Tool in Cancer Prevention and Therapy. Front. Oncol. 2020, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative activity of probiotics. Arch. Med. Sci. 2019, 17, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.E.; Stuer-Lauridsen, B.; Eskesen, D. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12(®). Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef]
- Khavari-Daneshvar, H.; Mosavi, M.; Khodayari, H.; Rahimi, E.; Ranji, P.; Mohseni, A.H.; Mahmudian, R.; Shidfar, F.; Agah, S.; Alizadeh, A.M. Modifications of mice gut microflora following oral consumption of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics. Turk. J. Med. Sci. 2017, 47, 689–694. [Google Scholar] [CrossRef]
- Shanahan, F. A commentary on the safety of probiotics. Gastroenterol. Clin. N. Am. 2012, 41, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The Role of Probiotics in Colorectal Cancer Management. Evid. Based Complement. Altern. Med. 2020, 2020, 3535982. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Jing, S.; Zhang, Q.; Wu, G. Pterostilbene protects against acute renal ischemia reperfusion injury and inhibits oxidative stress, inducible nitric oxide synthase expression and inflammation in rats via the Toll-like receptor 4/nuclear factor-κB signaling pathway. Exp. Ther. Med. 2018, 15, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.L.; Chew, M.T.; Ngeow, Y.F.; Lim, W.W.D.; Peh, S.C. Colon Carcinogenesis: The Interplay between Diet and Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 603086. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.F.; Feitosa, M.R.; Rocha, J.J.R.; Féres, O. A review of experimental models in colorectal carcinogenesis. J. Coloproctol. 2016, 36, 53–57. [Google Scholar] [CrossRef]
- Gois, E., Jr.; Daniel, R.A.; Parra, R.S.; Almeida, A.L.; Rocha, J.J.; Garcia, S.B.; Féres, O. Hyperbaric oxygen therapy reduces COX-2 expression in a dimethylhydrazine-induced rat model of colorectal carcinogenesis. Undersea Hyperb. Med. 2012, 39, 693–698. [Google Scholar]
- Perše, M.; Cerar, A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J. Biomed. Biotechnol. 2011, 2011, 473964. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Muhammad, J.S.; Siddiqui, R. The role of gut microbiome in cancer genesis and cancer prevention. Health Sci. Rev. 2022, 2, 100010. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, Y.J. The role of microbiome in colorectal carcinogenesis and its clinical potential as a target for cancer treatment. Intest. Res. 2022, 20, 31–42. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Patyar, S.; Patyar, R.R.; Medhi, B.; Khanduja, K.L. Chemopreventive effect of artesunate in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. J. Adv. Pharm. Technol. Res. 2017, 8, 102–107. [Google Scholar] [CrossRef]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987, 37, 147–151. [Google Scholar] [CrossRef]
- MacPherson, B.R.; Pfeiffer, C.J. Experimental production of diffuse colitis in rats. Digestion 1978, 17, 135–150. [Google Scholar] [CrossRef]
- Bird, R.P.; Good, C.K. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol. Lett. 2000, 112–113, 395–402. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Hsu, S.M.; Raine, L.; Fanger, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar] [CrossRef]
- Scopsi, L.; Larsson, L.I. Increased sensitivity in peroxidase immunocytochemistry. A comparative study of a number of peroxidase visualization methods employing a model system. Histochemistry 1986, 84, 221–230. [Google Scholar] [CrossRef]
- Brey, E.M.; Lalani, Z.; Johnston, C.; Wong, M.; McIntire, L.V.; Duke, P.J.; Patrick, C.W., Jr. Automated selection of DAB-labeled tissue for immunohistochemical quantification. J. Histochem. Cytochem. 2003, 51, 575–584. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Domijan, A.M.; Ralić, J.; Radić Brkanac, S.; Rumora, L.; Žanić-Grubišić, T. Quantification of malondialdehyde by HPLC-FL—Application to various biological samples. Biomed. Chromatogr. 2015, 29, 41–46. [Google Scholar] [CrossRef]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Zalewska, A.; Pryczynicz, A.; Matowicka-Karna, J.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef]
- Wang, H.; Fan, C.; Zhao, Z.; Zhai, Z.; Hao, Y. Anti-inflammatory effect of Bifidobacterium animalis subsp lactis A6 on DSS-induced colitis in mice. J. Appl. Microbiol. 2022, 133, 2063–2073. [Google Scholar] [CrossRef]
- Bousserouel, S.; Kauntz, H.; Gossé, F.; Bouhadjar, M.; Soler, L.; Marescaux, J.; Raul, F. Identification of gene expression profiles correlated to tumor progression in a preclinical model of colon carcinogenesis. Int. J. Oncol. 2010, 36, 1485–1490. [Google Scholar] [CrossRef]
- Kauntz, H.; Bousserouel, S.; Gosse, F.; Marescaux, J.; Raul, F. Silibinin, a natural flavonoid, modulates the early expression of chemoprevention biomarkers in a preclinical model of colon carcinogenesis. Int. J. Oncol. 2012, 41, 849–854. [Google Scholar] [CrossRef]
- Paul, S.; DeCastro, A.J.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Zhou, R.; Rimando, A.M.; Suh, N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the beta-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis 2010, 31, 1272–1278. [Google Scholar] [CrossRef]
- Jansson, A.; Gentile, M.; Sun, X.F. p53 Mutations are present in colorectal cancer with cytoplasmic p53 accumulation. Int. J. Cancer 2001, 92, 338–341. [Google Scholar] [CrossRef]
- Brentnall, T.A.; Crispin, D.A.; Rabinovitch, P.S.; Haggitt, R.C.; Rubin, C.E.; Stevens, A.C.; Burmer, G.C. Mutations in the p53 gene: An early marker of neoplastic progression in ulcerative colitis. Gastroenterology 1994, 107, 369–378. [Google Scholar] [CrossRef]
- Humphries, A.; Wright, N.A. Colonic crypt organization and tumorigenesis. Nat. Rev. Cancer 2008, 8, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.S.; Rabinovitch, P.S.; Haggitt, R.C.; Blount, P.L.; Dean, P.J.; Rubin, C.E.; Reid, B.J. Distribution of aneuploid cell populations in ulcerative colitis with dysplasia or cancer. Gastroenterology 1991, 101, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yoshimi, N.; Hirose, Y.; Matsunaga, K.; Katayama, M.; Sakata, K.; Shimizu, M.; Kuno, T.; Mori, H. Sequential analysis of morphological and biological properties of beta-catenin-accumulated crypts, provable premalignant lesions independent of aberrant crypt foci in rat colon carcinogenesis. Cancer Res. 2001, 61, 1874–1878. [Google Scholar]
- Yamada, Y.; Yoshimi, N.; Hirose, Y.; Hara, A.; Shimizu, M.; Kuno, T.; Katayama, M.; Qiao, Z.; Mori, H. Suppression of occurrence and advancement of beta-catenin-accumulated crypts, possible premalignant lesions of colon cancer, by selective cyclooxygenase-2 inhibitor, celecoxib. Jpn. J. Cancer Res. 2001, 92, 617–623. [Google Scholar] [CrossRef]
- Furihata, T.; Kawamata, H.; Kubota, K.; Fujimori, T. Evaluation of the malignant potential of aberrant crypt foci by immunohistochemical staining for beta-catenin in inflammation-induced rat colon carcinogenesis. Int. J. Mol. Med. 2002, 9, 353–358. [Google Scholar] [CrossRef]
- Suh, N.; Paul, S.; Hao, X.; Simi, B.; Xiao, H.; Rimando, A.M.; Reddy, B.S. Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin. Cancer Res. 2007, 13, 350–355. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Tsai, M.L.; Wang, Y.J.; Cheng, A.C.; Lai, W.M.; Badmaev, V.; Ho, C.T.; Pan, M.H. Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxymethane-treated mice. J. Agric. Food Chem. 2010, 58, 8833–8841. [Google Scholar] [CrossRef]
- Li, F.; Wang, Q.; Han, Y.; Song, M.; Cai, X.; Goulette, T.; Xiao, H. Dietary Pterostilbene Inhibited Colonic Inflammation in Dextran-Sodium-Sulfate-Treated Mice: A Perspective of Gut Microbiota. Infect. Microbes Dis. 2021, 3, 22–29. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, X.; Cai, X.; Song, M.; Zheng, J.; Pan, C.; Qiu, P.; Zhang, L.; Zhou, S.; Tang, Z.; et al. Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol. Nutr. Food Res. 2016, 60, 1924–1932. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wu, X.; Li, F.; Song, M.; Wang, M.; Cai, X.; Li, Z.; Gao, Z.; Zheng, J.; et al. Gastrointestinal biotransformation and tissue distribution of pterostilbene after long-term dietary administration in mice. Food Chem. 2022, 372, 131213. [Google Scholar] [CrossRef]
- Mohania, D.; Kansal, V.K.; Shah, D. Probiotic Dahi Containing Lactobacillus acidophilus and Lactobacillus plantarum Suppresses DMH Induced Preneoplastic lesions in early Colorectal Carcinogenesis in Wistar Rats. Am. J. Cancer Biol. 2013, 17, 325–333. [Google Scholar]
- Rao, C.V.; Sanders, M.E.; Indranie, C.; Simi, B.; Reddy, B.S. Prevention of colonic preneoplastic lesions by the probiotic Lactobacillus acidophilus NCFMTM in F344 rats. Int. J. Oncol. 1999, 14, 939–944. [Google Scholar] [CrossRef]
- Chang, J.H.; Shim, Y.Y.; Cha, S.K.; Reaney, M.J.T.; Chee, K.M. Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J. Med. Microbiol. 2012, 61 Pt 3, 361–368. [Google Scholar] [CrossRef]
- Štofilová, J.; Szabadosová, V.; Hrčková, G.; Salaj, R.; Bertková, I.; Hijová, E.; Strojný, L.; Bomba, A. Co-administration of a probiotic strain Lactobacillus plantarum LS/07 CCM7766 with prebiotic inulin alleviates the intestinal inflammation in rats exposed to N,N-dimethylhydrazine. Int. Immunopharmacol. 2015, 24, 361–368. [Google Scholar] [CrossRef]
- Jacouton, E.; Chain, F.; Sokol, H.; Langella, P.; Bermúdez-Humarán, L.G. Probiotic Strain Lactobacillus casei BL23 Prevents Colitis-Associated Colorectal Cancer. Front. Immunol. 2017, 8, 1553. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, H.M.; Yang, K.M.; Kim, S.A.; Kim, S.K.; An, M.J.; Park, J.J.; Lee, S.K.; Kim, T.I.; Kim, W.H.; et al. Bifidobacterium lactis inhibits NF-kappaB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflamm. Bowel Dis. 2010, 16, 1514–1525. [Google Scholar] [CrossRef]
- Vivinus-Nébot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef]
- Marzo, F.; Jauregui, P.; Barrenetxe, J.; Martínez-Peñuela, A.; Ibañez, F.C.; Milagro, F.I. Effect of a Diet Supplemented with Sphingomyelin and Probiotics on Colon Cancer Development in Mice. Probiotics Antimicrob. Proteins 2022, 14, 407–414. [Google Scholar] [CrossRef]
- Foo, N.P.; Ou Yang, H.; Chiu, H.H.; Chan, H.Y.; Liao, C.C.; Yu, C.K.; Wang, Y.J. Probiotics prevent the development of 1,2-dimethylhydrazine (DMH)-induced colonic tumorigenesis through suppressed colonic mucosa cellular proliferation and increased stimulation of macrophages. J. Agric. Food Chem. 2011, 59, 13337–13345. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Viladomiu, M.; Pedragosa, M.; De Simone, C.; Hontecillas, R. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS ONE 2012, 7, e34676. [Google Scholar] [CrossRef]
- Liboredo, J.C.; Anastácio, L.R.; Pelúzio Mdo, C.; Valente, F.X.; Penido, L.C.; Nicoli, J.R.; Correia, M.I. Effect of probiotics on the development of dimethylhydrazine-induced preneoplastic lesions in the mice colon. Acta Cirúrgica Bras. 2013, 28, 367–372. [Google Scholar] [CrossRef]
- Agah, S.; Alizadeh, A.M.; Mosavi, M.; Ranji, P.; Khavari-Daneshvar, H.; Ghasemian, F.; Bahmani, S.; Tavassoli, A. More Protection of Lactobacillus acidophilus Than Bifidobacterium bifidum Probiotics on Azoxymethane-Induced Mouse Colon Cancer. Probiotics Antimicrob. Proteins 2019, 11, 857–864. [Google Scholar] [CrossRef]
- Winawer, S.J. Natural history of colorectal cancer. Am. J. Med. 1999, 106, 3S–6S; discussion 50S–51S. [Google Scholar] [CrossRef]
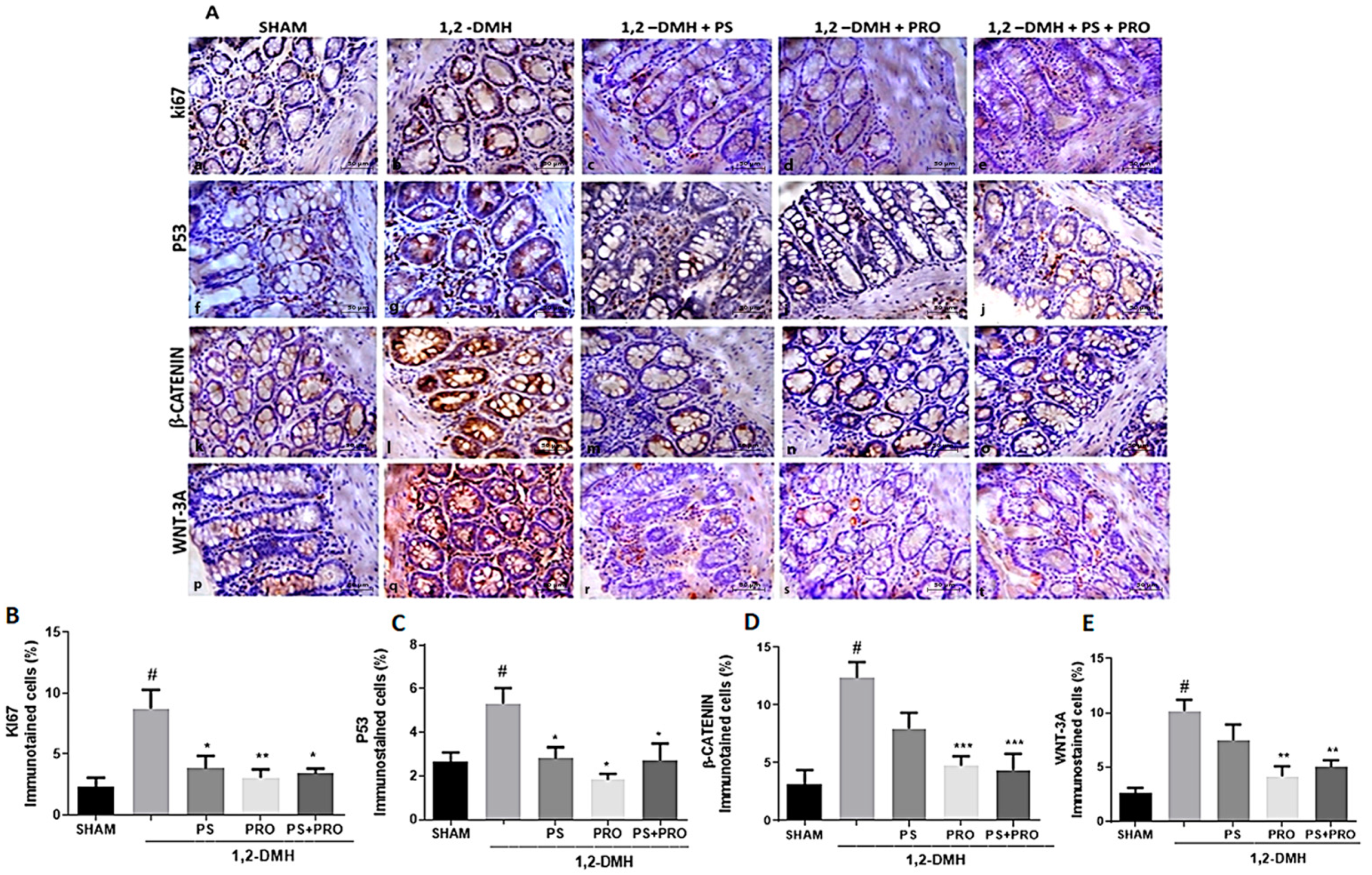
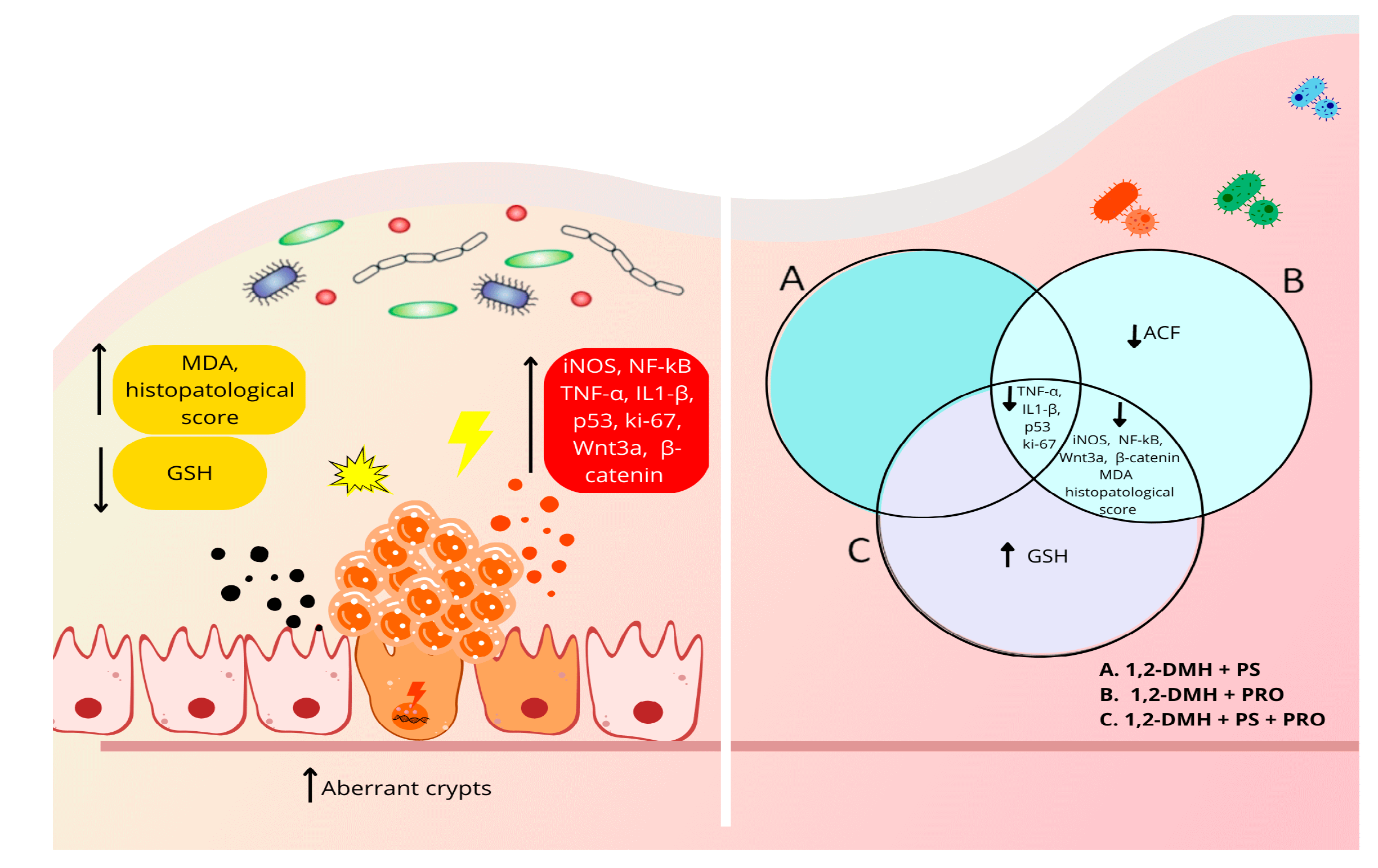
| Intestinal Segments | Experimental Groups | ||||
|---|---|---|---|---|---|
| Sham | 1,2-DMH | 1,2-DMH + PS | 1,2-DMH + PRO | 1,2-DMH + PS + PRO | |
| Proximal | 0.0 (0–0.1) | 1.8 (0.5–3.0) | 0.5 (0–2) | 0.5 (0–2) | 0.5 (0–1) |
| Medium | 0.0 (0–1) | 1.9 (1.4–3.0) # | 1.0 (0–2.8) | 0.126 (0–1.4) * | 0.25 (0–1) * |
| Distal | 1.1 (0.3–2) | 4.3 (3–6) # | 2.25 (1–5) | 2.0 (0.5–3.33) * | 3.0 (1–5) |
| Intestinal Segments | Experimental Groups | ||||
|---|---|---|---|---|---|
| Sham | 1,2-DMH | 1,2-DMH + PS | 1,2-DMH + PRO | 1,2-DMH + PS + PRO | |
| Proximal | 1 (0–1) | 3 (1–3) # | 2 (1–3) | 2 (1–3) | 2 (1–2) |
| Medium | 0 (0–1) | 2 (1–3) # | 2 (0–2) | 1 (1–2) * | 1 (0–2) * |
| Distal | 0 (0–1) | 3 (2–3) # | 2 (1–3) | 1 (1–2) ** | 2 (1–2) ** |
| Studies | Bacteria Strains | Concentration | Supplementation Time | Benefits |
|---|---|---|---|---|
| Wang et al., 2022 [45] | B. lactis A6 | 4 × 109 CFU/day | 3 weeks | ↓ MDA, ↑ SOD, GSH, and ↓ TNFα, IL-1β and IL-6 levels in colon tissues |
| Kim et al., 2010 [67] | B. lactis | 5 × 109 CFU/g | 9 weeks | ↓ NF-kB and COX-2 expression |
| Mohania et al., 2013 [62] | L. acidophilus and L. plantarum | 2 × 109 CFU/g | 32 weeks | ↓ Number of ACF |
| Rao et al., 1999 [63] | L. acidophilus | diet containing 0.2% or 4% lyophilized cultures | 10 weeks | ↓ Number of ACF |
| Chang et al., 2012 [64] | L. acidophilus | 2 × 109 CFU/mL | 10 weeks | ↓ Number of ACF |
| Štofilová et al., 2015 [65] | L. plantarum | 1 × 109 CFU/mL | 28 weeks | ↓ Pro-inflammatory cytokines (IL-2, IL-6, IL-17, and TNF-α,), NF- κB, COX-2, and iNOS proteins; ↑ goblet cell |
| Jacouton et al., 2017 [66] | L. casei BL23 | 5 × 109 CFU/mL | 46 days | ↓ Ki-67 immunolabeling |
| Marzo et al., 2022 [69] | L. casei and B. bifidum | 1 × 109 CFU/mL | 66 days | ↓ Number of ACF |
| Foo et al., 2011 [70] | L. gasseri and B. longum | 1 × 1011 CFU/g and 5 × 109 CFU/g | 24 weeks | ↓ Number of ACF |
| Bassaganya-Riera et al., 2012 [71] | L.casei, L. plantarum, L. bulgaricus, L. acidophilus, B.longum, B. breve, B. in-fantil, and Streptococcus thermophilus | 1.2 billion bacteria per mouse/day | 68 days | ↓ Adenoma and adenocarcinoma formation; ↑ mRNA expression of TNF-a |
| Liboredo et al., 2013 [72] | L. delbrueckii or B. lactis | 3 × 108 CFU/mL | 14 weeks | ↓ Number of ACF (55.7% vs. 45.1%, respectively). |
| Agah et al., 2019 [73] | L. acidophilus or B. bifidum | 1 × 109 CFU/g | 5 months | ↓ Incidence of colonic lesions (57% vs. 27%, respectively), CEA, and CA19-9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreira, M.A.; Campelo, M.W.S.; da Silva Martins Rebouças, C.; Duarte, A.S.G.; Barbosa, M.L.L.; da Cruz Fonseca, S.G.; Queiroz, R.R.; Holanda, É.U.; de Vasconcelos, A.B.A.; de Sousa Araújo, V.J.G.; et al. Pterostilbene and Probiotic Complex in Chemoprevention of Putative Precursor Lesions for Colorectal Cancer in an Experimental Model of Intestinal Carcinogenesis with 1,2-Dimethylhydrazine. Cancers 2023, 15, 2401. https://doi.org/10.3390/cancers15082401
Barreira MA, Campelo MWS, da Silva Martins Rebouças C, Duarte ASG, Barbosa MLL, da Cruz Fonseca SG, Queiroz RR, Holanda ÉU, de Vasconcelos ABA, de Sousa Araújo VJG, et al. Pterostilbene and Probiotic Complex in Chemoprevention of Putative Precursor Lesions for Colorectal Cancer in an Experimental Model of Intestinal Carcinogenesis with 1,2-Dimethylhydrazine. Cancers. 2023; 15(8):2401. https://doi.org/10.3390/cancers15082401
Chicago/Turabian StyleBarreira, Márcio Alencar, Márcio Wilker Soares Campelo, Conceição da Silva Martins Rebouças, Antoniella Souza Gomes Duarte, Maria Lucianny Lima Barbosa, Said Gonçalves da Cruz Fonseca, Raphaela Ribeiro Queiroz, Érica Uchoa Holanda, Ana Beatriz Aragão de Vasconcelos, Vitória Jannyne Guimarães de Sousa Araújo, and et al. 2023. "Pterostilbene and Probiotic Complex in Chemoprevention of Putative Precursor Lesions for Colorectal Cancer in an Experimental Model of Intestinal Carcinogenesis with 1,2-Dimethylhydrazine" Cancers 15, no. 8: 2401. https://doi.org/10.3390/cancers15082401
APA StyleBarreira, M. A., Campelo, M. W. S., da Silva Martins Rebouças, C., Duarte, A. S. G., Barbosa, M. L. L., da Cruz Fonseca, S. G., Queiroz, R. R., Holanda, É. U., de Vasconcelos, A. B. A., de Sousa Araújo, V. J. G., Diniz, G. M., Oriá, R. B., & de Vasconcelos, P. R. L. (2023). Pterostilbene and Probiotic Complex in Chemoprevention of Putative Precursor Lesions for Colorectal Cancer in an Experimental Model of Intestinal Carcinogenesis with 1,2-Dimethylhydrazine. Cancers, 15(8), 2401. https://doi.org/10.3390/cancers15082401







