Surgical and Oncological Outcomes of Salvage Hepatectomy for Locally Recurrent Hepatocellular Carcinoma after Locoregional Therapy: A Single-Institution Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diagnostic Criteria of Recurrent HCC
2.3. Treatment Strategy
2.4. Definition of LR-HCC and OR-HCC
2.5. Statistical Analysis
3. Results
3.1. Patient and Tumor Background
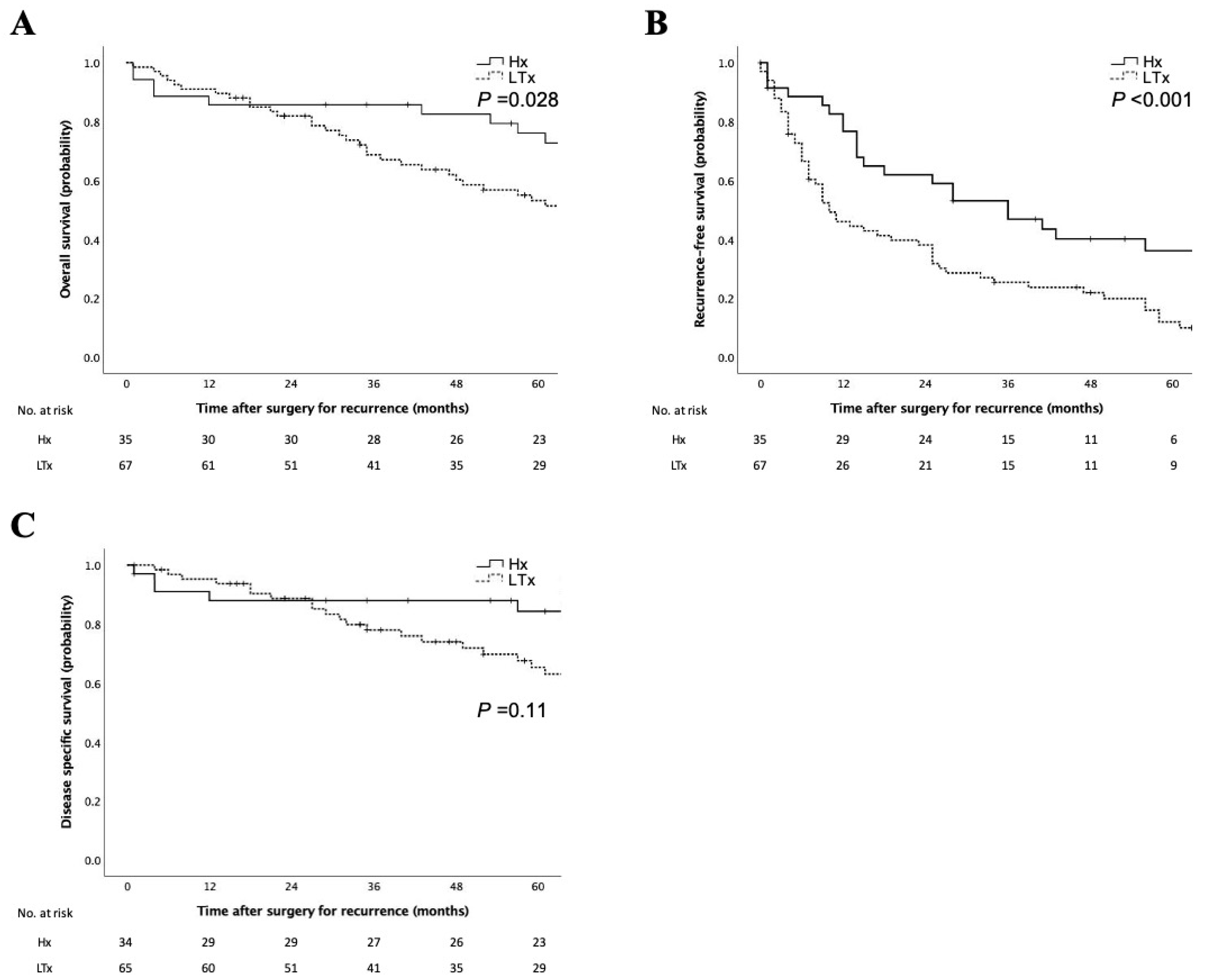
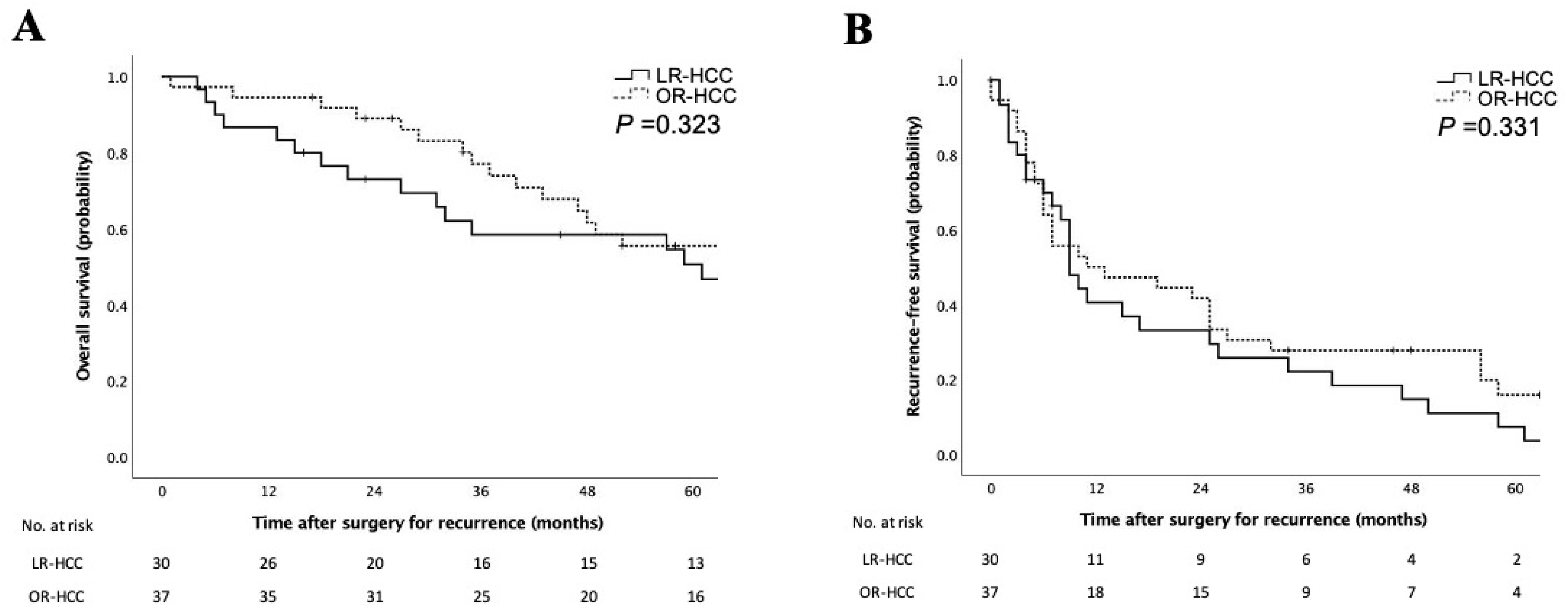
3.2. Clinicopathologic Characteristics of Patients with LR-HCC
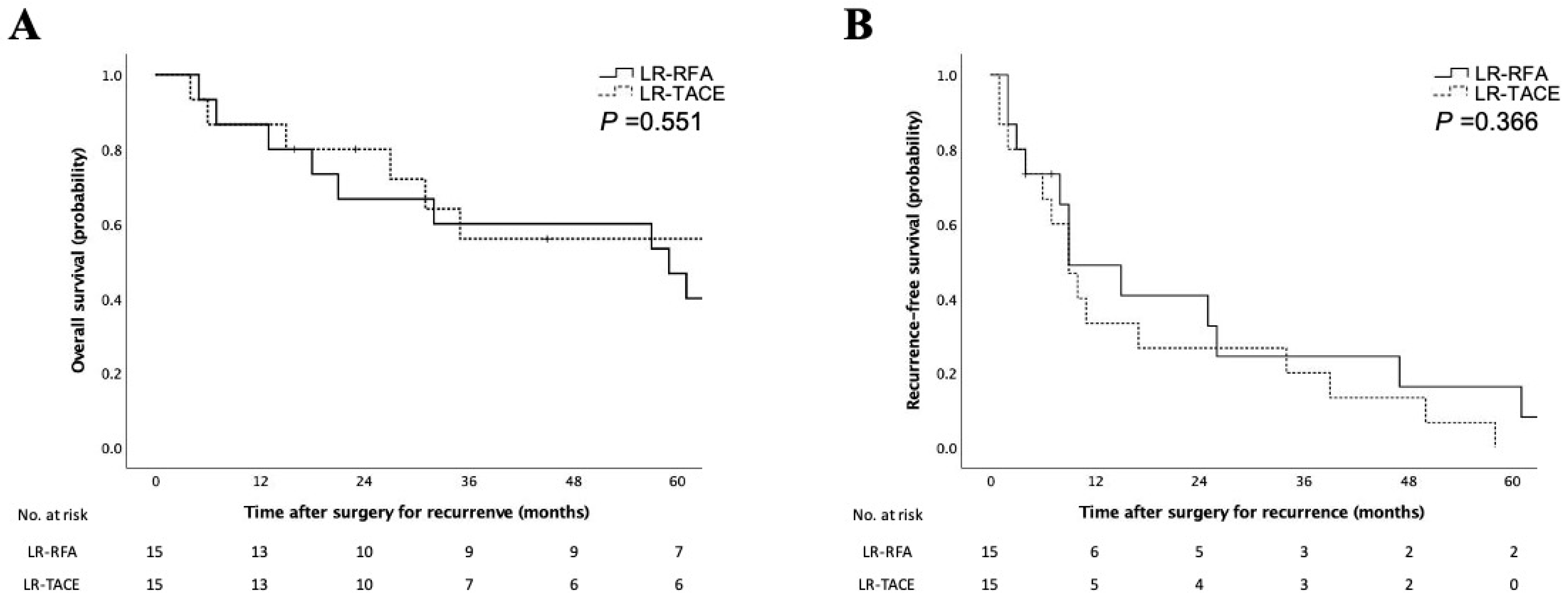
3.3. Characteristics and Prognosis of Patients with LR-HCC after RFA and TACE
3.4. Prognostic Factors for the RFS of Recurrent HCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arii, S.; Teramoto, K.; Kawamura, T.; Okamoto, H.; Kaido, T.; Mori, A.; Imamura, M. Characteristics of recurrent hepatocellular carcinoma in Japan and our surgical experience. J. Hepatobiliary Pancreat. Surg. 2001, 8, 397–403. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; European Association for the Study of the Liver: EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers (Version 5.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 13 January 2023).
- Torzilli, G.; Del Fabbro, D.; Palmisano, A.; Marconi, M.; Makuuchi, M.; Montorsi, M. Salvage hepatic resection after incomplete interstitial therapy for primary and secondary liver tumours. Br. J. Surg. 2007, 94, 208–213. [Google Scholar] [CrossRef]
- Masuda, T.; Beppu, T.; Ishiko, T.; Horino, K.; Baba, Y.; Mizumoto, T.; Hayashi, H.; Okabe, H.; Horlad, H.; Doi, K.; et al. Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J. Hepatobiliary Pancreat. Surg. 2008, 15, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Portolani, N.; Baiocchi, G.L.; Coniglio, A.; Grazioli, L.; Frassi, E.; Gheza, F.; Giulini, S.M. Sequential multidisciplinary treatment of hepatocellular carcinoma: The role of surgery as rescue therapy for failure of percutaneous ablation therapies. J. Surg. Oncol. 2009, 100, 580–584. [Google Scholar] [CrossRef]
- Sugo, H.; Ishizaki, Y.; Yoshimoto, J.; Imamura, H.; Kawasaki, S. Salvage hepatectomy for local recurrent hepatocellular carcinoma after ablation therapy. Ann. Surg. Oncol. 2012, 19, 2238–2245. [Google Scholar] [CrossRef]
- Imai, K.; Beppu, T.; Chikamoto, A.; Mima, K.; Okabe, H.; Hayashi, H.; Nitta, H.; Ishiko, T.; Baba, H. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol. Res. 2014, 44, E335–E345. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 updates by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Kishi, Y.; Shimada, K.; Nara, S.; Esaki, M.; Kosuge, T. Role of hepatectomy for recurrent or initially unresectable hepatocellular carcinoma. World J. Hepatol. 2014, 6, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Taura, K.; Ikai, I.; Hatano, E.; Fujii, H.; Uyama, N.; Shimahara, Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: An analysis of 610 patients over 16 years old. Ann. Surg. 2006, 244, 265–273. [Google Scholar] [CrossRef]
- Poon, R.T.P.; Ngan, H.; Lo, C.M.; Liu, C.L.; Fan, S.T.; Wong, J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J. Surg. Oncol. 2000, 73, 109–114. [Google Scholar] [CrossRef]
- Choi, D.; Lim, H.K.; Rhim, H.; Kim, Y.S.; Yoo, B.C.; Paik, S.W.; Joh, J.W.; Park, C.K. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: Long-term results and prognostic factors. Ann. Surg. Oncol. 2007, 14, 2319–2329. [Google Scholar] [CrossRef]
- Okuwaki, Y.; Nakazawa, T.; Kokubu, S.; Hidaka, H.; Tanaka, Y.; Takada, J.; Watanabe, M.; Shibuya, A.; Minamino, T.; Saigenji, K. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am. J. Gastroenterol. 2009, 104, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, F.; Fantini, C.; Ratti, F.; Lauro, R.; Tranchart, H.; Halls, M.; Scuderi, V.; Barkhatov, L.; Edwin, B.; Troisi, R.I.; et al. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg. Endosc. 2018, 32, 617–626. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Hilal, M.A.; Berardi, G.; Ciria, R.; Abe, Y.; Aoki, T.; Asbun, H.J.; Chan, A.C.Y.; et al. The Tokyo 2020 terminology of liver anatomy and resections: Updates of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Sci. 2022, 29, 6–15. [Google Scholar] [CrossRef]
- Arita, J.; Yamamoto, H.; Kokudo, T.; Hasegawa, K.; Miyata, H.; Toh, Y.; Gotoh, M.; Kokudo, N.; Kakeji, Y.; Seto, Y. Impact of board certification system and adherence to the clinical practice guidelines for liver cancer on post-hepatectomy risk-adjusted mortality rate in Japan: A questionnaire survey of departments registered with the National Clinical Database. J. Hepatobiliary Pancreat. Sci. 2021, 28, 801–811. [Google Scholar] [CrossRef]
- Guerrini, G.P.; Esposito, G.; Olivieri, T.; Magistri, P.; Ballarin, R.; Di Sandro, S.; Di Benedetto, F. Salvage versus primary liver transplantation for hepatocellular carcinoma: A twenty-year experience meta-analysis. Cancers 2022, 14, 3465. [Google Scholar] [CrossRef]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, G.H.; Na, D.C.; Ahn, E.Y.; Kim, G.I.; Lee, J.E.; Cho, J.Y.; Yoo, J.E.; Choi, J.S.; Park, Y.N. Human hepatocellular carcinomas with “Stemness”-related marker expression: Keratin 19 expression and a poor prognosis. Hepatology 2011, 54, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ji, J.; Budhu, A.; Forgues, M.; Yang, W.; Wang, H.Y.; Jia, H.; Ye, Q.; Wauthier, E.; Reid, L.M.; et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009, 136, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, H.; Masugi, Y.; Yamazaki, K.; Itano, O.; Kitagawa, Y.; Sakamoto, M. Immunohistochemical molecular analysis indicates hepatocellular carcinoma subgroups that reflect tumor aggressiveness. Hum. Pathol. 2016, 50, 24–33. [Google Scholar] [CrossRef]
| Variables | Recurrence after Hepatectomy | Recurrence after Locoregional Therapy | p-Value |
|---|---|---|---|
| (n = 35) | (n = 67) | ||
| Age (year, median) | 70 (33–82) | 71 (50–86) | 0.73 |
| Sex | 0.58 | ||
| Female | 9 | 14 | |
| Male | 26 | 53 | |
| Etiology | 0.114 | ||
| HBV | 14 | 16 | |
| HBV + HCV | 0 | 1 | |
| HCV | 12 | 38 | |
| NBNC | 9 | 9 | |
| Child–Pugh classification | 0.296 | ||
| A | 35 | 63 | |
| B | 0 | 4 | |
| Liver damage | 0.206 | ||
| A | 32 | 53 | |
| B | 3 | 13 | |
| C | 0 | 1 | |
| Platelet count (×103/µL, median) | 13.6 (5.1–28.5) | 11.8 (4.1–37.4) | 0.347 |
| AFP (ng/mL, median) | 6 (2–18,000) | 10 (0–80,977) | 0.286 |
| AFP-L3 (%, median) | 7.2 (0–50.3) | 9.2 (0–84.9) | 0.304 |
| DCP (mAU/mL, median) | 21 (9–5220) | 25 (7–23,000) | 0.956 |
| Number of pretreatments | 1 (1–19) | 3 (1–10) | <0.001 |
| Surgical approach | 0.883 | ||
| Open | 23 | 45 | |
| Laparoscopic | 12 | 22 | |
| Procedures | 0.13 | ||
| Partial resection | 27 | 44 | |
| Segmentectomy | 1 | 5 | |
| Sectionectomy | 6 | 8 | |
| Hemihepatectomy | 1 | 10 | |
| Operation time (min, median) | 291 (118–780) | 359 (84–1500) | 0.087 |
| Estimated blood loss (g, median) | 300 (1–4560) | 300 (1–16,156) | 0.915 |
| Morbidities (Clavien–Dindo ≥ IIIa) | 0.048 | ||
| No | 28 | 40 | |
| Yes | 7 | 26 | |
| Postoperative hospital stay | 13 (6–40) | 15 (4–217) | 0.313 |
| (day, median) | |||
| Tumor multiplicity | 0.236 | ||
| Solitary | 20 | 30 | |
| Multiple | 15 | 37 | |
| Tumor size (mm, median) | 18 (6–42) | 20 (7–140) | 0.018 |
| Histology | 0.429 | ||
| Well | 4 | 7 | |
| Moderate | 23 | 50 | |
| Poor | 7 | 10 | |
| Other | 1 | 0 | |
| Portal venous invasion | 0.532 | ||
| No | 19 | 32 | |
| Yes | 16 | 35 | |
| Hepatic venous invasion | 0.658 | ||
| No | 34 | 63 | |
| Yes | 1 | 4 | |
| Hepatic arterial invasion | 1 | ||
| No | 35 | 66 | |
| Yes | 0 | 1 | |
| Bile duct invasion | 0.658 | ||
| No | 34 | 63 | |
| Yes | 1 | 4 | |
| Surgical margin | 0.678 | ||
| Negative | 26 | 48 | |
| Positive | 7 | 16 | |
| Background liver condition | 0.002 | ||
| Normal | 2 | 0 | |
| Chronic hepatitis | 24 | 30 | |
| Cirrhosis | 7 | 33 | |
| Initial recurrence site | 0.136 | ||
| Liver | 19 | 41 | |
| Extrahepatic | 0 | 5 | |
| Both | 3 | 11 | |
| Recurrence pattern in liver | 0.662 | ||
| Intrahepatic metastasis/ | 22 | 47 | |
| Multicentric occurrence | |||
| Local recurrence | 1 | 5 |
| Variables | LR-HCC | OR-HCC | p-Value |
|---|---|---|---|
| (n = 30) | (n = 37) | ||
| Age (year, median) | 68 (52–86) | 70 (50–80) | 0.94 |
| Sex | 0.871 | ||
| Female | 6 | 8 | |
| Male | 24 | 29 | |
| Etiology | 0.031 | ||
| HBV | 12 | 4 | |
| HBV + HCV | 0 | 1 | |
| HCV | 14 | 24 | |
| NBNC | 4 | 8 | |
| Child–Pugh classification | 1 | ||
| A | 28 | 35 | |
| B | 2 | 2 | |
| Liver damage | 0.548 | ||
| A | 24 | 29 | |
| B | 6 | 7 | |
| C | 0 | 1 | |
| Platelet count (×103/µL, median) | 11.1 (5.1–19.1) | 12.8 (5.1–28.5) | 0.57 |
| AFP (ng/mL, median) | 35 (4–47,598) | 7 (0–314) | 0.031 |
| AFP-L3 (%, median) | 17.3 (0–84.9) | 7.3 (0–50.3) | 0.033 |
| DCP (mAU/mL, median) | 32 (10–10,520) | 19 (7–23,000) | 0.897 |
| Number of pretreatments | 3 (1–7) | 3 (1–10) | 0.253 |
| Surgical approach | 0.938 | ||
| Open | 20 | 25 | |
| Laparoscopic | 10 | 12 | |
| Procedures | 0.297 | ||
| Partial resection | 16 | 28 | |
| Segmentectomy | 3 | 2 | |
| Sectionectomy | 5 | 3 | |
| Hemihepatectomy | 6 | 4 | |
| Operation time (min, median) | 363 (155–650) | 310 (84–1500) | 0.123 |
| Estimated blood loss (g, median) | 475 (1–2537) | 275 (1–16,156) | 0.197 |
| Morbidities (Clavien–Dindo ≥IIIa) | 0.07 | ||
| No | 14 | 26 | |
| Yes | 15 | 11 | |
| Postoperative hospital stay | 16 (7–43) | 12 (4–217) | 0.232 |
| (day, median) | |||
| Tumor multiplicity | 0.439 | ||
| Solitary | 15 | 15 | |
| Multiple | 15 | 22 | |
| Tumor size (mm, median) | 25 (7–50) | 22 (10–80) | 0.705 |
| Histology | 0.577 | ||
| Well | 3 | 4 | |
| Moderate | 21 | 29 | |
| Poor | 6 | 4 | |
| Other | 0 | 0 | |
| Portal venous invasion | 0.102 | ||
| No | 11 | 21 | |
| Yes | 19 | 16 | |
| Hepatic venous invasion | 0.318 | ||
| No | 27 | 36 | |
| Yes | 3 | 1 | |
| Hepatic arterial invasion | 0.448 | ||
| No | 29 | 37 | |
| Yes | 1 | 0 | |
| Bile duct invasion | 1 | ||
| No | 28 | 35 | |
| Yes | 2 | 2 | |
| Surgical margin | 0.081 | ||
| Negative | 18 | 30 | |
| Positive | 10 | 6 | |
| Background liver condition | 0.268 | ||
| Normal | 0 | 0 | |
| Chronic hepatitis | 16 | 14 | |
| Cirrhosis | 13 | 20 | |
| Initial recurrence site | 0.689 | ||
| Liver | 18 | 23 | |
| Extrahepatic | 3 | 2 | |
| Both | 6 | 5 | |
| Recurrence pattern in the liver | 1 | ||
| Intrahepatic metastasis/ | 22 | 25 | |
| Multicentric occurrence | |||
| Local recurrence | 2 | 3 |
| Variables | Local Recurrence after RFA | Local Recurrence after TACE | p-Value |
|---|---|---|---|
| (n = 15) | (n = 15) | ||
| Age (year, median) | 64 (52–86) | 73 (54–79) | 0.713 |
| Sex | 1 | ||
| Female | 3 | 3 | |
| Male | 12 | 12 | |
| Etiology | 0.513 | ||
| HBV | 6 | 6 | |
| HCV | 8 | 6 | |
| NBNC | 1 | 3 | |
| Child–Pugh classification | 0.483 | ||
| A | 13 | 15 | |
| B | 2 | 0 | |
| Liver damage | 0.651 | ||
| A | 11 | 13 | |
| B | 4 | 2 | |
| Platelet count (×103/µL, median) | 14.2 (5.1–25.8) | 11.6 (7.1–29.0) | 0.624 |
| AFP (ng/mL, median) | 71 (3–47,598) | 9 (1–80,977) | 0.367 |
| AFP-L3 (%, median) | 14.1 (0–84.9) | 22.6 (0–58.0) | 0.591 |
| DCP (mAU/mL, median) | 45 (10–6060) | 15 (9–10,520) | 0.041 |
| Number of pretreatments | 3 (1–5) | 3 (1–11) | 0.217 |
| Surgical approach | 1 | ||
| Open | 10 | 10 | |
| Laparoscopic | 5 | 5 | |
| Procedures | 0.4 | ||
| Partial resection | 8 | 8 | |
| Segmentectomy | 1 | 2 | |
| Sectionectomy | 4 | 1 | |
| Hemihepatectomy | 2 | 4 | |
| Operation time (time, median) | 351 (155–691) | 403 (246–801) | 0.505 |
| Estimated blood loss (g, median) | 400 (1–2537) | 510 (1–7100) | 0.88 |
| Morbidities (Clavien-Dindo ≥IIIa) | 0.858 | ||
| No | 7 | 7 | |
| Yes | 8 | 7 | |
| Postoperative hospital stay | 21 (7–160) | 16 (6–101) | 0.935 |
| (day, median) | |||
| Tumor multiplicity | 0.273 | ||
| Solitary | 9 | 6 | |
| Multiple | 6 | 9 | |
| Tumor size (mm, median) | 20 (7–55) | 23 (13–140) | 0.806 |
| Histology | |||
| Well | 2 | 1 | 0.587 |
| Moderate | 11 | 10 | |
| Poor | 2 | 4 | |
| Portal venous invasion | 0.256 | ||
| No | 7 | 4 | |
| Yes | 8 | 11 | |
| Hepatic venous invasion | 1 | ||
| No | 14 | 13 | |
| Yes | 1 | 2 | |
| Hepatic arterial invasion | 0.309 | ||
| No | 15 | 14 | |
| Yes | 0 | 1 | |
| Bile duct invasion | 0.483 | ||
| No | 15 | 13 | |
| Yes | 0 | 2 | |
| Surgical margin | 1 | ||
| Negative | 9 | 9 | |
| Positive | 5 | 5 | |
| Background liver condition | 0.34 | ||
| Normal | 0 | 0 | |
| Chronic hepatitis | 7 | 9 | |
| Cirrhosis | 8 | 5 | |
| Initial recurrence site | 0.453 | ||
| Liver | 7 | 11 | |
| Extrahepatic | 1 | 2 | |
| Both | 4 | 2 | |
| Recurrence pattern in the liver | 0.482 | ||
| Intrahepatic metastasis/ | 11 | 11 | |
| Multicentric occurrence | |||
| Local recurrence | 0 | 2 |
| Median | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Variables | n | RFS | p-Value | HR (95% CI) | p-Value |
| Etiology | 0.208 | ||||
| HBV | 30 | 26 | |||
| HBV + HCV | 1 | 56 | |||
| HCV | 50 | 11 | |||
| NBNC | 21 | 27 | |||
| Child–Pugh classification | 0.946 | ||||
| A | 98 | 17 | |||
| B | 4 | 32 | |||
| Liver damage | 0.404 | ||||
| A | 85 | 18 | |||
| B + C | 17 | 10 | |||
| AFP (ng/mL, median) | 0.009 | 0.061 | |||
| <20 | 69 | 25 | 1 (ref) | ||
| ≥20 | 33 | 10 | 1.65 (0.98–2.77) | ||
| AFP-L3 (%, median) | 0.04 | 0.388 | |||
| <10 | 81 | 25 | 1 (ref) | ||
| ≥10 | 21 | 10 | 1.31 (0.71–2.39) | ||
| DCP (mAU/mL, median) | 0.076 | 0.899 | |||
| <40 | 67 | 23 | 1 (ref) | ||
| ≥40 | 35 | 13 | 1.04 (0.60–1.80) | ||
| Number of pretreatments | 0.018 | 0.712 | |||
| 1 | 47 | 25 | 1 (ref) | ||
| ≥2 | 55 | 11 | 1.10 (0.67–1.79) | ||
| Pretreatment modality | <0.001 | 0.005 | |||
| Hepatectomy | 35 | 36 | 1 (ref) | ||
| Locoregional therapy | 67 | 10 | 2.04 (1.24–3.39) | ||
| Tumor multiplicity | <0.001 | <0.001 | |||
| Solitary | 50 | 34 | 1 (ref) | ||
| Multiple | 52 | 8 | 2.78 (1.71–4.49) | ||
| Tumor size (mm) | 0.029 | 0.577 | |||
| ≤20 | 56 | 25 | 1 (ref) | ||
| >20 | 46 | 14 | 1.20 (0.66–2.19) | ||
| Portal venous invasion | <0.001 | 0.001 | |||
| No | 51 | 25 | 1 (ref) | ||
| Yes | 51 | 10 | 2.27 (1.39–3.71) | ||
| Hepatic venous invasion | 0.281 | ||||
| No | 97 | 17 | |||
| Yes | 5 | 11 | |||
| Hepatic arterial invasion | 0.495 | ||||
| No | 101 | 17 | |||
| Yes | 1 | 11 | |||
| Bile duct invasion | <0.001 | 0.19 | |||
| No | 97 | 19 | 1 (ref) | ||
| Yes | 5 | 2 | 1.95 (0.72–5.28) | ||
| Surgical margin | 0.049 | 0.521 | |||
| Negative | 74 | 25 | 1 (ref) | ||
| Positive | 23 | 7 | 1.22 (0.67–2.23) | ||
| Background liver condition | 0.411 | ||||
| Normal/Chronic hepatitis | 56 | 25 | |||
| Cirrhosis | 40 | 17 | |||
| Recurrence pattern | 0.014 | 0.644 | |||
| Local recurrence | 30 | 9 | 1.15 (0.64–2.08) | ||
| Other types of recurrence | 72 | 25 | 1 (ref) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minagawa, T.; Itano, O.; Kitago, M.; Abe, Y.; Yagi, H.; Hibi, T.; Shinoda, M.; Ojima, H.; Sakamoto, M.; Kitagawa, Y. Surgical and Oncological Outcomes of Salvage Hepatectomy for Locally Recurrent Hepatocellular Carcinoma after Locoregional Therapy: A Single-Institution Experience. Cancers 2023, 15, 2320. https://doi.org/10.3390/cancers15082320
Minagawa T, Itano O, Kitago M, Abe Y, Yagi H, Hibi T, Shinoda M, Ojima H, Sakamoto M, Kitagawa Y. Surgical and Oncological Outcomes of Salvage Hepatectomy for Locally Recurrent Hepatocellular Carcinoma after Locoregional Therapy: A Single-Institution Experience. Cancers. 2023; 15(8):2320. https://doi.org/10.3390/cancers15082320
Chicago/Turabian StyleMinagawa, Takuya, Osamu Itano, Minoru Kitago, Yuta Abe, Hiroshi Yagi, Taizo Hibi, Masahiro Shinoda, Hidenori Ojima, Michiie Sakamoto, and Yuko Kitagawa. 2023. "Surgical and Oncological Outcomes of Salvage Hepatectomy for Locally Recurrent Hepatocellular Carcinoma after Locoregional Therapy: A Single-Institution Experience" Cancers 15, no. 8: 2320. https://doi.org/10.3390/cancers15082320
APA StyleMinagawa, T., Itano, O., Kitago, M., Abe, Y., Yagi, H., Hibi, T., Shinoda, M., Ojima, H., Sakamoto, M., & Kitagawa, Y. (2023). Surgical and Oncological Outcomes of Salvage Hepatectomy for Locally Recurrent Hepatocellular Carcinoma after Locoregional Therapy: A Single-Institution Experience. Cancers, 15(8), 2320. https://doi.org/10.3390/cancers15082320






