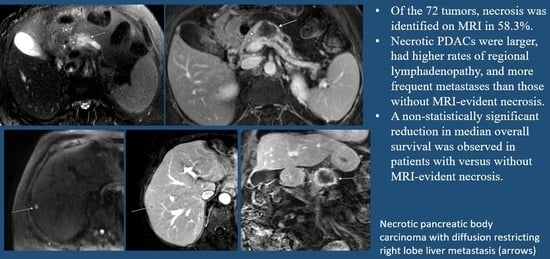
MRI-Based Tumor Necrosis Depiction in Pancreatic Ductal Adenocarcinoma: Can It Predict Tumor Aggressiveness?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients’ Demographics and Tumor Characteristics
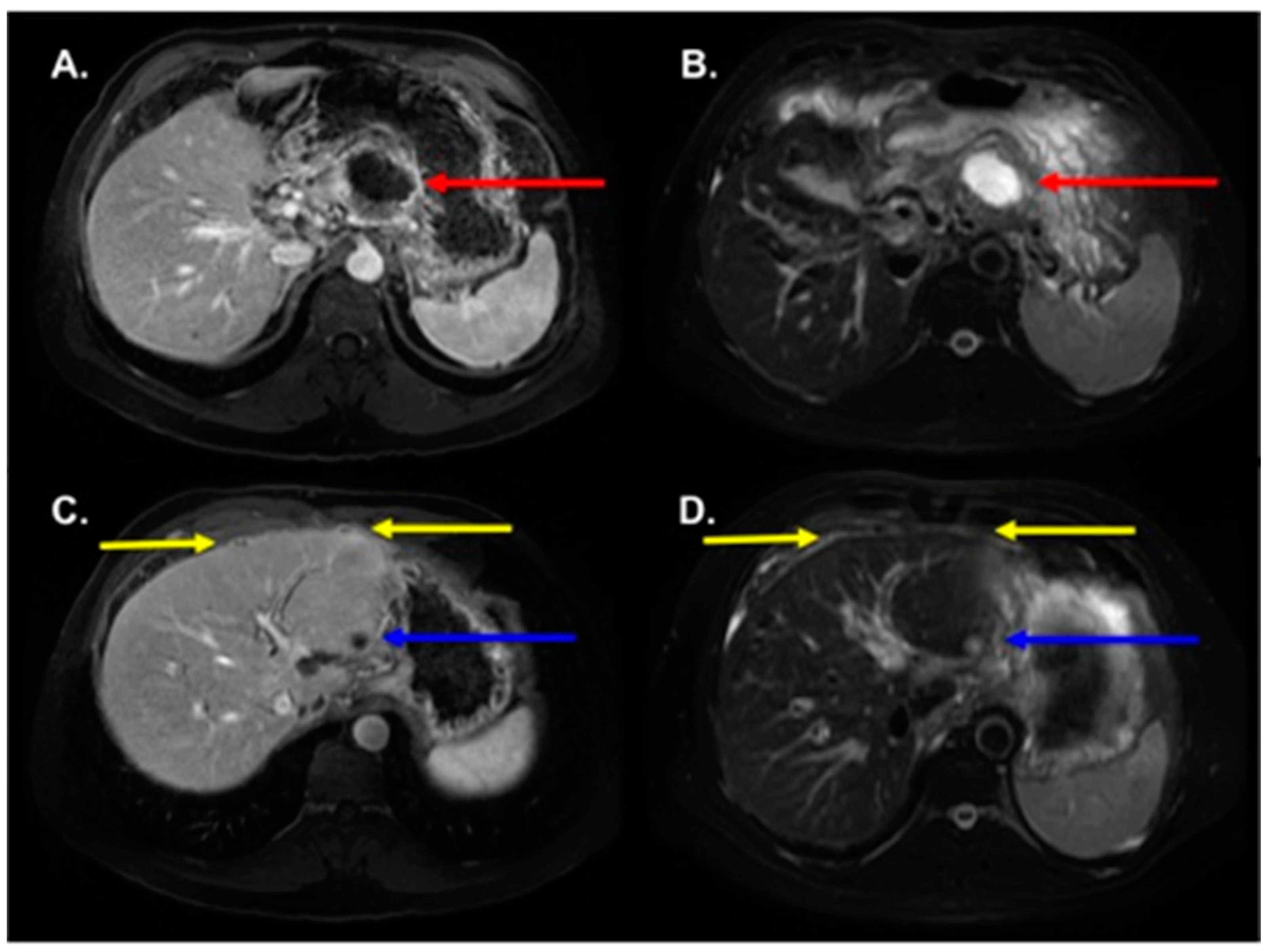
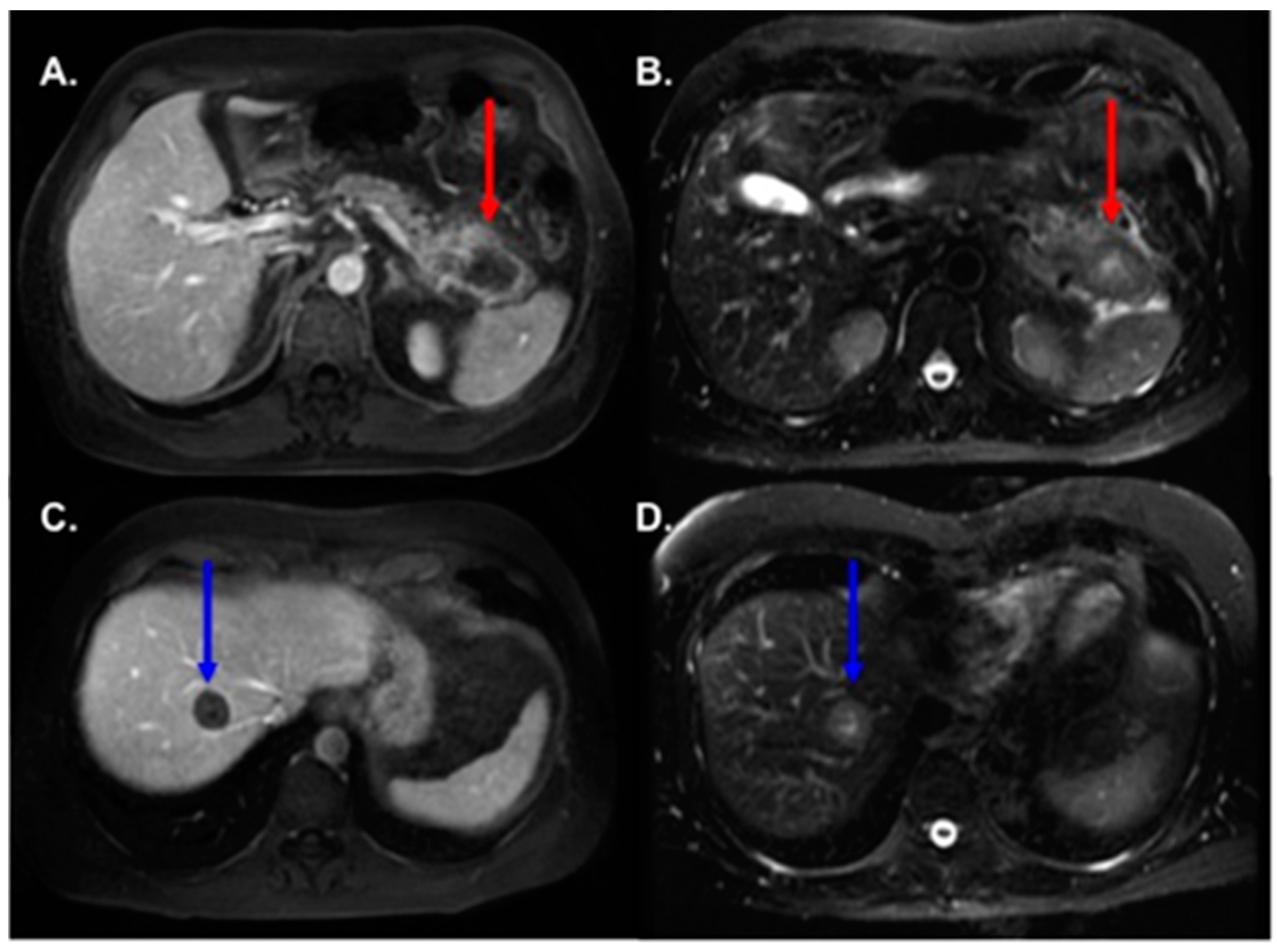
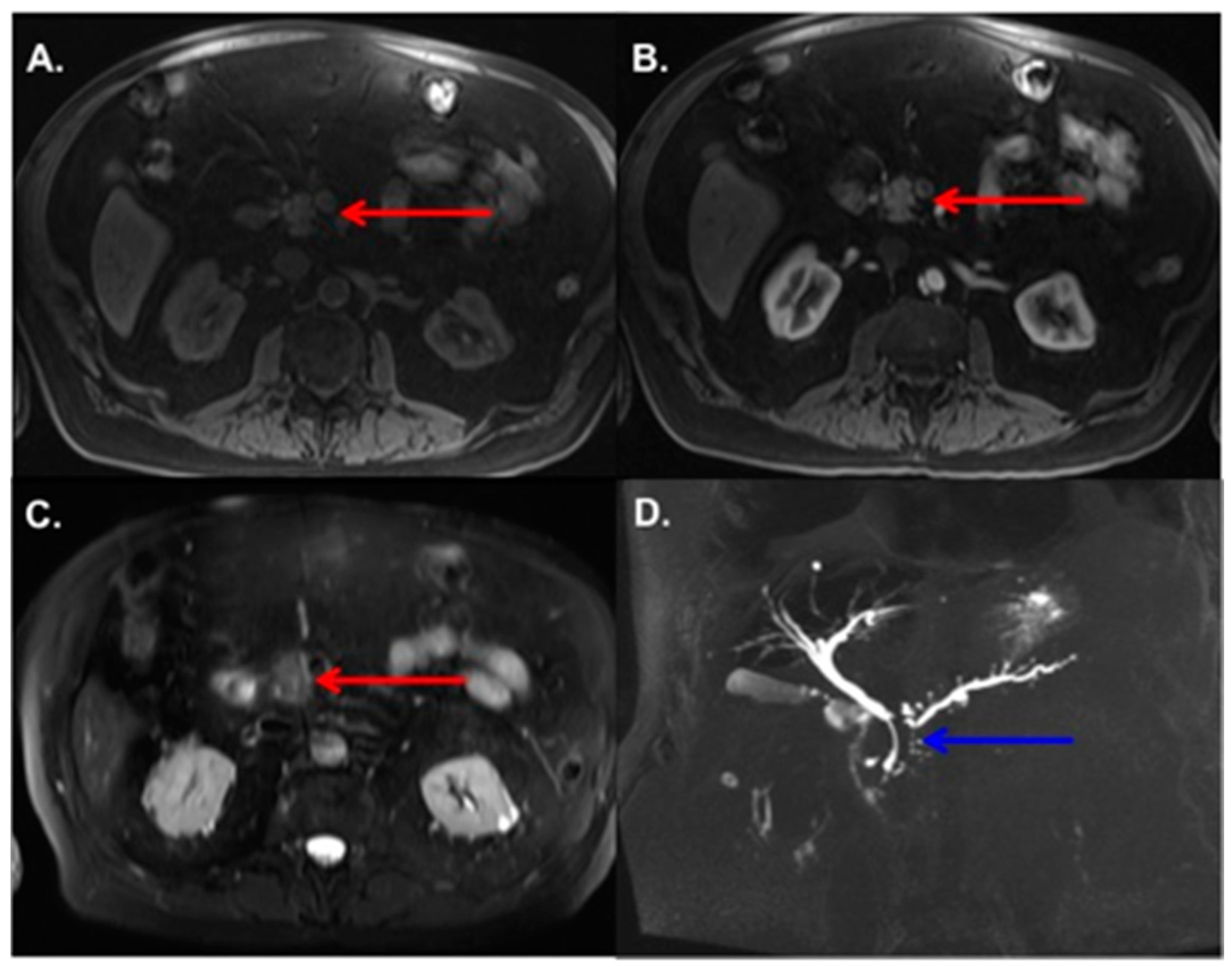
3.2. MRI Findings
3.3. Radiologic–Pathologic Correlation
3.4. Patients Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Ritchey, J.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: Report from the National Cancer Database. Cancer 2007, 110, 738–744. [Google Scholar] [CrossRef]
- Becker, A.E.; Hernandez, Y.G.; Frucht, H.; Lucas, A.L. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. World J. Gastroenterol. 2014, 20, 11182–11198. [Google Scholar] [CrossRef]
- Zhang, X.M.; Mitchell, D.G.; Byun, J.H.; Verma, S.K.; Bergin, D.; Witkiewicz, A. MR imaging for predicting the recurrence of pancreatic carcinoma after surgical resection. Eur. J. Radiol. 2010, 73, 572–578. [Google Scholar] [CrossRef]
- Elias, J., Jr.; Semelka, R.C.; Altun, E.; Tsurusaki, M.; Pamuklar, E.; Zapparoli, M.; Voultsinos, V.; Armao, D.M.; Rubinas, T. Pancreatic cancer: Correlation of MR findings, clinical features, and tumor grade. J. Magn. Reson. Imaging 2007, 26, 1556–1563. [Google Scholar] [CrossRef]
- Lauenstein, T.C.; Martin, D.R.; Sarmiento, J.M.; Kalb, B.; Moreira, R.; Carew, J.; Salman, K.; Adsay, V. Pancreatic adenocarcinoma tumor grade determination using contrast-enhanced magnetic resonance imaging. Pancreas 2010, 39, 71–75. [Google Scholar] [CrossRef]
- Frank, I.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Weaver, A.L.; Zincke, H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J. Urol. 2002, 168, 2395–2400. [Google Scholar] [CrossRef]
- Pollheimer, M.J.; Kornprat, P.; Lindtner, R.A.; Harbaum, L.; Schlemmer, A.; Rehak, P.; Langner, C. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum. Pathol. 2010, 41, 1749–1757. [Google Scholar] [CrossRef]
- Swinson, D.E.; Jones, J.L.; Richardson, D.; Cox, G.; Edwards, J.G.; O’Byrne, K.J. Tumour necrosis is an independent prognostic marker in non-small cell lung cancer: Correlation with biological variables. Lung Cancer 2002, 37, 235–240. [Google Scholar] [CrossRef]
- Leek, R.D.; Landers, R.J.; Harris, A.L.; Lewis, C.E. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br. J. Cancer 1999, 79, 991–995. [Google Scholar] [CrossRef]
- Kudo, M.; Kobayashi, T.; Gotohda, N.; Konishi, M.; Takahashi, S.; Kobayashi, S.; Sugimoto, M.; Okubo, S.; Martin, J.; Cabral, H.; et al. Clinical Utility of Histological and Radiological Evaluations of Tumor Necrosis for Predicting Prognosis in Pancreatic Cancer. Pancreas 2020, 49, 634–641. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, S.S.; Kim, S.O.; Kim, J.H.; Kim, H.J.; Byun, J.H.; Yoo, C.; Kim, K.P.; Song, K.B.; Kim, S.C. Estimating Recurrence after Upfront Surgery in Patients with Resectable Pancreatic Ductal Adenocarcinoma by Using Pancreatic CT: Development and Validation of a Risk Score. Radiology 2020, 296, 541–551. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.H.; Park, H.K.; Jang, K.T.; Hwang, J.A.; Kim, S. Pancreatic Ductal Adenocarcinoma: Rim Enhancement at MR Imaging Predicts Prognosis after Curative Resection. Radiology 2018, 288, 456–466. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.H.; Song, I.H.; Youn, S.Y.; Kim, B.; Oh, S.N.; Choi, J.-I.; Rha, S.E. Identification of intratumoral fluid-containing area by magnetic resonance imaging to predict prognosis in patients with pancreatic ductal adenocarcinoma after curative resection. Eur. Radiol. 2022, 32, 2518–2528. [Google Scholar] [CrossRef]
- Allen, P.J.; Kuk, D.; Castillo, C.F.; Basturk, O.; Wolfgang, C.L.; Cameron, J.L.; Lillemoe, K.D.; Ferrone, C.R.; Morales-Oyarvide, V.; He, J.; et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann. Surg. 2017, 265, 185–191. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Asbun, H.; Bain, A.; Behrman, S.W.; Benson, A.B., 3rd; Binder, E.; Cardin, D.B.; Cha, C.; et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1028–1061. [Google Scholar] [CrossRef]
- Beddy, P.; Genega, E.M.; Ngo, L.; Hindman, N.; Wei, J.; Bullock, A.; Bhatt, R.S.; Atkins, M.B.; Pedrosa, I. Tumor necrosis on magnetic resonance imaging correlates with aggressive histology and disease progression in clear cell renal cell carcinoma. Clin. Genitourin. Cancer 2014, 12, 55–62. [Google Scholar] [CrossRef]
- Monsky, W.L.; Jin, B.; Molloy, C.; Canter, R.J.; Li, C.S.; Lin, T.C.; Borys, D.; Mack, W.; Kim, I.; Buonocore, M.H.; et al. Semi-automated volumetric quantification of tumor necrosis in soft tissue sarcoma using contrast-enhanced MRI. Anticancer Res. 2012, 32, 4951–4961. [Google Scholar]
- Hiraoka, N.; Ino, Y.; Sekine, S.; Tsuda, H.; Shimada, K.; Kosuge, T.; Zavada, J.; Yoshida, M.; Yamada, K.; Koyama, T.; et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br. J. Cancer 2010, 103, 1057–1065. [Google Scholar] [CrossRef]
- Semenza, G.L. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today 2007, 12, 853–859. [Google Scholar] [CrossRef]
- Nordsmark, M.; Overgaard, M.; Overgaard, J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother. Oncol. 1996, 41, 31–39. [Google Scholar] [CrossRef]
- Montemagno, C.; Cassim, S.; De Leiris, N.; Durivault, J.; Faraggi, M.; Pagès, G. Pancreatic ductal adenocarcinoma: The dawn of the era of nuclear medicine? Int. J. Mol. Sci. 2021, 15, 6413. [Google Scholar] [CrossRef]
- Shannon, A.M.; Bouchier-Hayes, D.J.; Condron, C.M.; Toomey, D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003, 29, 297–307. [Google Scholar] [CrossRef]
- Pedrosa, I.; Sun, M.R.; Spencer, M.; Genega, E.M.; Olumi, A.F.; Dewolf, W.C.; Rofsky, N.M. MR imaging of renal masses: Correlation with findings at surgery and pathologic analysis. Radiographics 2008, 28, 985–1003. [Google Scholar] [CrossRef]
| Feature | Necrotic (n = 42) | Non-Necrotic (n = 30) | p Value |
|---|---|---|---|
| Age (Years) | 62.8 ± 10.1 (47–85) | 64.3 ± 10.0 (43–83) | 0.49 |
| Sex (Male: Female) | 26:15 | 21:9 | 0.62 |
| Location | 0.01 * | ||
| Head | 16 (38%) | 15 (50%) | |
| Uncinate | 8 (19%) | 1 (3%) | |
| Neck | 5 (12%) | 1 (3%) | |
| Body | 6 (14%) | 12 (40%) | |
| Tail | 7 (17%) | 1 (3%) | |
| Tumor Stage | 0.02 * | ||
| IA | 0 (0%) | 1 (3%) | |
| IB | 4 (10%) | 3 (10%) | |
| IIA | 1 (2%) | 0 (0%) | |
| IIB | 1 (2%) | 5 (17%) | |
| III | 1 (2%) | 4 (13%) | |
| IV | 35 (84%) | 17 (57%) | |
| Metastases | 33 (79%) | 12 (40%) | 0.0001 * |
| Liver | 32 | 10 | |
| Lung | 5 | 2 | |
| Peritoneum | 5 | 1 | |
| Adrenal | 1 | 1 | |
| Bone | 1 | 0 |
| Feature | Necrotic (n = 42) | Non-Necrotic (n = 30) | p Value |
|---|---|---|---|
| Margins | 0.03 * | ||
| Ill-Defined | 16 (38%) | 21 (70%) | |
| Partially Defined | 22 (52%) | 8 (27%) | |
| Well-Defined | 4 (10%) | 1 (3%) | |
| Tumor Size (mm) | 44.6 ± 15.7 (21.7–96.8) | 34.5 ± 19.6 (14.0–82.0) | 0.002 * |
| T1 Signal Intensity | 0.08 | ||
| Hypointense | 36 (86%) | 20 (67%) | |
| Isointense | 6 (14%) | 10 (33%) | |
| T2 Signal Intensity | <0.0001 * | ||
| Hypointense | 3 | 13 | |
| Isointense | 7 | 15 | |
| Hyperintense | 32 | 2 | |
| Arterial Enhancement | 0.42 | ||
| Hypo-enhancement | 42 (100%) | 29 (97%) | |
| Iso-enhancement | 0 (0%) | 1 (3%) | |
| PV Enhancement | 0.0005 * | ||
| Hypo-enhancement | 42 (100%) | 22 (73%) | |
| Iso-enhancement | 0 (0%) | 8 (27%) | |
| Equilibrium Enhancement | <0.0001 * | ||
| Hypo-enhancement | 42 (100%) | 15 (50%) | |
| Iso-enhancement | 0 (0%) | 15 (50%) | |
| PD Dilatation | 31 (74%) | 27 (90%) | 0.13 |
| CBD Dilatation | 12 (29%) | 11 (37%) | 0.61 |
| Pancreatic Tail Atrophy | 16 (38%) | 20 (67%) | 0.03 * |
| Extra-Pancreatic Extension | 32 (76%) | 26 (89%) | 0.37 |
| Vascular Involvement | 24 (57%) | 12 (40%) | 0.23 |
| Lymphadenopathy | 29 (69%) | 8 (27%) | 0.0007 * |
| ADC value (×10−3 mm2/s) | 1.51 ± 0.58 (0.37–3.45) | 1.48 ± 0.72 (0.37–3.63) | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, M.A.; Knipp, D.E.; Noda, Y.; Kamran, S.C.; Baliyan, V.; Kordbacheh, H.; Hong, T.S.; Kambadakone, A. MRI-Based Tumor Necrosis Depiction in Pancreatic Ductal Adenocarcinoma: Can It Predict Tumor Aggressiveness? Cancers 2023, 15, 2313. https://doi.org/10.3390/cancers15082313
Anderson MA, Knipp DE, Noda Y, Kamran SC, Baliyan V, Kordbacheh H, Hong TS, Kambadakone A. MRI-Based Tumor Necrosis Depiction in Pancreatic Ductal Adenocarcinoma: Can It Predict Tumor Aggressiveness? Cancers. 2023; 15(8):2313. https://doi.org/10.3390/cancers15082313
Chicago/Turabian StyleAnderson, Mark A., David E. Knipp, Yoshifumi Noda, Sophia C. Kamran, Vinit Baliyan, Hamed Kordbacheh, Theodore S. Hong, and Avinash Kambadakone. 2023. "MRI-Based Tumor Necrosis Depiction in Pancreatic Ductal Adenocarcinoma: Can It Predict Tumor Aggressiveness?" Cancers 15, no. 8: 2313. https://doi.org/10.3390/cancers15082313
APA StyleAnderson, M. A., Knipp, D. E., Noda, Y., Kamran, S. C., Baliyan, V., Kordbacheh, H., Hong, T. S., & Kambadakone, A. (2023). MRI-Based Tumor Necrosis Depiction in Pancreatic Ductal Adenocarcinoma: Can It Predict Tumor Aggressiveness? Cancers, 15(8), 2313. https://doi.org/10.3390/cancers15082313