1. Introduction
Pleural mesothelioma, previously known as malignant pleural mesothelioma or MPM, is a rare but fatal cancer that forms on the thin membrane that lines the lungs and thoracic cavity [
1]. It continues to have one of the poorest survival rates of any cancer, with a median survival of typically less than a year after diagnosis for untreated patients [
1,
2] or 16 to 18 months for patients receiving treatment [
3,
4], and a five-year survival rate of around 7% [
5]. The primary cause of pleural mesothelioma is exposure to asbestos fibres, a group of naturally occurring fibrous minerals with excellent physical and electrochemical insulating properties and a long history of industrial applications [
5,
6]. Despite a progressive ban on asbestos production and usage in over 50 countries worldwide since the 1980s, the incidence of pleural mesothelioma continues to rise due to the long latency period (20 to 50 years) between asbestos exposure and the development of the tumor [
5,
6,
7]. As a result, pleural mesothelioma is common in the elderly population and in males with a history of occupational exposure to asbestos [
5].
The diagnosis of pleural mesothelioma can be a complex process requiring multiple techniques, such as invasive tissue biopsy, cytological examination of the pleural fluid, and thoracoscopy, accompanied by immunohistochemistry staining on a wide panel of markers to differentiate pleural mesothelioma from other tumors [
8]. Consequently, pleural mesothelioma is often diagnosed at an advanced stage and has a poor prognosis. Currently, the performance status and histological subtype are the only factors used as prognostic indicators [
9,
10], with three histologic subtypes validated in clinical practice. Epithelioid is the most common subtype of pleural mesothelioma, constituting up to two-thirds of cases, with a survival of 19 months [
2,
11]; sarcomatoid is the least common subtype with the worst survival of 4 to 6 months [
11], and biphasic is a mixture of epithelioid and sarcomatoid subtypes, with the survival dependent on the ratio of epithelioid and sarcomatoid cells present in the tumor [
11].
Until 2020, the treatment for pleural mesothelioma was limited to systemic combination chemotherapy, typically platinum or cisplatin and pemetrexed with or without bevacizumab, with only modest improvements in survival [
9,
10,
12]. In recent years, immunotherapy using immune checkpoint inhibitors has transformed the treatment landscape of pleural mesothelioma. In October 2020, the US Food and Drug Administration approved the combination immunotherapy drugs, nivolumab (anti-programmed death-1 antibody) plus ipilimumab (anti-cytotoxic T-lymphocyte antigen-4 antibody), as first-line treatment for patients with unresectable pleural mesothelioma. This came following the results of the CHECKMATE-743 trial, which showed a median overall survival of 18.1 months for patients who received combination immunotherapy versus 14.1 months for patients who received chemotherapy [
4]. A recent study with a minimum of three years of follow-up further showed that nivolumab plus ipilimumab continued to provide long-term survival benefit over chemotherapy, regardless of histology [
13], indicating that immunotherapy is the new treatment modality for pleural mesothelioma. Nevertheless, not all patients benefit from immunotherapy, and although significant progress has been made for the treatment of pleural mesothelioma, there remains an urgent need for biomarkers and molecular targets that could help with early diagnosis, improve prognostication, help stratify patients to targeted treatments, and help select patients who might benefit from immunotherapy. There is also an important clinical need for non-invasive or minimally invasive techniques for detecting biomarkers without using invasive tissue biopsies.
During the formation and growth of the tumor, various components may be released into the body fluids due to apoptosis, necrosis, or active release of particles [
14]. These include circulating tumor cells, circulating tumor DNA, circulating tumor RNA, and extracellular vesicles (EVs) [
14]. These offer a means to identify biomarkers from liquid biopsies. EVs have demonstrated enormous potential as biomarkers for cancer, including for pleural mesothelioma. EVs are membrane-contained particles secreted by malignant and non-malignant cells to transport biological information from one cell to another and mediate intercellular communication [
15,
16,
17,
18]. Their cargo composition consists of different classes of proteins, lipids, and genetic material including DNA, RNA, mRNA, and miRNA [
15,
16,
17,
18]. EVs reported in the literature are typically classified according to their size and biogenesis; however, there is substantial overlap. Although there is currently no standardized nomenclature for different EV populations, the most common EV subtypes reported in the literature include small EVs (50–150 nm) [
19], commonly called exosomes, formed from the endosomal biogenesis pathways and typically isolated using a centrifugal force ranging from 100,000×
g to 200,000×
g; larger-sized EVs (50–1000 nm), previously termed microparticles and later changed to microvesicles, formed by blebbing from the plasma membrane and typically isolated using a centrifugal force of 18,000×
g; and apoptotic bodies (1–4 µm), which are shed from dying cells and are typically isolated using a centrifugal force of 2000–3000×
g [
15,
19,
20]. Recently, EVs containing oncogenic cargo have been characterized, named oncosomes (100–400 nm) and large oncosomes (1–10 µm), which are thought to be shed exclusively from cancer cells [
21]. These so-called large oncosomes have been isolated using a centrifugal force of 10,000×
g [
15,
20,
21].
EVs circulate freely in almost all body fluids, including blood, urine, milk, and saliva [
19], making them an ideal candidate for non-invasive or minimally invasive biopsies. Furthermore, EVs are encapsulated by a phospholipid membrane, which protects their contents from degradation, making them highly stable in body fluids [
22]. Growing evidence suggests that the behavior and functional roles of EVs are specific, not random, which can help reveal the tumor-specific immune suppression mechanisms for individual patients [
15,
16,
17,
18]. These qualities make EVs ideal biomarker candidates for pleural mesothelioma over tissue-based biomarkers, due to the invasiveness of tissue biopsies, the difficulty in capturing the tumor heterogeneity of pleural mesothelioma and its subtypes from only a section of the tumor tissue, and the limited access to tissue samples from mostly elderly patients who are typically already at an advanced stage of disease and are usually poor candidates for surgery [
14]. EVs have a major advantage of being available in body fluids, offering a novel pathway to perform liquid biopsies for pleural mesothelioma.
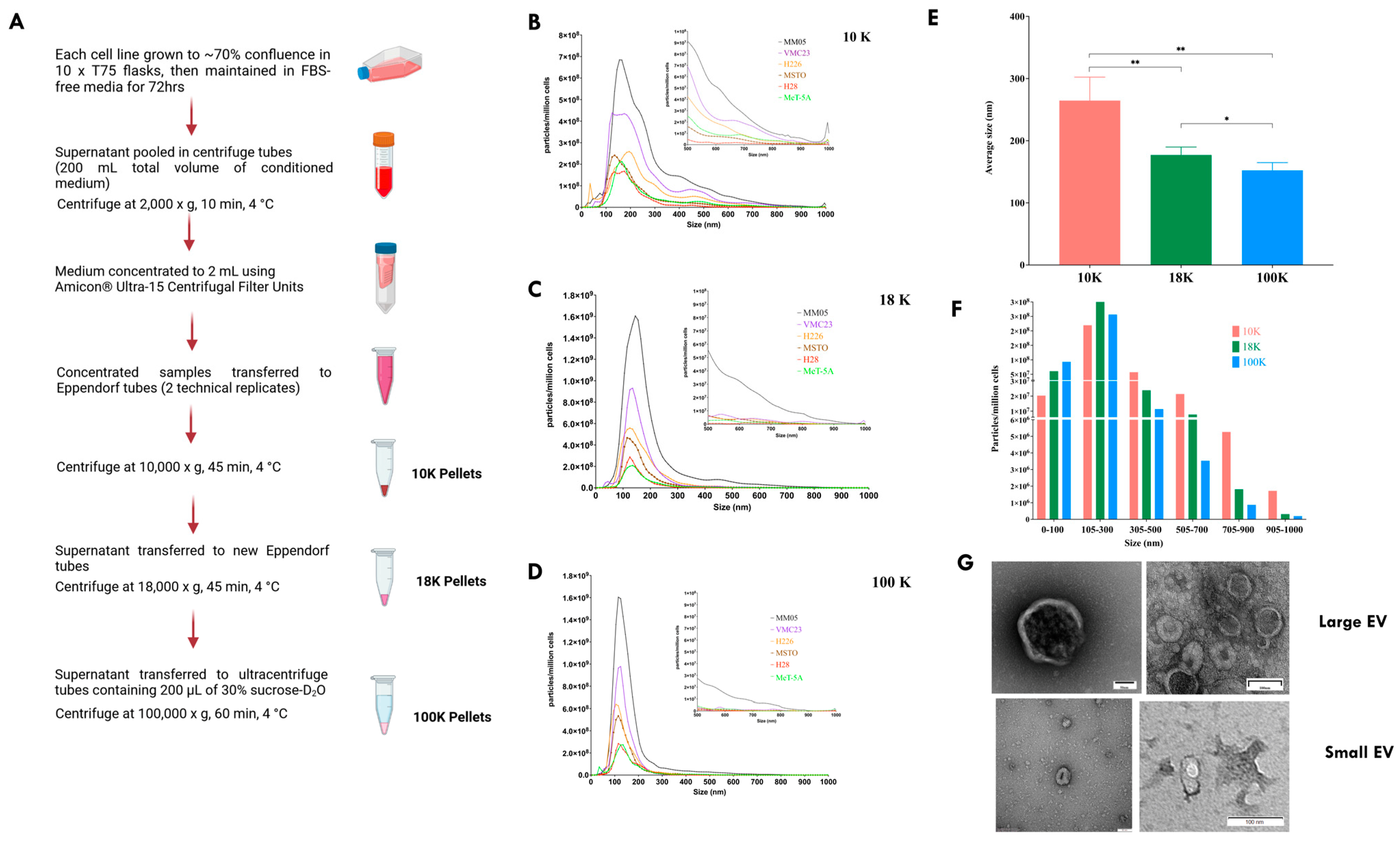
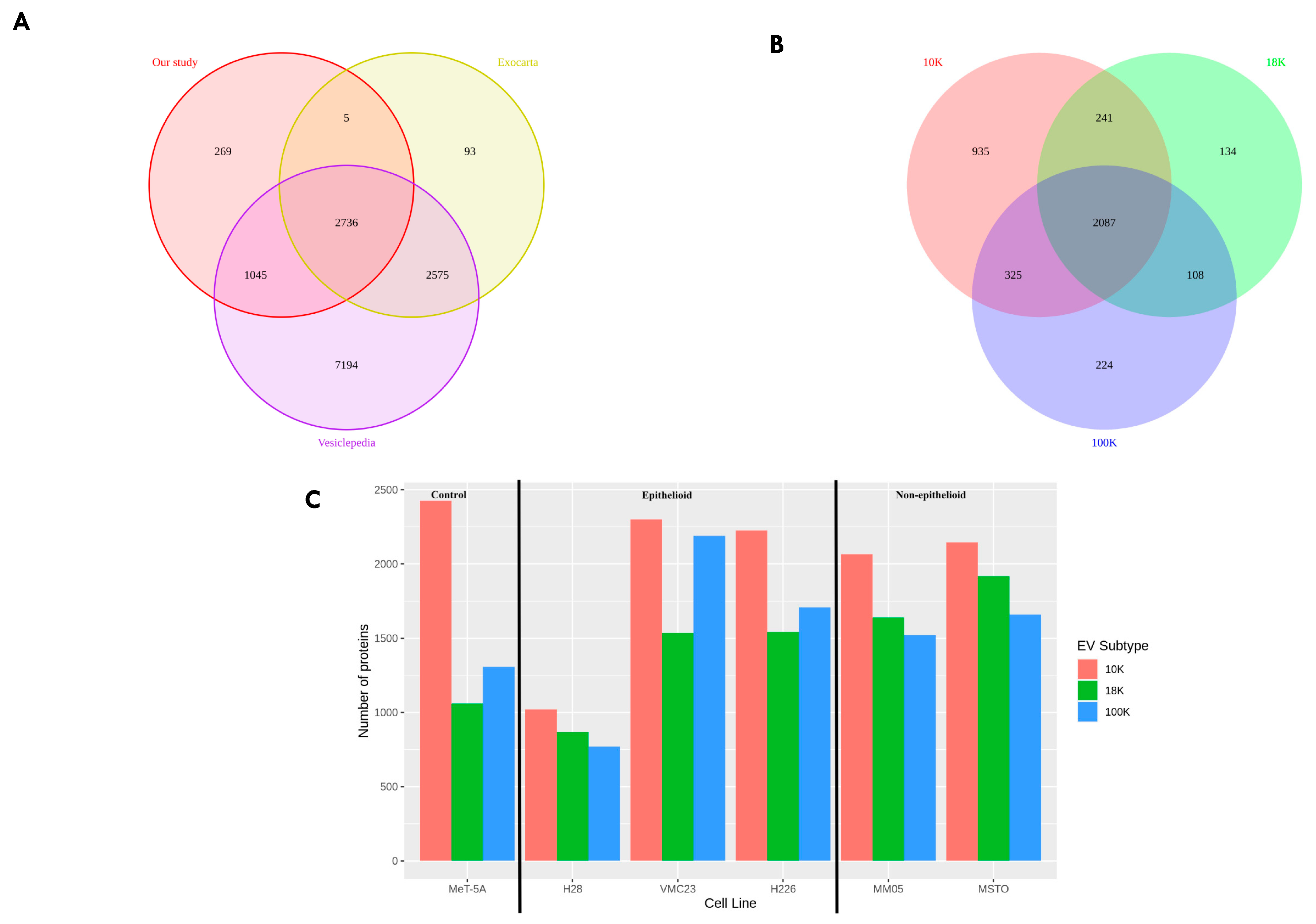
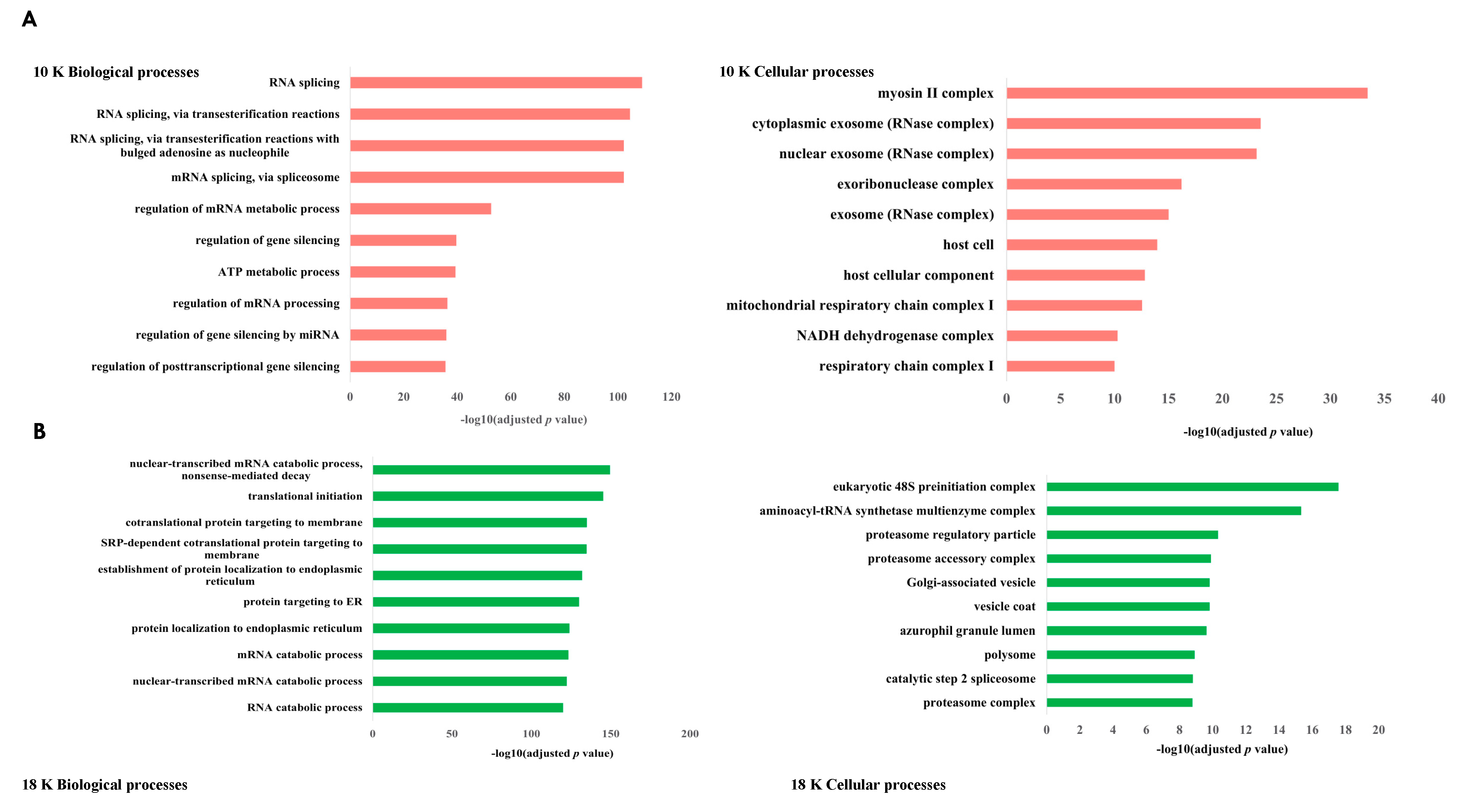
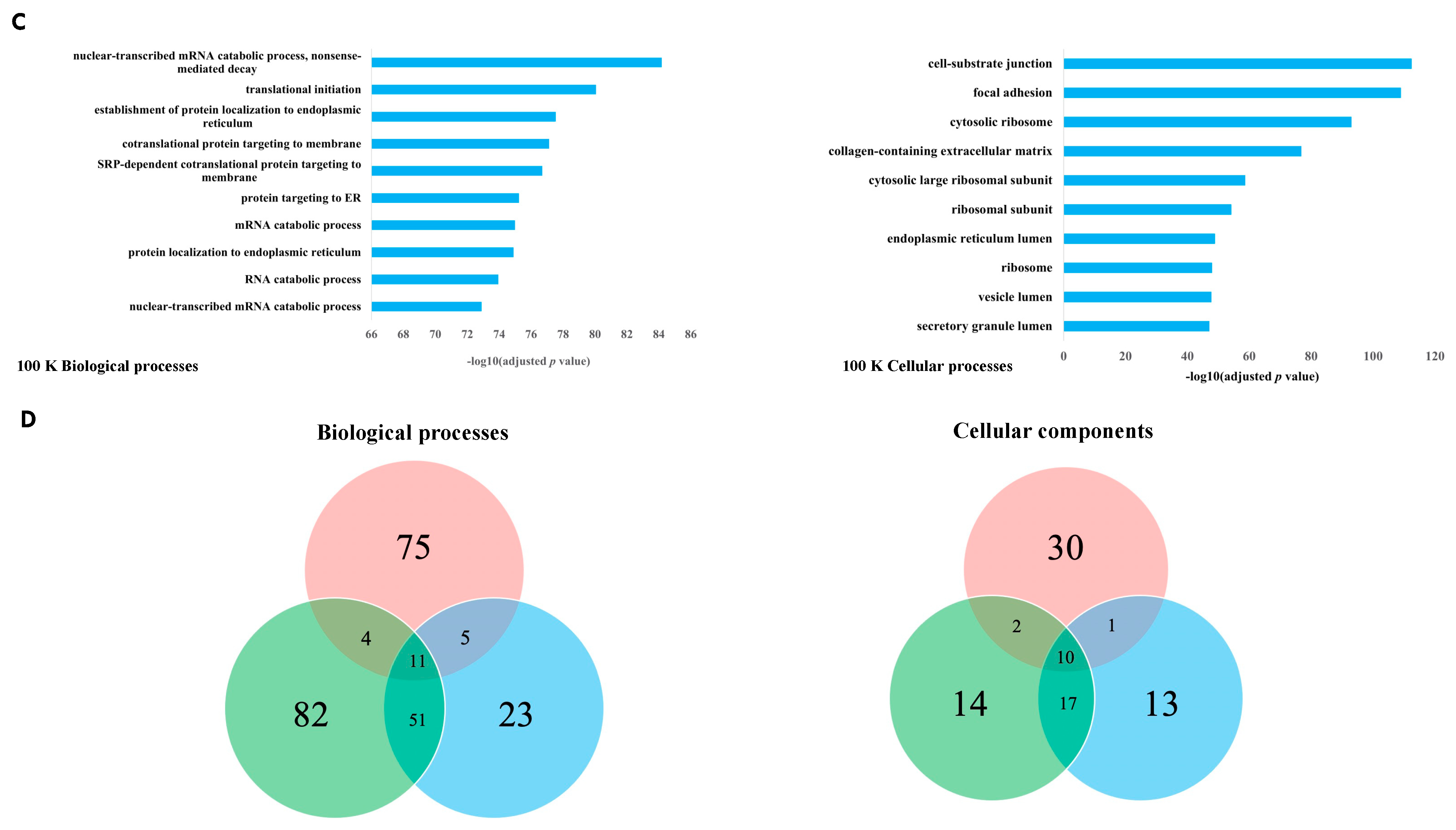
Our study had two main objectives. Firstly, we aimed to characterize and distinguish the proteomes of three subtypes of EVs derived from pleural mesothelioma cell lines using different centrifugal forces: large-sized EVs isolated at 10,000× g (10 K) and at 18,000× g (18 K), and then small-sized EVs isolated at 100,000× g (100 K). Secondly, we aimed to identify unique cancer-specific proteins that are contained within the different EV subtypes derived from pleural mesothelioma cell lines, which could offer a novel means to extract specific biomarkers of interest from liquid biopsies. To our knowledge, this is the first study that distinguishes three subtypes of EVs, and the first to characterize larger-sized EVs in pleural mesothelioma.
4. Discussion
In this study, we have demonstrated that different subtypes of EVs are released by mesothelioma cancer cells and their cargo can uncover new diagnostic, prognostic, or predictive biomarkers for cancer therapy.
Only two proteomic studies have been published to date on EVs derived from mesothelioma cell lines. However, to our knowledge, there are currently no studies that have investigated EVs derived from the cell lines used in this study. Our study is also the first to investigate different subtypes of EVs from mesothelioma cell lines. In the previous proteomic studies, the focus has been on small EVs, termed exosomes, whereas medium- to large-sized EVs have not yet been studied in mesothelioma cell lines. Greening et al. (2016) [
90] conducted quantitative proteomics on pleural mesothelioma-derived exosomes (isolated at 100,000×
g for 2 h) from four malignant mesothelioma cell lines (J038, LO68, OLD1612, and JU77). They defined a selective mesothelioma oncogenic exosomal signature (mEXOS) consisting of 570 proteins [
90]. Of the 570 proteins in mEXOS, 344 proteins (approximately 60%) were identified as complementary DNA (cDNA) and 3 proteins were uncharacterized protein fragments. Of the remaining 224 proteins in mEXOS, 48 proteins were also identified in our proteomics results in this study, most of which were found in all our EV subtypes, with some exceptions (
Supplementary Table S1). For example, Annexin A6, which is a calcium-dependent membrane-binding protein that is closely associated with several cancers such as melanoma, epithelial carcinoma, cervical, breast and prostate cancer [
91], was not identified in our 100 K EVs, but was found mostly in our 10 K EVs and in only one 18 K EVs derived from the MM05 cell line. This suggests the possibility for membrane-binding proteins to be localized to the larger-sized EVs due to their biogenesis through blebbing from the plasma membrane. Furthermore, ADAMTS12 (which has shown both pro- and anti-tumor roles and associated with immune cells [
92]), neuropilin 2 and interleukin-7 (both of which act on immune cells and have shown great potential in cancer immunotherapy [
93,
94]) were only detected in our 10 K EVs and 100 K EVs, but not in any of our 18 K EVs, suggesting the presence of immune-associated proteins localized to the cargo of large (10 K) and small (100 K) EVs. Furthermore, OASL (potential prognostic biomarker in breast cancer [
95]) was only found in one of our 10 K EVs, suggesting that the cargo of 10 EVs contains oncogenic material. Overall, our results complement the protein signature characterized in mEXOS in Greening et al.’s study and uncovers a larger portfolio of proteins of biomarker value for mesothelioma. Our results further show that certain markers are localized to specific subsets of EVs, which can help streamline biomarker identification and tracking for mesothelioma.
Greening et al. also observed 16 mesothelioma-associated proteins in the cargo of their small EVs, the so-called exosomes, of which 14 were also found in the protein cargo of EVs in our study (as indicated in
Table 10). These include mesothelin, calretinin, and vimentin. These proteins are already known to be expressed in mesothelioma tumor tissue microenvironment and have been suggested as biomarkers for the differential diagnosis of pleural mesothelioma [
96,
97,
98]. Their presence in EVs across two studies from different mesothelioma cell lines, especially in specific subsets of EVs in our results, opens an exciting pathway to detect and extract these important biomarkers for pleural mesothelioma without invasive tissue biopsy techniques.
In another proteomics study, Hegmans et al. analyzed exosomes derived from two mesothelioma cells (PMR-MM7 and PMRMM8) [
99]. They did not find mesothelioma-associated antigens; however, they found 19 proteins that were associated with antigen presentation, including MHC class I molecules. Interestingly, we found antigen presentation via MHC class I exclusively enriched in our 18 K EVs. These results provide insight into the select oncogenic cargo of EV subtypes and support the notion that cancer-specific biomarkers are localized to specific subtypes of EVs, where proteins associated with antigen presentation more likely to be localized to the cargo of the 18 K EVs. In a recent study involving pleural biopsies of 44 mesothelioma patients, Kosari et al. found that multiple antigen processing and presentation gene sets were predictive of overall survival, which could help facilitate patient selection for immunotherapy [
100]. Our study suggests that circulating 18 K EVs may offer novel predictive biomarker potential for immunotherapy for mesothelioma patients.
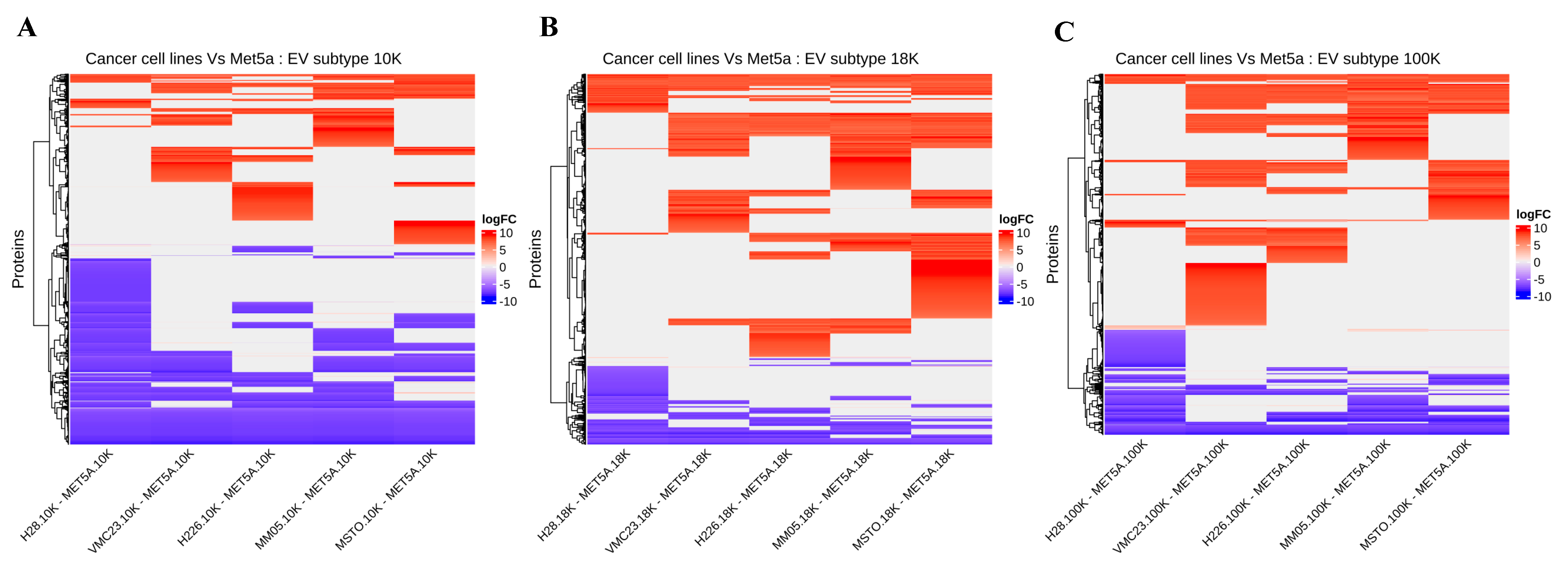
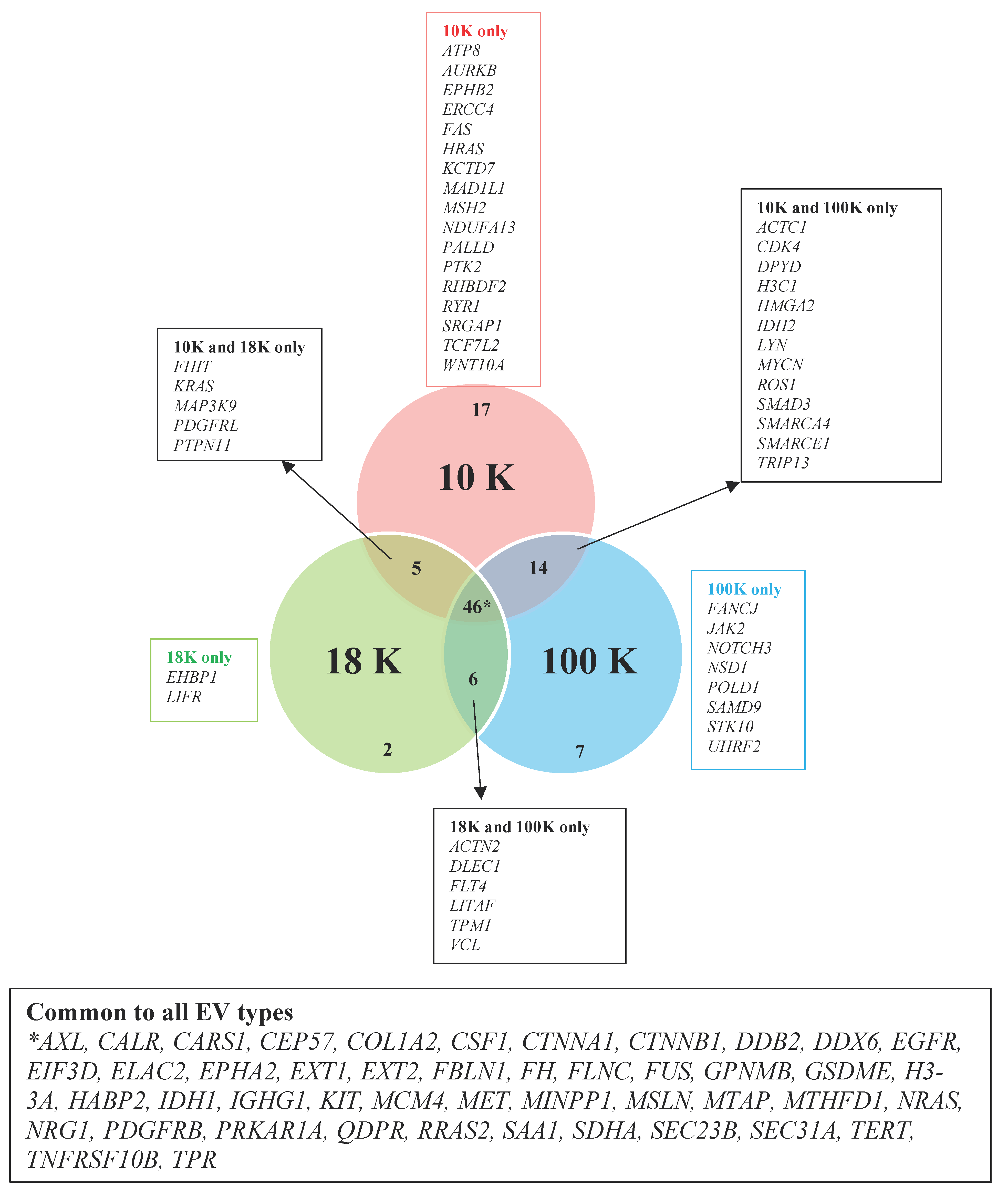
We have uncovered several novel biomarker candidates for pleural mesothelioma by identifying the top upregulated and downregulated proteins that were consistently expressed in each of the EV subtypes derived from all pleural mesothelioma cell lines, compared to the corresponding EVs of the control cell line. Each of these highly over- and under-expressed proteins are involved in cancer pathogenesis and have reported diagnostic, prognostic, or predictive biomarker potential in other cancers from previous reports, suggesting that they have great potential as cancer biomarkers for pleural mesothelioma. In addition, we identified several cancer-associated proteins that were present in all the EV pellets from all five mesothelioma cell lines and absent in all the EV pellets from the control cell line. Among these are proteins such as AXL, which has recently been discovered as a potential target in cancer treatment as it has important signaling functions that drive cancer cell survival, proliferation, migration and invasion and whose aberrant expression has been found in several malignancies including breast cancer, chronic lymphocytic leukemia, and pancreatic cancer [
31]; and calreticulin, a protein found in the endoplasmic reticulum that is involved in a spectrum of cellular process including folding of proteins that help stressed and dying cells release co-stimulatory signals to immune cells [
32].
Our results further suggest that mitochondrial metabolism may play an important role in pleural mesothelioma tumorigenesis. The mitochondrial enzymes succinate dehydrogenase (
SDHA), fumarate hydratase (
FH), and isocitrate dehydrogenase (
IDH1) were detected in all our EV pellets (10 K, 18 K, and 100 K) derived from all our mesothelioma cell lines but were absent in all EV pellets derived from our control cell line, MeT-5A. It has previously been reported that mitochondrial function is essential for cancer cell viability and that mutations in
SDHA,
FH, and
IDH1 [
101] can change mitochondrial metabolism and allow cancer cells to adapt to changing environments. They could be potential biomarkers for pleural mesothelioma. Although we detected these enzymes in all EV pellets derived from all our pleural mesothelioma cell lines, the mitochondria were an enriched cellular component exclusively in the 10 K EVs. One hypothesis is that mitochondrial EV cargo may have originated from the large EVs and passed on to other EV types for cellular transport, which warrants further investigation. It has also been suggested that mitochondrial proteins are present in EV cargo even in unstimulated conditions, or that there may be mitochondria-derived vesicles (MDVs), which are small vesicles that carry mitochondrial proteins to other organelles. One study showed that cells selectively package damaged mitochondrial components in MDVs for lysosomal degradation to prevent the release of damaged components or pro-inflammatory content [
102]. Although we found mitochondrial proteins in all our EV subtypes, especially in the 10 K EVs, we did not characterize MDVs as this was outside the scope of our study. Our results warrant further investigation into the role of MDVs as a potential EV subtype that may confer biomarker potential.
We also searched for known pleural mesothelioma markers that have been established from tissue samples, to determine if we can detect them in our EVs. Mesothelin and the programmed death ligand-1 (PD-L1) are the most widely reported biomarkers in pleural mesothelioma [
103,
104]. Mesothelin is a cell surface protein that is expressed normally in mesothelial cells and is elevated in the serum of pleural mesothelioma patients, particularly for epithelioid subtype. Soluble mesothelin has gained regulatory approval for monitoring of patients who are diagnosed with epithelioid or biphasic pleural mesothelioma, albeit in limited situations [
97]. It currently lacks the required sensitivity for routine clinical use. PD-L1 is an immune-suppressing receptor that is expressed on tumor cells. It binds to the corresponding PD-1 receptor on T cells to suppress their tumor-killing function. PD-L1 expression in the tumor tissue has been established as marker of poor prognosis in pleural mesothelioma [
105]. It is currently being investigated as a predictive biomarker for immunotherapy in multiple clinical trials [
106], including in our recent study evaluating the safety and efficacy of pembrolizumab in mesothelioma patients [
106]. However, results have so far been equivocal. Recently, Chiarucci et al. (2020) investigated the soluble form of PD-L1 in the sera of pleural mesothelioma patients from the NIBIT-MESO-1 clinical trial [
107]. They found elevated levels of soluble PD-L1 in the immunotherapy-treated patients vs. baseline (
p < 0.001). The study provides the basis to investigate soluble PD-L1 as opposed to tumor PD-L1 expression levels for biomarker potential. This is especially important for pleural mesothelioma due to the lack of tumor availability for robust biomarker studies. Although the origins of the soluble form of PD-L1 is not yet fully understood in pleural mesothelioma, our study alludes to EVs as one possible mechanism. Previously, PD-L1 has only been reported in small EVs, the so-called exosomes, in melanoma [
108], non-small cell lung cancer [
109], and gastric cancer [
110]. Our study is the first to investigate PD-L1 in large EVs, where we found a higher enrichment of PD-L1 expression in our large EV pellets, 10 K, relative to our small EV pellets, 100 K. We did not detect PD-L1 in our 18 K EV pellets. Similarly, we detected mesothelin in our EV pellets. Interestingly, for some of our cell lines, a higher enrichment of mesothelin was detected in our small EVs, 100 K, relative to the 10 K and 18 K EV pellets. Our results give preliminary evidence that PD-L1 and mesothelin are localized to specific subtypes of EVs in pleural mesothelioma, which warrants further investigation. Our results also encourage further investigation of PD-L1 in the cargo of 10 K EVs as a source of biomarker for pleural mesothelioma.
While our large 10 K EVs were mostly enriched with mitochondrial components, interestingly, both our 18 K EVs and 100 K EVs were mostly associated with the endoplasmic reticulum (ER). Some of the top biological processes associated with the 18 K EVs and 100 K EVs were protein targeting to the ER, protein localization to the ER, and SRP-dependent co-translational protein targeting to the membrane, which is a protein that binds to the ER [
111]. The ER lumen was also one of the top enriched components in the 18 K EVs and 100 K EVs. It is known that protein handling and folding in the ER are critical processes for cell function and survival. In the tumor microenvironment, oncogenic and metabolic abnormalities can create aberrant activation of ER stress signals that could in turn activate aberrate signaling pathways for tumor growth [
112]. ER stress is therefore an important characteristic of the tumor microenvironment. Some studies suggest that the modulation of ER stress can make aggressive tumors sensitive to cytotoxic drugs and immunotherapies [
112]; however, this needs to be investigated further. We observed that there are many overlaps in the biological processes and cellular components between 18 K EVs and 100 K EVs; however, 10 K EVs in comparison do not share many common biological processes or enriched cellular components to either the 18 K EVs or the 100 K EVs. Our results suggest that EV isolation at 10 K results in the isolation of more distinct EV subtypes that carry unique oncogenic cargo and should be investigated more in future EV studies.
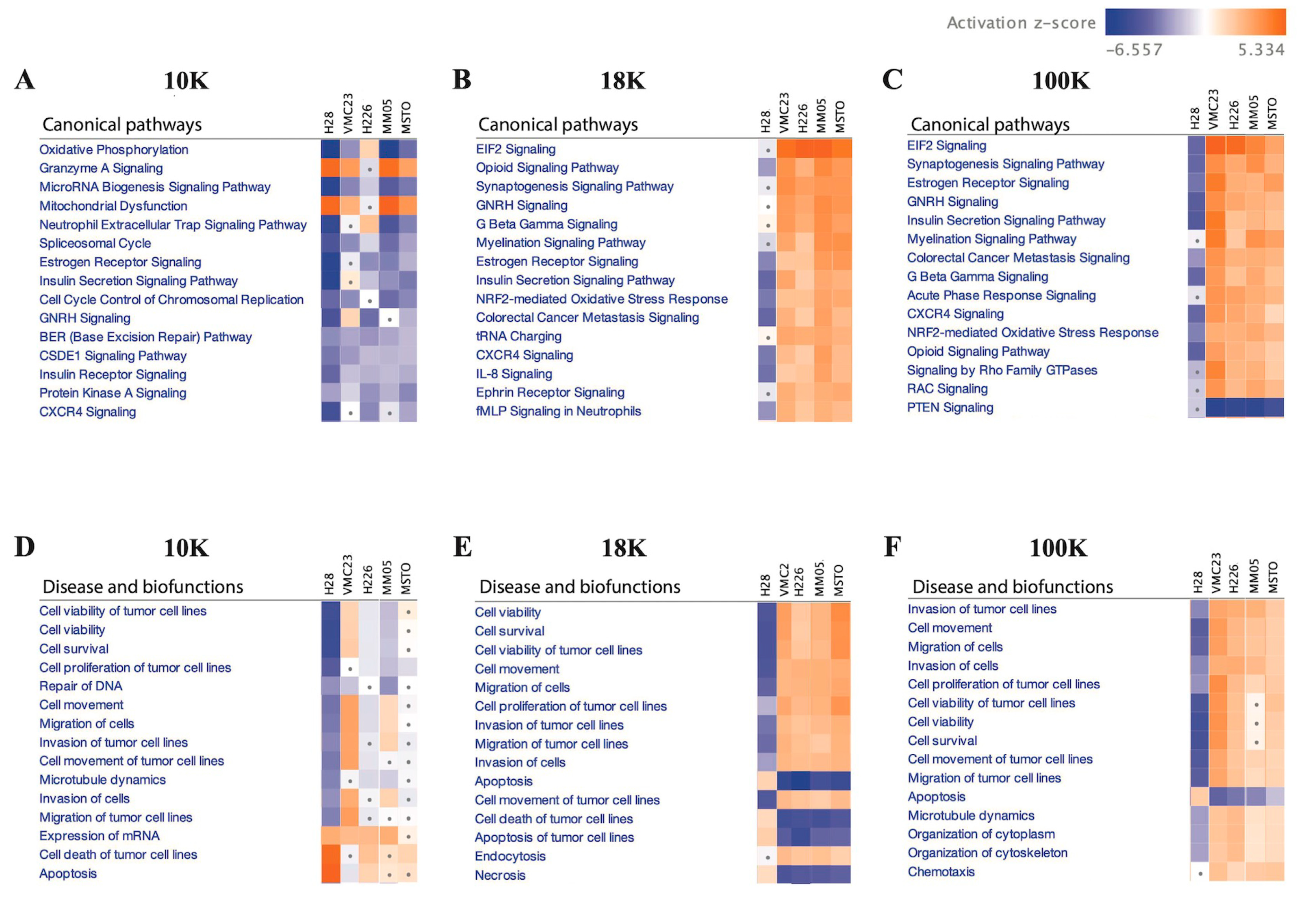
Additionally, IPA analysis of the data revealed that 10 K EV pellets exhibit a distinct proteomic profile, characterized by decreased activities of oxidative phosphorylation, microRNA biogenesis signaling, and neutrophil extracellular trap signaling pathways. These pathways are known to interplay and promote tumor growth, progression, and metastasis when activated [
113,
114]. The observed reduction in protein activation in these pathways may account for the decreased cell viability, cell survival, cell proliferation, and DNA repair as seen in the 10 K EVs. Further investigations are required to verify these results; however, one possible explanation is that the exosomes may contain more carcinogenic materials and are primarily present in fractions isolated at speeds greater than 10 K [
115]. Nonetheless, we did report in this study that 10 AK EVs still carries potential protein biomarkers that warrants further investigation.
Conversely, a majority of the 18 K and 100 K EV pellets exhibited increased activation of various pathways, including the EIF2 signaling and NRF2-mediated oxidative stress response pathways. Our findings align with prior research that demonstrated that increased expression and activity of EIF2 [
116] can promote the growth of mesothelioma. NRF2 has also been reported to play a critical role in the proliferation of MSTO-211H cell lines by elevating intracellular ROS levels [
117]. These results are consistent with our observation that hallmark cancer features, such as cell viability, cell migration, and cell proliferation, are enhanced, while apoptotic activity is decreased in the 18 K and 100 K EVs. Overall, our results suggest that EV isolation at 10 K results in the isolation of more distinct EV subtypes that carry limited but unique oncogenic cargo and should be investigated more in future EV studies.
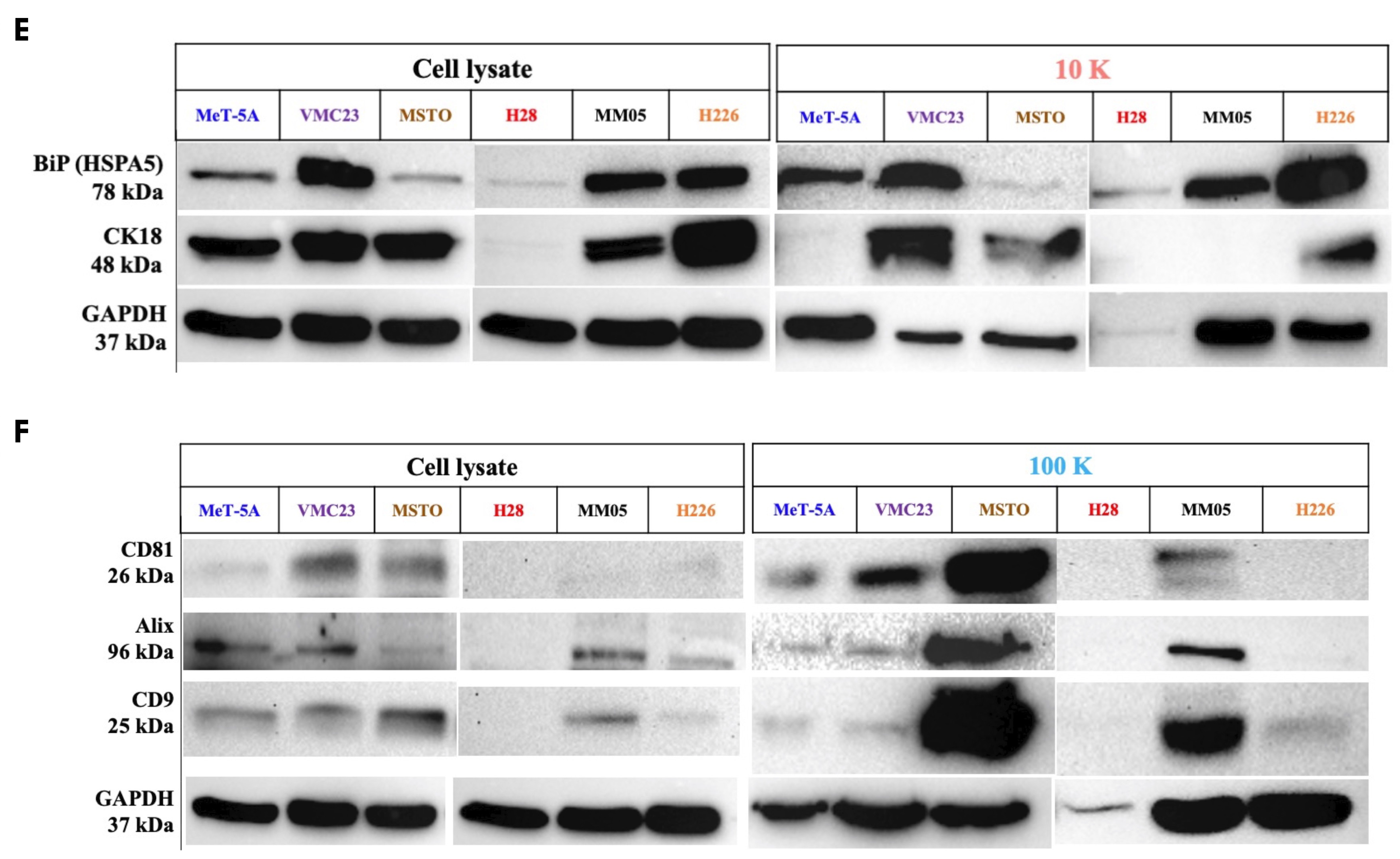
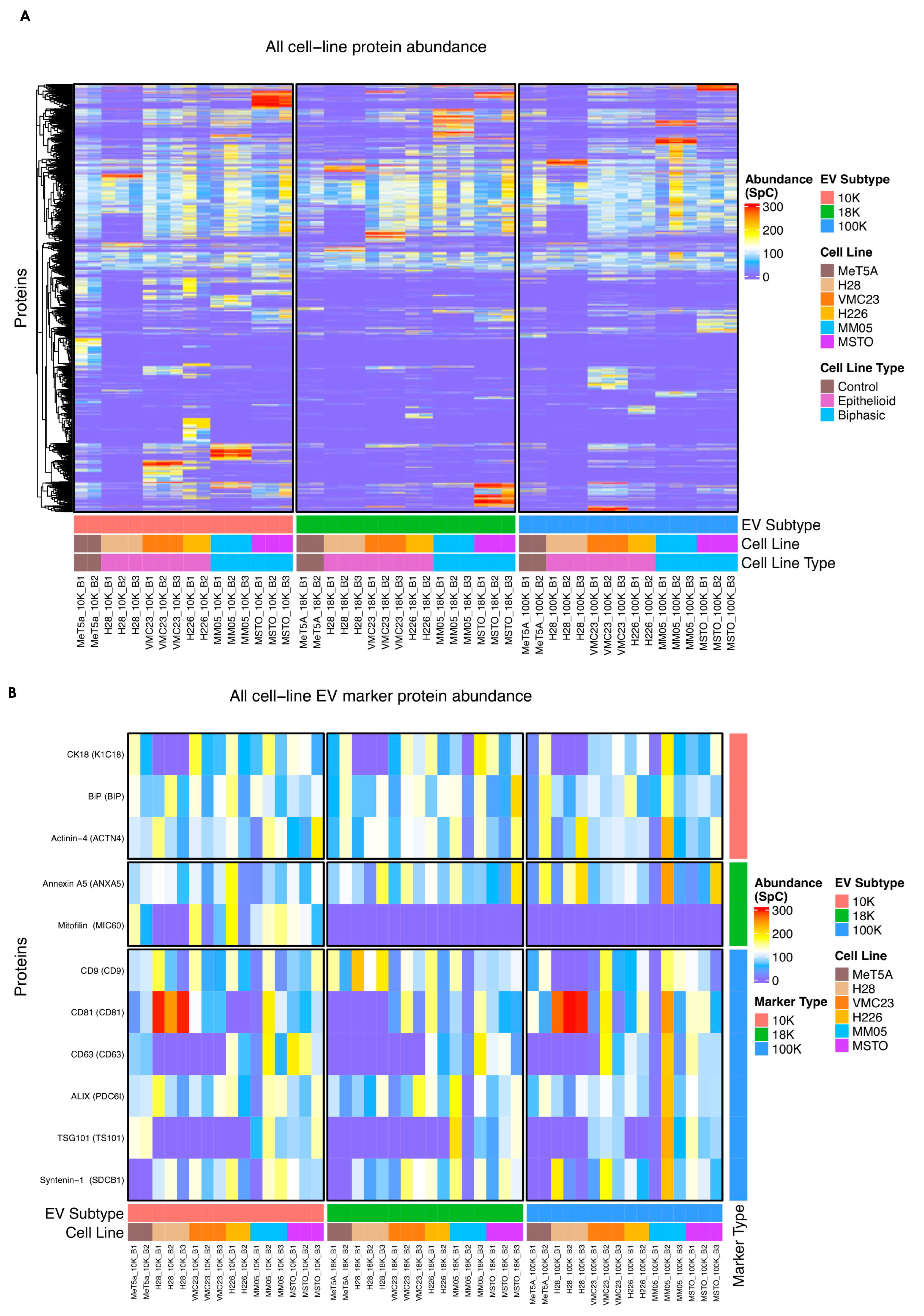
There are currently limited reports in the literature on different EV subtypes and their role in cancer. Our study encourages further research especially in 10 K EVs, as we have shown important oncogenic cargo to be present exclusively in 10 K EVs derived from pleural mesothelioma cells. In other cancers, there is a dominant focus on small EVs, the so-called exosomes, in the EV literature, with relatively limited reports on larger-sized EVs. Perhaps one of the barriers to studying sub-populations of EVs is the lack of unique markers that can characterize and distinguish them from each other. Crescitelli et al. (2020) investigated subpopulations of EVs derived from metastatic melanoma tissue by isolating EVs at 16,500×
g for 20 min and then at 118,000×
g for 2.5 h to isolate large and small EVs, respectively [
16]. They found mitofilin (a mitochondrial inner membrane protein) exclusively enriched in the large EVs. Similarly in our results, mitofilin was found only in the 10 K EVs derived from all but H28 cell lines, and absent across the 18 K and 100 K EVs derived from all cell lines, suggesting that it could potentially be a unique marker of 10 K EVs. We also detected HSPA5 (BiP) only in our 10 K EVs, whereas CK18 (previously reported as a marker of large oncosomes [
21]) was detected in some of our 10 K EVs and in some of our 100 K EVs. Our results suggest that mitofilin and BiP may be better markers of 10 K EVs. We further characterized 18 K and 10 K EVs using flow cytometry with the EVs stained with Annexin V, which has a high affinity to PS. PS is a phospholipid that is located on the inner membrane of cells or EVs and is translocated to the outer membrane in response to an event or stimuli, such as a vesicular event [
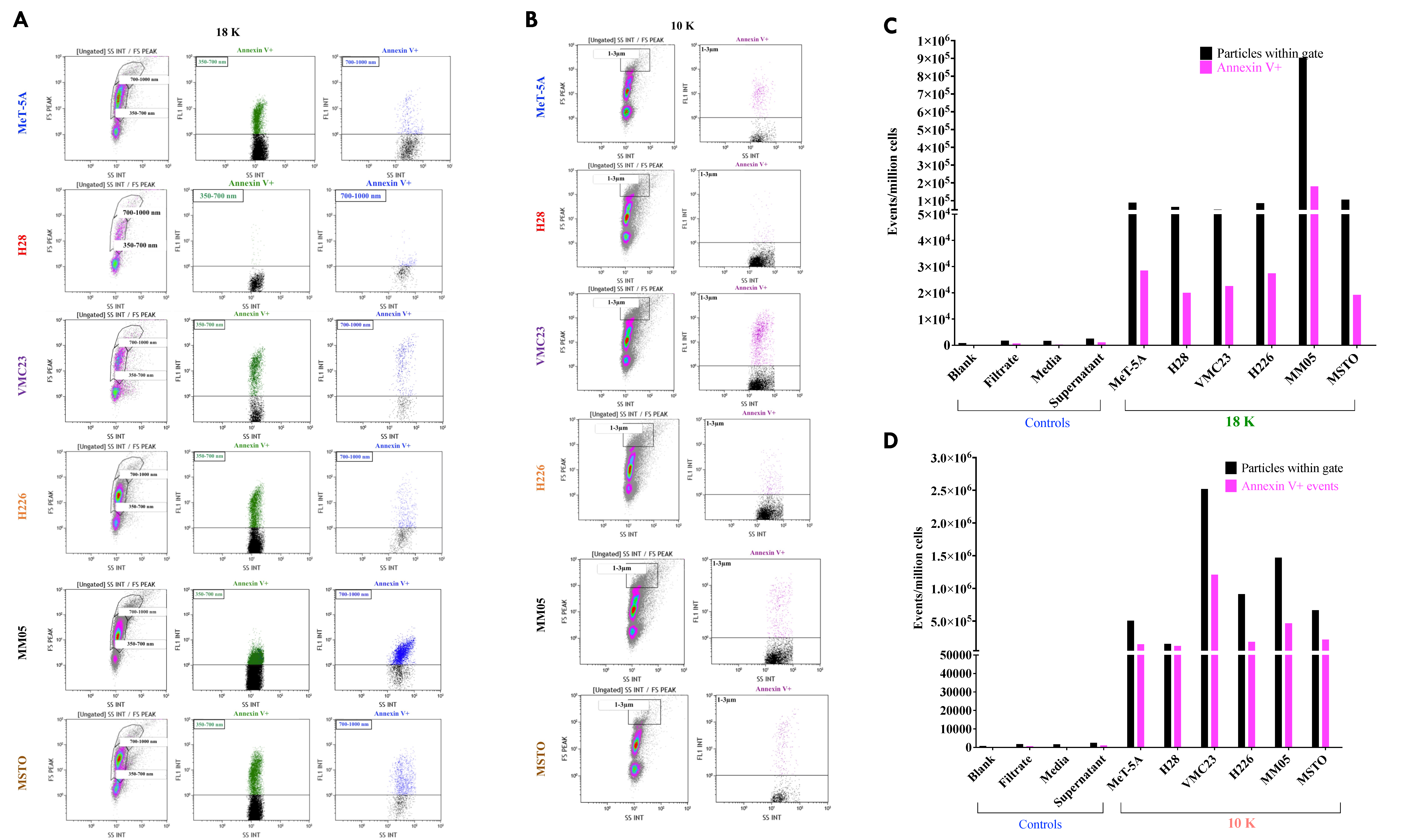
118]. EVs, which form from blebbing or shedding of the plasma membrane, are typically characterized by the presence of PS on their outer membrane [
119], which prompted us to further characterize 18 K and 10 K EVs using this technique.
For small EVs, tetraspanins including CD9, CD81, and CD63 have been commonly reported as markers of small EVs, or so-called exosomes. However, tetraspanins have been shown to induce plasma membrane curvature, making them present in shedding vesicles [
120]. This may explain why we observed enrichment of CD9 in our 10 K EVs (
Supplementary Figure S1). This is consistent with the results from Javadi et al. (2021), who conducted a proteomics study of EV subpopulations derived from pleural mesothelioma pleural effusion samples [
121] and found enrichment of cell surface tetraspanins CD9 in their large EV pellet (isolated at 10,000×
g for 10 min and referred to as microvesicles), and enrichment of CD81 in their small EV pellet (isolated 100,000×
g for 90 min and referred to as exosomes). We also observed enrichment of CD81 in our 100 K EVs, suggesting that CD81 may be a better marker of 100 K EVs. It is noted that currently, there is no single marker that can uniquely identify EVs [
122,
123]. It should also be noted that EVs can also contain proteins that are considered pan-EV markers, i.e., they are common for most EV subtypes [
123], and our results suggest that most of the commonly used EV markers could possibly be pan-EV markers. Our results also showed that the enriched biological processes and cellular processes are distinct in the 10 K EVs compared to 18 K and 100 K EV subtypes. Furthermore, our NTA and TEM results demonstrate that there is substantial overlap in the size of EVs across the three subtypes. This suggests that biogenesis pathway may be a better way of classifying EV subtypes, rather than by the size and the currently available makers.
The main strength of our study is that we performed a pooled analysis of potential biomarkers for pleural mesothelioma from five mesothelioma cell lines of different histological subtypes and an immortalized control cell line, each with at least two biological replicates, and two technical replicates within every biological sample. To our knowledge, this is the largest proteomics study conducted on EVs derived from mesothelioma cell lines to date. This study took a discovery approach to uncover the proteomic profile of pleural mesothelioma and identify a comprehensive list of markers that have cancer biomarker potential. We have revealed several oncogenic targets for mesothelioma, and while we have argued that each of the enriched proteins have associations with cancer pathogenesis from other reports, a follow-up study is strongly encouraged to validate the biomarker potential of each of the key targets discovered in this study for mesothelioma.
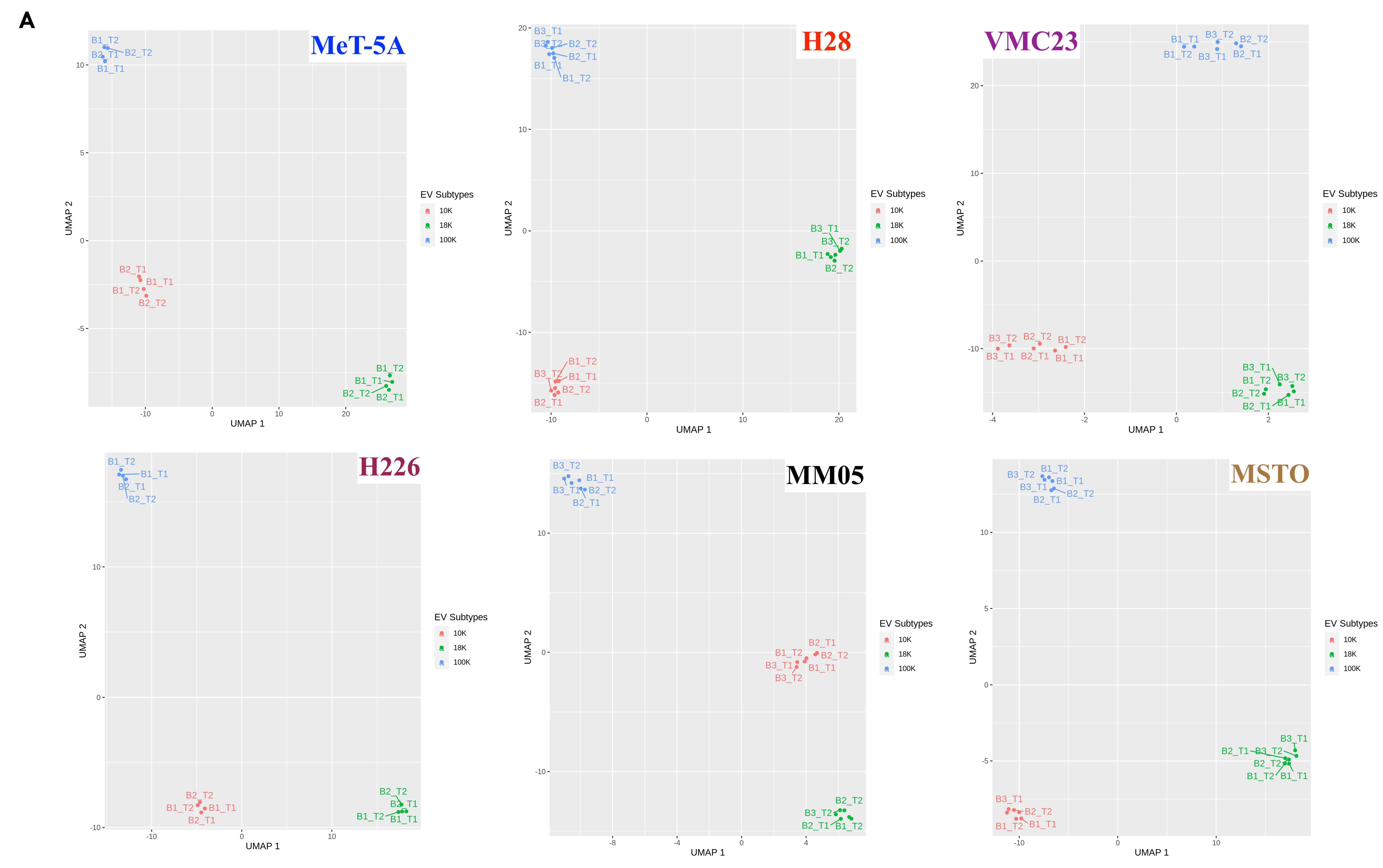
The other key strength of our study is that we studied potential biomarkers within different EV populations, which enabled us to reveal multiple targets and uncover the distinct roles of three EV subtypes. Our study opens a pathway of accessing key proteins of biomarker potential more directly from the oncogenic cargo of specific EV subtypes, rather than from a pooled pellet of EVs, or via invasive tissue extraction techniques. This would be a significant step towards improving biomarker access for pleural mesothelioma. We acknowledge that there is vast heterogeneity in the size and populations of EV subtypes that are still under research and not fully understood. In this study, we show that at least three EV subtypes can be isolated using different centrifugal forces, i.e., 10,000× g, 18,000× g and 100,000× g to isolate 10 K, 18 K, and 100 K EVs, respectively. These three subtypes showed many overlapping similarities, as well as many unique properties.
There were also limitations to our study that should be considered for future studies. Firstly, it is now recognized that the methods of EV isolation have limited association with the method of EV formation, and EV isolation protocols typically adopt a purification step. We chose not to use density gradient separation techniques for isolating 10 K and 18 K EVs but chose to use a sucrose cushion for isolating the 100 K EVs. While gradient separation techniques offer higher specificity and are intended to remove as many non-vesicular components and debris as possible, it is acknowledged that this technique is still under research, has the risk of producing low EV yield or recovery, and has not been optimized for 10 K and 18 K EVs. Therefore, we chose to isolate 18 K and 10 K using the differential centrifugation method, which was originally the primary EV isolation technique reported in the literature. This was to ensure that we capture a higher EV yield, at the risk of potentially compromising the purity of the EV pellets retrieved [
122]. Our isolation technique for 10 K and 18 K may have caused some of the overlap in the presence of surface EV markers we observed in both the large and small EV pellets. We were also limited with the NTA technology for the particle size distribution because large particles are less likely to be reliably detected by the NTA. Therefore, we were limited to a particle size of 1 μm in our current study and relied on flow cytometry with a specific membrane marker and gating strategy to detect larger-sized EVs. Further optimization of isolation techniques is needed for future EV studies. Despite this, our results showed a predominantly large EV population in our 10 K pellets, a relatively smaller EV population in our 18 K pellets, and small EV populations in our 100 K pellets. Furthermore, our UMAP plots show a clear separation between the three EV subtypes, indicating major differences in the cargo between 10 K, 18 K, and 100 K EVs. Furthermore, MeT-5A may not have been a robust control cell line for mesothelioma. MeT-5A is an immortalized cell line that has been transfected with the simian virus 40 (SV40), which has been proposed as a risk factor for mesothelioma [
124]. Although a direct causal link between SV40 and human mesothelioma has not been established, future experiments should consider the use of a mesothelial control cell line without any confounding risk factors.
Given the lack of standardized nomenclature and isolation techniques for EVs, we avoided assigning naming conventions to our EV pellets. We were also limited with the NTA technology for the particle size distribution because large particles are less likely to be reliably detected using the NTA. Therefore, we were limited to a particle size of 1 µm in our current study and relied on flow cytometry with a specific membrane marker and gating strategy to detect larger-sized EVs. Improvements in EV technologies are needed to detect particles larger than 1 µm.
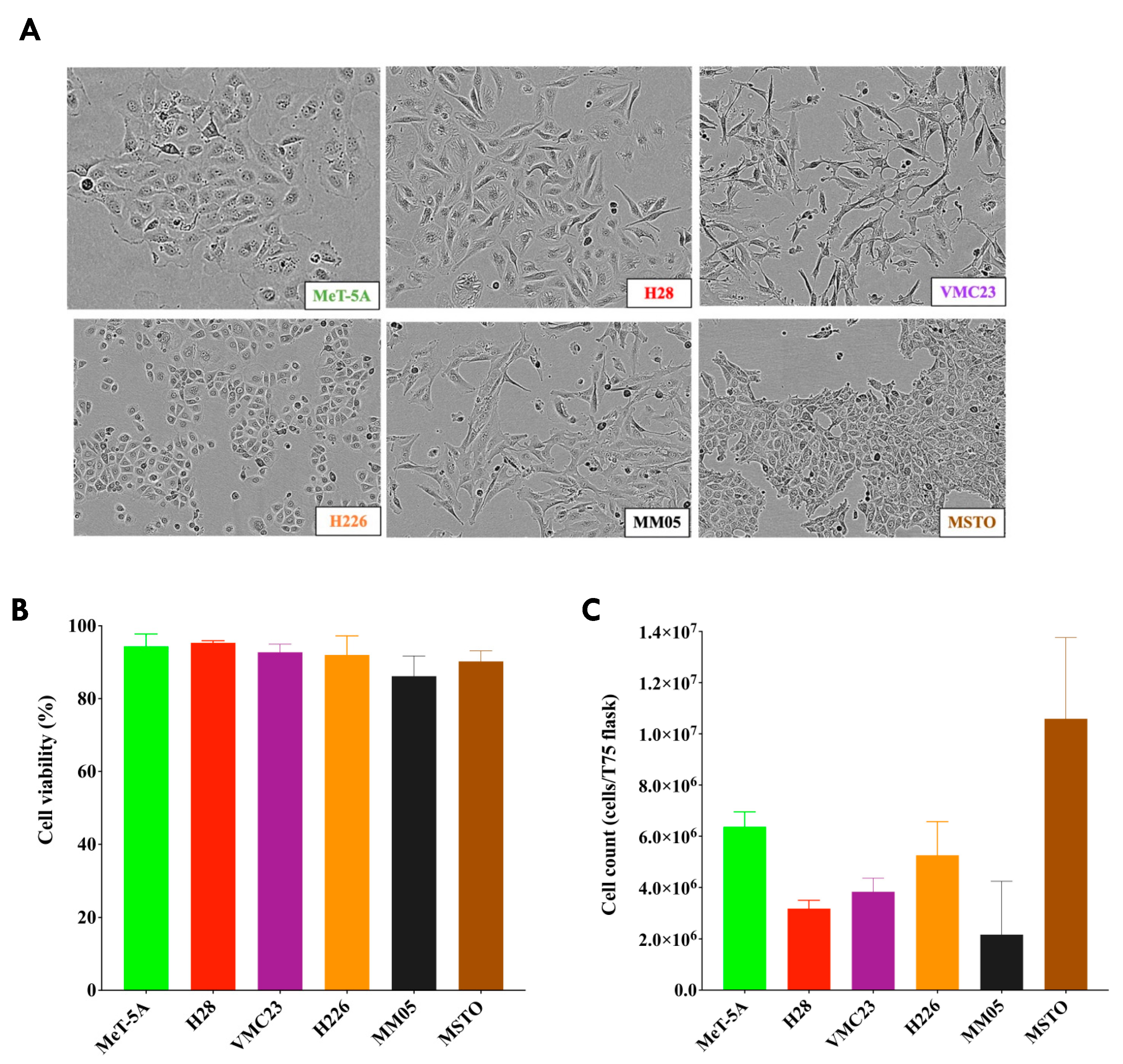
We note that the morphology of our cell lines did not represent the typical characteristics of epithelioid and non-epithelioid subtypes from pleural mesothelioma tumor histology. Epithelioid cells are typically round or polygonal, while sarcomatoid cells are spindle-like, and biphasic cells are a mixture of both. These characteristics have been observed in tissues from patient samples, whereas cells in culture may not be true representations of the histological subtypes of pleural mesothelioma as they are not in their ideal condition of cell growth. Therefore, we were unable to establish clear distinctions between epithelioid and non-epithelioid subtypes in our current study; however, our results encourage further research to investigate biomarkers unique to each subtype, perhaps from patient plasma or pleural effusion samples.