Persistent Immunity against SARS-CoV-2 in Individuals with Oncohematological Diseases Who Underwent Autologous or Allogeneic Stem Cell Transplantation after Vaccination
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Statement
2.3. Samples Processing and Materials
2.4. SARS-CoV-2 Serology
2.5. Pseudotyped SARS-CoV-2 Neutralization Assays
2.6. Phenotyping of B Cells
2.7. Antibody-Dependent Cellular Cytotoxicity Assay
2.8. Direct Cellular Cytotoxicity Assay
2.9. Statistical Analysis
3. Results
3.1. Patients’ Cohorts
3.2. SARS-CoV-2 Breakthrough Infection
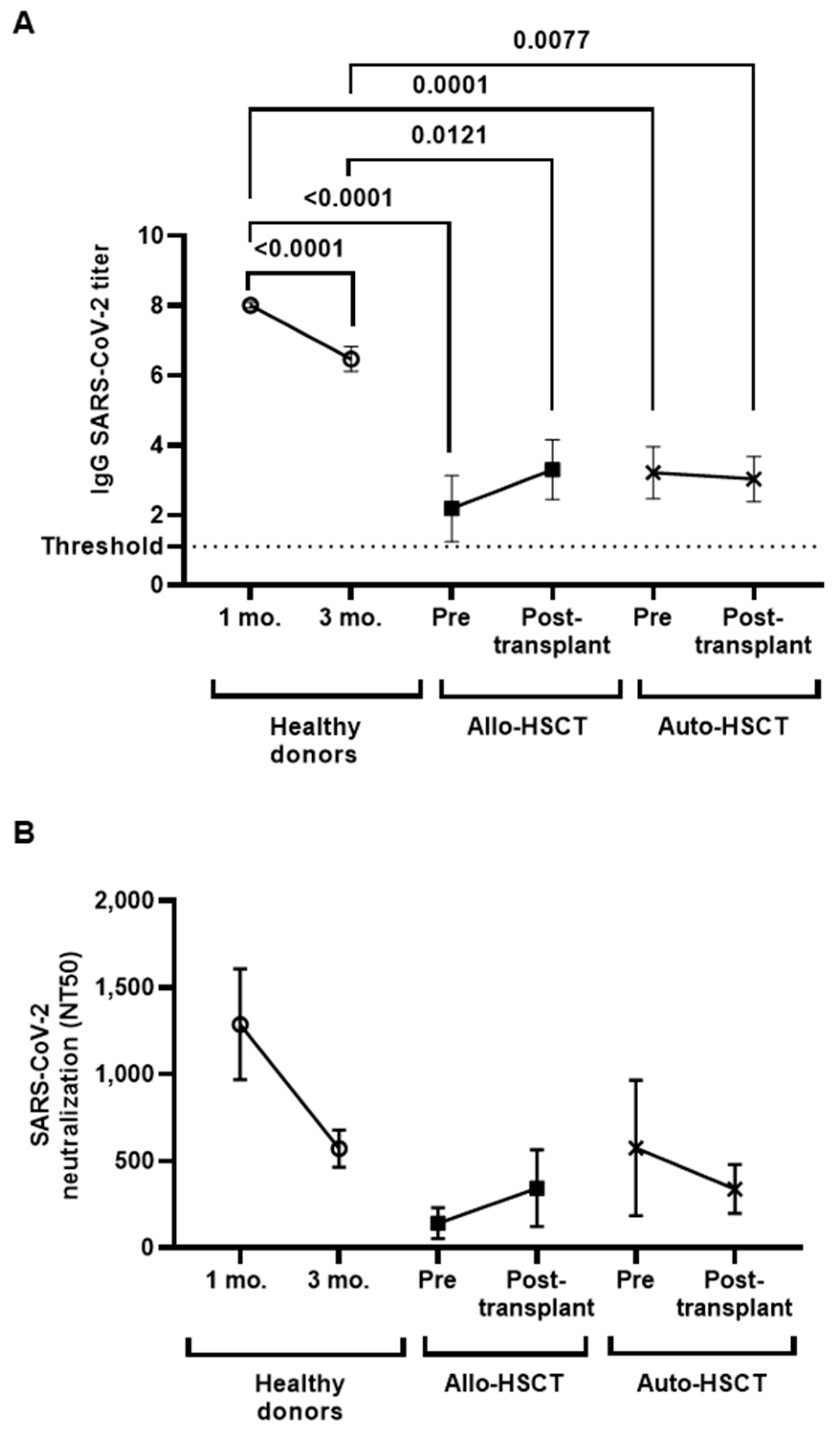
3.3. Levels of IgGs against SARS-CoV-2 before and after HSCT
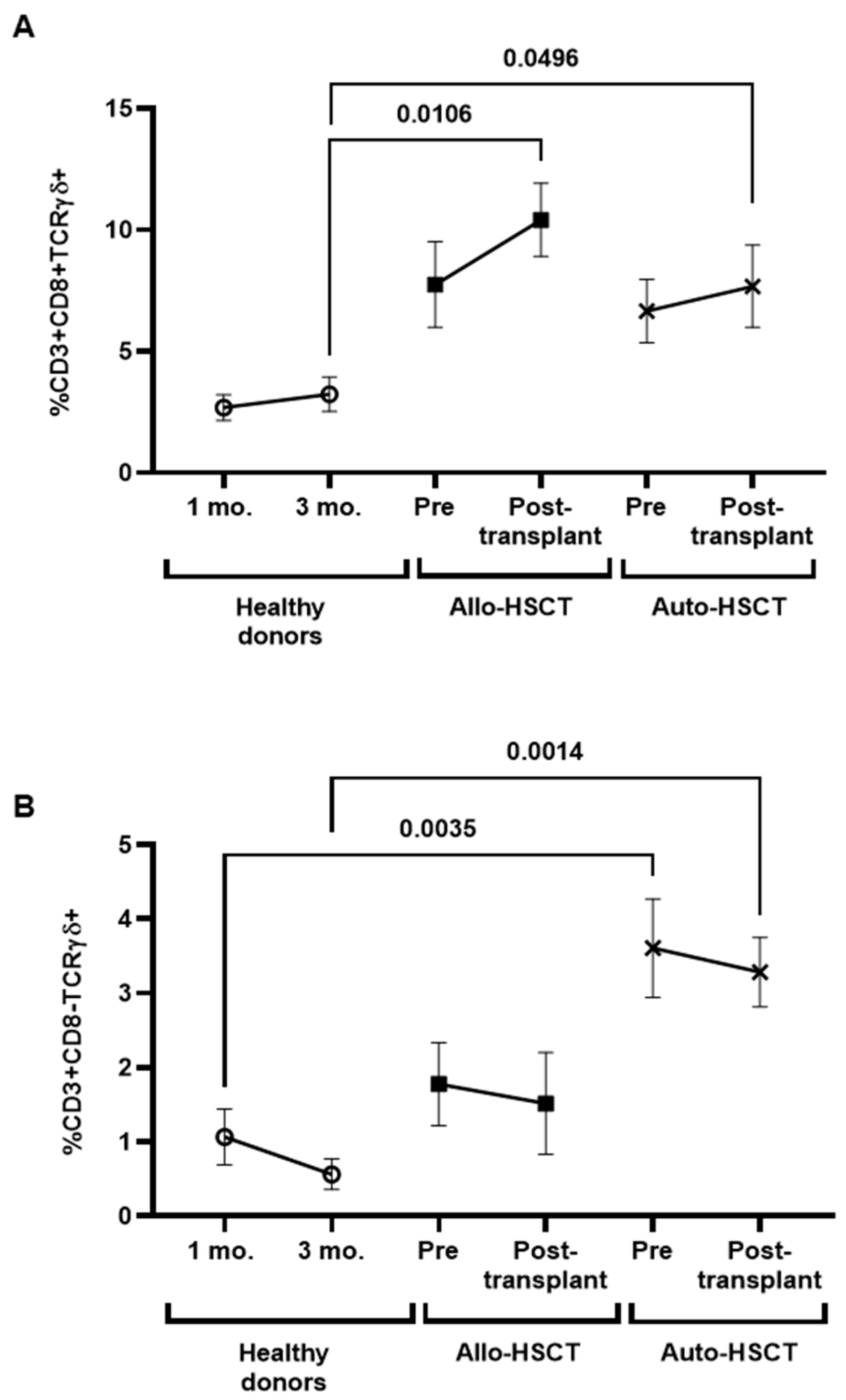
3.4. Changes in B Cell Subpopulations after HSCT
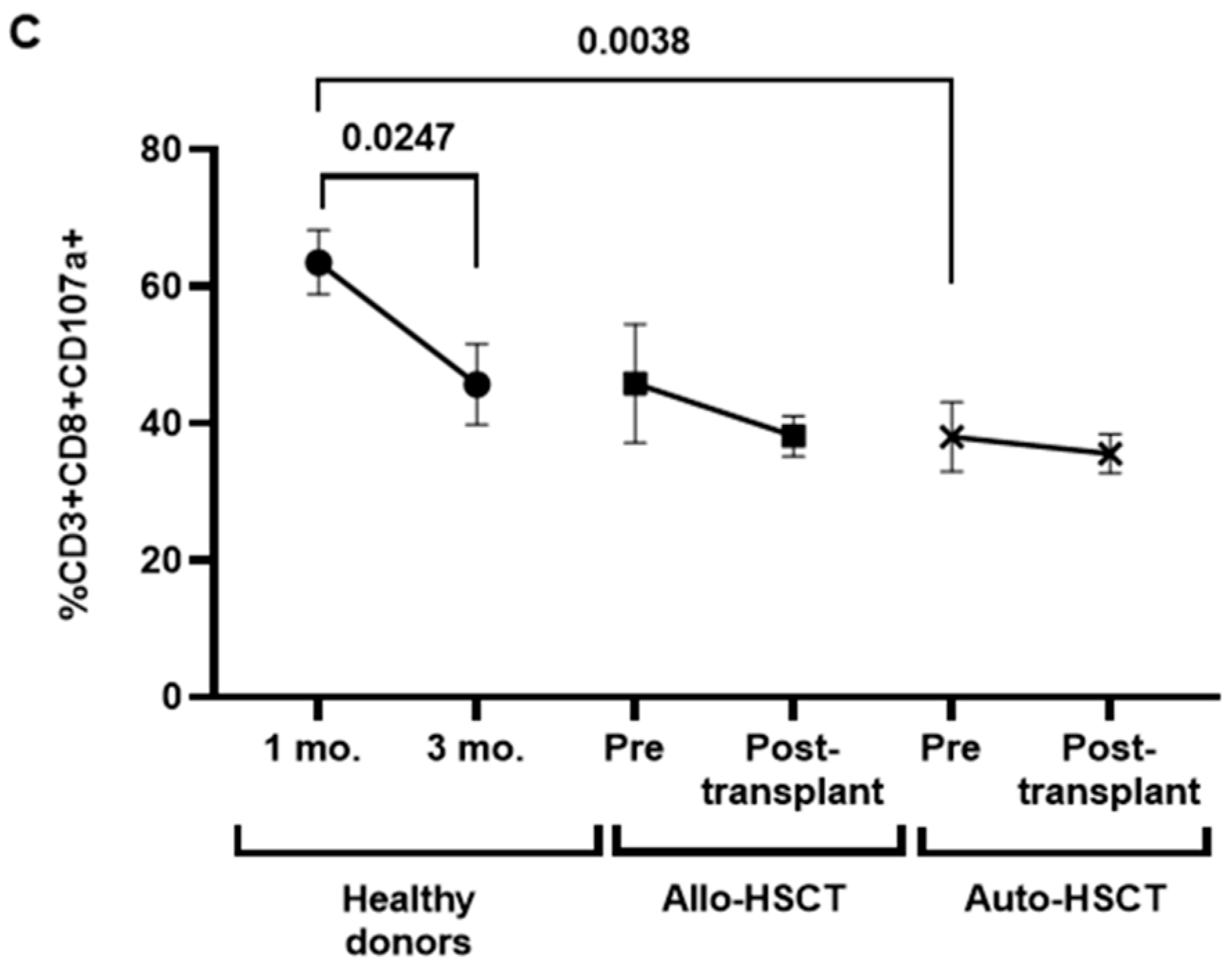
3.5. Cytotoxic Cellular Immune Responses against SARS-CoV-2 before and after HSCT
3.6. Characterization of Cytotoxic Cell Populations in PBMCs of Transplanted Individuals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; De Lima Lopes, G.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhatt, N.S.; St Martin, A.; Abid, M.B.; Bloomquist, J.; Chemaly, R.F.; Dandoy, C.; Gauthier, J.; Gowda, L.; Perales, M.A.; et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021, 8, e185–e193. [Google Scholar] [CrossRef] [PubMed]
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martín-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Orchard, K.; Dignan, F.L.; Lee, J.; Pearce, R.; Desai, M.; McFarlane, E.; Parkin, A.; Shearn, P.; Snowden, J.A. The NICE COVID-19 rapid guideline on haematopoietic stem cell transplantation: Development, implementation and impact. Br. J. Haematol. 2021, 192, 467–473. [Google Scholar] [CrossRef]
- Coronavirus Disease COVID-19: EBMT Recommendations (Update May 27, 2021). 2021. Available online: https://www.ebmt.org/ebmt/documents/coronavirus-disease-covid-19-ebmt-recommendations-update-may-27-2021 (accessed on 1 February 2023).
- Janssen, M.; Bruns, A.; Kuball, J.; Raijmakers, R.; van Baarle, D. Vaccine Responses in Adult Hematopoietic Stem Cell Transplant Recipients: A Comprehensive Review. Cancers 2021, 13, 6140. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Rezvani, K. Immune reconstitution post allogeneic transplant and the impact of immune recovery on the risk of infection. Virulence 2016, 7, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Meerveld-Eggink, A.; van der Velden, A.M.T.; Ossenkoppele, G.J.; van de Loosdrecht, A.A.; Biesma, D.H.; Rijkers, G.T. Antibody Response to Polysaccharide Conjugate Vaccines after Nonmyeloablative Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2009, 15, 1523–1530. [Google Scholar] [CrossRef]
- Giebink, G.S.; Warkentin, P.I.; Norma, N.K.; Kersey, J.H. Titers of antibody to pneumococci in allogeneic bone marrow transplant recipients before and after vaccination with pneumococcal vaccine. J. Infect. Dis. 1986, 154, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Garcia Garrido, H.M.; van Aalst, M.; Schinkel, J.; Koen, G.; Defoer, J.M.; Hazenberg, M.D.; Nur, E.; Grobusch, M.P.; Zeerleder, S.S.; Goorhuis, A.; et al. Early loss of immunity against measles following allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2019, 94, E270–E272. [Google Scholar] [CrossRef] [PubMed]
- Bögeholz, J.; Russkamp, N.F.; Wilk, C.M.; Gourri, E.; Haralambieva, E.; Schanz, U.; Mueller, N.J.; Manz, M.G.; Müller, A.M.S. Long-Term Follow-Up of Antibody Titers Against Measles, Mumps, and Rubella in Recipients of Allogenic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 581–592. [Google Scholar] [CrossRef]
- Piekarska, A.; Wisniewski, P.; Lewandowski, K.; Gil, L.; Trzonkowski, P.; Bieniaszewska, M.; Zaucha, J.M. Immune Status Against Hepatitis B in Patients After Allogeneic Hematopoietic Cell Transplantation—Factors Affecting Early and Long-Lasting Maintenance of Protective Anti-HBs Titers. Front. Immunol. 2020, 11, 586523. [Google Scholar] [CrossRef] [PubMed]
- Parkkali, T.; Ruutu, T.; Stenvik, M.; Kuronen, T.; Käyhty, H.; Hovi, T.; Ölander, R.M.; Volin, L.; Ruutu, P. Loss of protective immunity to polio, diphtheria and Haemophilus influenzae type b after allogeneic bone marrow transplantation. J. Pathol. Microbiol. Immunol. 1996, 104, 383–388. [Google Scholar] [CrossRef]
- Pauksen, K.; Hammarstrom, V.; Ljungman, P.; Sjolin, J.; Oberg, G.; Lonnerholm, G.; Magnius, L.; Simonsson, B. Immunity to Poliovirus and Immunization with Inactivated Poliovirus Vaccine after Autologous Bone Marrow Transplantation. Clin. Infect. Dis. 1994, 18, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.; Carlo Dufour, C.; Mohty, M.; Kröger, N. The EBMT Handbook. Hematopoietic Stem Cell Transplantation and Cellular Therapies; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; De Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e200–e212. [Google Scholar] [CrossRef] [PubMed]
- Chiarucci, M.; Paolasini, S.; Isidori, A.; Guiducci, B.; Loscocco, F.; Capalbo, M.; Visani, G. Immunological Response Against SARS-COV-2 after BNT162b2 Vaccine Administration Is Impaired in Allogeneic but Not in Autologous Stem Cell Transplant Recipients. Front. Oncol. 2021, 11, 6–9. [Google Scholar] [CrossRef]
- Hill, J.A. Humoral Immunity After mRNA SARS-CoV-2 Vaccination in Allogeneic HCT Recipients-Room for Improvement and Much to Learn. JAMA Netw. Open 2021, 4, e2127454. [Google Scholar] [CrossRef]
- Mamez, A.C.; Pradier, A.; Giannotti, F.; Petitpas, A.; Urdiola, M.F.; Vu, D.L.; Masouridi-Levrat, S.; Morin, S.; Dantin, C.; Clerc-Renaud, D.; et al. Antibody responses to SARS-CoV2 vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transpl. 2021, 56, 3094–3096. [Google Scholar] [CrossRef]
- Huang, A.; Cicin-Sain, C.; Pasin, C.; Epp, S.; Audigé, A.; Müller, N.J.; Nilsson, J.; Bankova, A.; Wolfensberger, N.; Vilinovszki, O.; et al. Antibody Response to SARS-CoV-2 Vaccination in Patients following Allogeneic Hematopoietic Cell Transplantation. Transpl. Cell. Ther. 2022, 28, e1–e214. [Google Scholar] [CrossRef]
- Leclerc, M.; Redjoul, R.; le Bouter, A.; Beckerich, F.; Robin, C.; Parinet, V.; Pautas, C.; Menouche, D.; Bouledroua, S.; Roy, L.; et al. Impact of donor vaccination on recipient response to early SARS-CoV-2 mRNA vaccination after allogeneic HSCT. Lancet Haematol. 2022, 9, e318–e321. [Google Scholar] [CrossRef]
- Leclerc, M.; Redjoul, R.; le Bouter, A.; Beckerich, F.; Robin, C.; Parinet, V.; Pautas, C.; Menouche, D.; Bouledroua, S.; Roy, L.; et al. Determinants of SARS-CoV-2 waning immunity in allogeneic hematopoietic stem cell transplant recipients. J. Hematol. Oncol. 2022, 15, 27. [Google Scholar] [CrossRef]
- Vigón, L.; Fuertes, D.; García-Pérez, J.; Torres, M.; Rodríguez-Mora, S.; Mateos, E.; Pautas, C.; Menouche, D.; Bouledroua, S.; Roy, L.; et al. Impaired Cytotoxic Response in PBMCs From Patients with COVID-19 Admitted to the ICU: Biomarkers to Predict Disease Severity. Front. Immunol. 2021, 12, 665329. [Google Scholar] [CrossRef] [PubMed]
- Díez-Fuertes, F.; Iglesias-Caballero, M.; García-Pérez, J.; Monzón, S.; Jiménez, P.; Varona, S.; Cuesta, I.; Zaballos, A.; Jiménez, M.; Checa, L.; et al. A Founder Effect Led Early SARS-CoV-2 Transmission in Spain. J. Virol. 2021, 95, e01583. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus Dashboard. Available online: https://covid19.who.int/ (accessed on 16 January 2023).
- Piñana, J.L.; López-Corral, L.; Martino, R.; Montoro, J.; Vazquez, L.; Pérez, A.; Martín-Martín, G.; Facal-Malvar, A.; Ferrer, E.; Pascual, M.J.; et al. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: Prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am. J. Hematol. 2022, 97, 30–42. [Google Scholar] [CrossRef]
- Redjoul, R.; le Bouter, A.; Parinet, V.; Fourati, S.; Maury, S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021, 8, e681–e683. [Google Scholar] [CrossRef]
- Padoan, A.; Cosma, C.; Bonfante, F.; della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’Olmo, K.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing antibody titers six months after Comirnaty vaccination: Kinetics and comparison with SARS-CoV-2 immunoassays. Clin. Chem. Lab. Med. 2022, 60, 456–463. [Google Scholar] [CrossRef]
- Brisotto, G.; Muraro, E.; Montico, M.; Corso, C.; Evangelista, C.; Casarotto, M.; Caffau, C.; Vettori, R.; Cozzi, M.R.; Zanussi, S.; et al. IgG antibodies against SARS-CoV-2 decay but persist 4 months after vaccination in a cohort of healthcare workers. Clin. Chim. Acta 2021, 523, 476–482. [Google Scholar] [CrossRef]
- Vicenti, I.; Basso, M.; Gatti, F.; Scaggiante, R.; Boccuto, A.; Zago, D.; Modolo, E.; Dragoni, F.; Parisi, S.G.; Zazzi, M.; et al. Faster decay of neutralizing antibodies in never infected than previously infected healthcare workers three months after the second BNT162b2 mRNA COVID-19 vaccine dose. Int. J. Infect. Dis. 2021, 112, 40–44. [Google Scholar] [CrossRef]
- Wiegering, V.; Eyrich, M.; Winkler, B.; Schlegel, P.G. Comparison of Immune Reconstitution After Allogeneic Versus Autologous Stem Cell Transplantation in 182 Pediatric Recipients. J. Pediatr. Hematol. Oncol. 2019, 41, e302–e307. [Google Scholar] [CrossRef]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. GammaDelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Bernal, E.; Gimeno, L.; Alcaraz, M.J.; Quadeer, A.A.; Moreno, M.; Martínez-Sánchez, M.V.; Campillo, J.A.; Gomez, J.M.; Pelaez, A.; García, E.; et al. Activating Killer-Cell Immunoglobulin-like Receptors Are Associated with the Severity of Coronavirus Disease 2019. J. Infect. Dis. 2021, 224, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Valero, M.; Corton, M.; López-Rodríguez, R.; Mahillo-Fernández, I.; Ruiz-Hornillos, J.; Minguez, P.; Villaverde, C.; Pérez-Tomás, M.E.; Barreda-Sánchez, M.; Mancebo, E.; et al. Age-dependent association of clonal hematopoiesis with COVID-19 mortality in patients over 60 years. Geroscience 2023, 45, 543–553. [Google Scholar] [CrossRef] [PubMed]




| Allo-HSCT (n = 11) | Auto-HSCT (n = 18) | |||
|---|---|---|---|---|
| Median age at data collection, median years (IQR) | 60 (52–63) | 57 (46–65) | ||
| Gender, n (%) | ||||
| Male | 7 (64) | 13 (72) | ||
| Female | 4 (36) | 5 (28) | ||
| Underlying oncohematological disease, n (%) | ||||
| MDS | 3 (27) | MM | 10 (55) | |
| ALL | 6 (54) | NHL | 7 (39) | |
| Others | 2 (18) | HL | 1 (6) | |
| Pre-transplant parameters at the time of first sample collection | ||||
| Pre-transplant treatment with immunosuppressive potential | ||||
| Chemotherapy, n (%) | 9 (82) | 9 (50) | ||
| Targeted therapies, n (%) | 7 (64) | 6 (33) | ||
| Type of transplant according to the donor | NA | |||
| Related donor, n (%) | 10 (90) | |||
| Identical | 3 (27) | |||
| Haploidentical | 7 (64) | |||
| Non-related donor, n (%) | 1 (9) | |||
| Identical | 0 | |||
| Mismatch | 1 (9) | |||
| Conditioning, n (%) | ||||
| Myeloablative | 8 (73) | 12 (67) | ||
| Reduced intensity | 3 (27) | 6 (33) | ||
| GvHD prophylaxis, n (%) | NA | |||
| Cy post + Csa + MMF | 9 (82) | |||
| Csa + Mtx | 2 (18) | |||
| Vaccine type, n (%) | ||||
| Vaxzevria (AstraZeneca) | 1 (9) | 4 (22) | ||
| Comirnaty (Pfizer) | 2 (18) | 3 (17) | ||
| Spikevax (Moderna) | 8 (73) | 11 (61) | ||
| SARS-CoV-2 infection prior to transplant, n (%) | 4 (36) | 2 (11) | ||
| Mean time from vaccination of recipient to transplantation, median days (IQR) | 95 (21–298) | 105 (38–205) | ||
| Vaccinated donor, n (%) | 11 (100) | NA | ||
| Vaccine type of donors, n (%) | - | |||
| Comirnaty (Pfizer) | 7 (64) | - | ||
| Spikevax (Moderna) | 1 (9) | - | ||
| Vaxzevria (AstraZeneca) | 1 (9) | - | ||
| Jcovden (Janssen) | 1 (9) | - | ||
| Unknown | 1 (9) | - | ||
| Mean time from vaccination of the donor to transplantation, median days (IQR) | 46 (10–216) | NA | ||
| Immunoglobulin deficiency, n (%) | ||||
| IgG | 2 (18) | 7 (39) | ||
| IgM | 2 (18) | 13 (72) | ||
| IgA | 2 (18) | 9 (50) | ||
| Post-transplant parameters at the time of second sample collection | ||||
| Immunosuppressive medications after HSCT, n (%) | NA | |||
| Csa/Tacrolimus | 11 (100) | |||
| MMF | 4 (36) | |||
| Corticosteroids | 3 (27) | |||
| GvHD, n (%) | 2 (18) | NA | ||
| CMV replication, n (%) | 6 (54) | 0 | ||
| Disease relapse, n (%) | 1 (9) | 0 | ||
| Admitted to ICU, n (%) | 2 (18) | 0 | ||
| SARS-CoV-2 breakthrough infection after HSCT confirmed by PCR, n (%) | 4 (36) | 7 (39) | ||
| Severity of COVID-19, n (%) | ||||
| Mild | 3 (75) | 7 (100) | ||
| Hospitalized | 1 (25) | 0 | ||
| Healthy Donors (n = 18) | |
|---|---|
| Median age at data collection, median years (IQR) | 50 (42–62) |
| Gender, n (%) | |
| Male | 13 (72) |
| Female | 5 (28) |
| Underlying oncohematological disease, n (%) | 0 |
| Vaccine type, n (%) | |
| Comirnaty (Pfizer) | 16 (89) |
| Spikevax (Moderna) | 2 (11) |
| SARS-CoV-2 infection prior to vaccination, n (%) | 0 |
| Time from complete vaccination schedule to 1st sample, median days (IQR) | 28 (23–30) |
| Time from complete vaccination schedule to 2nd sample, median days (IQR) | 91 (86–93) |
| SARS-CoV-2 breakthrough infection after vaccination confirmed by PCR, n (%) | 8 (44) |
| Severity of COVID-19, n (%) | |
| Mild | 8 (100) |
| Hospitalized | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Mora, S.; Pérez-Lamas, L.; Sainero, M.S.; Torres, M.; Sánchez-Menéndez, C.; Corona, M.; Mateos, E.; Casado-Fernández, G.; Alcamí, J.; García-Pérez, J.; et al. Persistent Immunity against SARS-CoV-2 in Individuals with Oncohematological Diseases Who Underwent Autologous or Allogeneic Stem Cell Transplantation after Vaccination. Cancers 2023, 15, 2344. https://doi.org/10.3390/cancers15082344
Rodríguez-Mora S, Pérez-Lamas L, Sainero MS, Torres M, Sánchez-Menéndez C, Corona M, Mateos E, Casado-Fernández G, Alcamí J, García-Pérez J, et al. Persistent Immunity against SARS-CoV-2 in Individuals with Oncohematological Diseases Who Underwent Autologous or Allogeneic Stem Cell Transplantation after Vaccination. Cancers. 2023; 15(8):2344. https://doi.org/10.3390/cancers15082344
Chicago/Turabian StyleRodríguez-Mora, Sara, Lucía Pérez-Lamas, Miriam Solera Sainero, Montserrat Torres, Clara Sánchez-Menéndez, Magdalena Corona, Elena Mateos, Guiomar Casado-Fernández, José Alcamí, Javier García-Pérez, and et al. 2023. "Persistent Immunity against SARS-CoV-2 in Individuals with Oncohematological Diseases Who Underwent Autologous or Allogeneic Stem Cell Transplantation after Vaccination" Cancers 15, no. 8: 2344. https://doi.org/10.3390/cancers15082344
APA StyleRodríguez-Mora, S., Pérez-Lamas, L., Sainero, M. S., Torres, M., Sánchez-Menéndez, C., Corona, M., Mateos, E., Casado-Fernández, G., Alcamí, J., García-Pérez, J., Pérez-Olmeda, M., Murciano-Antón, M. A., López-Jiménez, J., García-Gutiérrez, V., & Coiras, M. (2023). Persistent Immunity against SARS-CoV-2 in Individuals with Oncohematological Diseases Who Underwent Autologous or Allogeneic Stem Cell Transplantation after Vaccination. Cancers, 15(8), 2344. https://doi.org/10.3390/cancers15082344







