The Prevalence of Back Pain in Patients Operated on Due to Colorectal Cancer Depending on the Type of Surgical Procedure Performed
Abstract
Simple Summary
Abstract
1. Introduction
2. Objective of the Study
3. Material and Methods
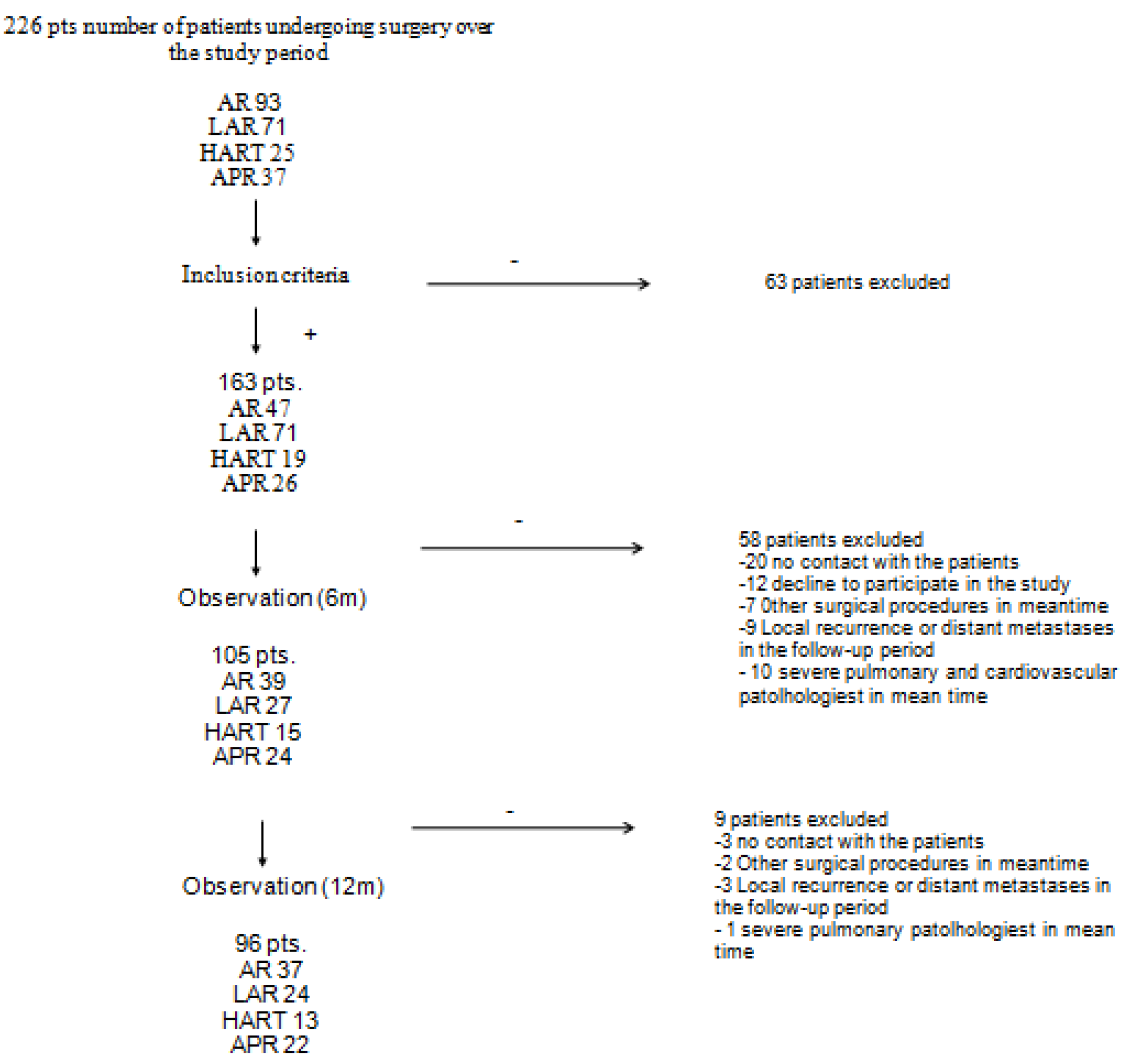
3.1. Study Design
3.2. Study Enrollment
- -
- consent to participate in the study;
- -
- age over 18 years;
- -
- colorectal cancer patients qualified for scheduled surgeries including anterior resection of rectum, laparoscopic anterior resection of the rectum, Hartmann’s procedure, or abdominoperineal resection of the rectum;
- -
- the primary character of colorectal cancer surgery;
- -
- good mobility, Zubrod-ECOG-WHO performance score of 0–1;
- -
- colorectal cancer of stage I–III as per preoperative assessment.
- -
- disseminated disease (stage IV cancer);
- -
- ASA score of 4 or higher;
- -
- intraoperative conversion from laparoscopic to open surgery;
- -
- severe cardiovascular, pulmonary, orthopedic, and neurological pathologies;
- -
- cognitive impairment;
- -
- local recurrence or distant metastases in the follow-up period.
- 0–20%—minimal disability;
- 21–40%—moderate disability;
- 41–60%—severe disability;
- 61–80%—crippled;
- 81–100%—complete motor impairment.
3.3. Statistical Methods
4. Results
5. Discussion
6. Study Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwenk, W.; Haase, O.; Neudecker, J.; Müller, J.M. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst. Rev. 2005, 2005, CD003145. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, J.; Zhang, S. Laparoscopic versus conventional open surgery for immune function in patients with colorectal cancer. Int. J. Color. Dis. 2011, 26, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. Colorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Panjabi, M.M. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J. Spinal Disord. 1992, 5, 383–389. [Google Scholar] [CrossRef]
- Bankoff, A.D.; Furlani, J. Electromyographic study of the rectus abdominis and external oblique muscles during exercises. Electroencephalogr. Clin. Neurophysiol. 1984, 24, 501–510. [Google Scholar]
- Lindner, U.K. Bettruhe—Einegefährliche “Therapie” [Bedrest—A dangerous “therapy”]. Der. Internist. 2000, 41, 698–703. [Google Scholar] [CrossRef]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Viswanath, O.; Jones, M.R.; Sidransky, M.A.; Spektor, B.; et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr. Pain Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef]
- Salibasic, M.; Pusina, S.; Bicakcic, E.; Pasic, A.; Gavric, I.; Kulovic, E.; Rovcanin, A.; Beslija, S. Colorectal Cancer Surgical Treatment, our Experience. Med. Arch. 2019, 73, 412–414. [Google Scholar] [CrossRef]
- Yee, T.J.; Fearer, K.J.; Oppenlander, M.E.; Kashlan, O.N.; Szerlip, N.; Buckingham, M.J.; Swong, K.; Chang, V.; Schwalb, J.M.; Park, P. Correlation Between the Oswestry Disability Index and the North American Spine Surgery Patient Satisfaction Index. World Neurosurg. 2020, 139, e724–e729. [Google Scholar] [CrossRef]
- Chotai, S.; Devin, C.J.; Archer, K.R.; Bydon, M.; McGirt, M.J.; Nian, H.; Harrell, F.E., Jr.; Dittus, R.S.; Asher, A.L.; QOD Vanguard Sites. Effect of patients’ functional status on satisfaction with outcomes 12 months after elective spine surgery for lumbar degenerative disease. Spine J. 2017, 17, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- DeVine, J.; Norvell, D.C.; Ecker, E.; Fourney, D.R.; Vaccaro, A.; Wang, J.; Andersson, G. Evaluating the correlation and responsiveness of patient-reported pain with function and quality-of-life outcomes after spine surgery. Spine 2011, 36 (Suppl. 21), S69–S74. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.M.; Kerr, D.P.; Lennon, S. Low back pain in people with a stoma: A postal survey. Disabil. Rehabil. 2009, 31, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.M.; Lennon, S.; McCrum-Gardner, E.; Kerr, D.P. Factors that influence low back pain in people with a stoma. Disabil. Rehabil. 2012, 34, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.; Stage, J.G.; Galbo, H.; Christensen, N.J.; Kehlet, H. Fatigue and cardiac and endocrine metabolic response to exercise after abdominal surgery. Surgery 1989, 105, 46–50. [Google Scholar]
- Schroeder, D.; Hill, G.L. Postoperative fatigue: A prospective physiological study of patients undergoing major abdominal surgery. ANZ J. Surg. 1991, 61, 774–779. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- da Rocha, I.M.G.; Marcadenti, A.; de Medeiros, G.O.C.; Bezerra, R.A.; Rego, J.F.M.; Gonzalez, M.C.; Fayh, A.P.T. Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J. Cachexia Sarcopenia Muscle 2019, 10, 445–454. [Google Scholar] [CrossRef]
- Anandavadivelan, P.; Brismar, T.B.; Nilsson, M.; Johar, A.M.; Martin, L. Sarcopenic obesity: A probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin. Nutr. 2016, 35, 724–730. [Google Scholar] [CrossRef]
- Stokes, I.A.; Gardner-Morse, M.G.; Henry, S.M. Abdominal muscle activation increases lumbar spinal stability: Analysis of contributions of different muscle groups. Clin. Biomech. 2011, 26, 797–803. [Google Scholar] [CrossRef]
- Blazek, D.; Stastny, P.; Maszczyk, A.; Krawczyk, M.; Matykiewicz, P.; Petr, M. Systematic review of intra-abdominal and intrathoracic pressures initiated by the Valsalva manoeuvre during high-intensity resistance exercises. Biol. Sport 2019, 36, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, A.G.; Blake, P.L.; Thorstensson, A. The effect of an abdominal muscle training program on intra-abdominal pressure. Scand. J. Rehabil. Med. 1994, 26, 79–86. [Google Scholar] [PubMed]
- Hlaing, S.S.; Puntumetakul, R.; Khine, E.E.; Boucaut, R. Effects of core stabilization exercise and strengthening exercise on proprioception, balance, muscle thickness and pain related outcomes in patients with subacute nonspecific low back pain: A randomized controlled trial. BMC MusculoskeletDisord. 2021, 22, 998. [Google Scholar] [CrossRef] [PubMed]
- Lewit, K.; Olsanska, O. Clinical importance of active scars: Abnormal scars as a cause of myofascial pain. J. Manip. Physiol. Ther. 2004, 27, 399–402. [Google Scholar] [CrossRef]
- Kelly, R.C.; Armstrong, M.; Bensky, A.; Foti, A.; Wasserman, J.B. Soft tissue mobilization techniques in treating chronic abdominal scar tissue: A quasi-experimental single subject design. J. Bodyw. Mov. Ther. 2019, 23, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Molegraaf, M.J.; Torensma, B.; Lange, C.P.; Lange, J.F.; Jeekel, J.; Swank, D.J. Twelve-year outcomes of laparoscopic adhesiolysis in patients with chronic abdominal pain: A randomized clinical trial. Surgery 2017, 161, 415–421. [Google Scholar] [CrossRef]
- Schiphorst, A.H.W.; Verweij, N.M.; Pronk, A.; BorelRinkes, I.H.M.; Hamaker, M.E. Non-surgical complications after laparoscopic and open surgery for colorectal cancer—A systematic review of randomised controlled trials. Eur. J. Surg. Oncol. 2015, 41, 1118–1127. [Google Scholar] [CrossRef]
- Del Rio, P.; Cataldo, C.; Cozzani, F.; Pedrazzi, G.; Bonati, E.; Dell’abate, P. Non-surgical complications in oncological colorectal surgery: A comparison between open and laparoscopic techniques. Ann. Ital. Chir. 2019, 90, 225–230. [Google Scholar]
- Kochi, M.; Hinoi, T.; Niitsu, H.; Ohdan, H.; Konishi, F.; Kinugasa, Y.; Kobatake, T.; Ito, M.; Inomata, M.; Yatsuoka, T.; et al. Japan Society of Laparoscopic Colorectal Surgery. Risk factors for postoperative pneumonia in elderly patients with colorectal cancer: A sub-analysis of a large, multicenter, case-control study in Japan. Surg. Today 2018, 48, 756–764. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ekblom-Bak, E.; Olsson, G.; Ekblom, Ö.; Ekblom, B.; Bergström, G.; Börjesson, M. The Daily Movement Pattern and Fulfilment of Physical Activity Recommendations in Swedish Middle-Aged Adults: The SCAPIS Pilot Study. PLoS ONE 2015, 10, e0126336. [Google Scholar] [CrossRef]
- Lois, F.; Lavand’homme, P.; Leonard, D.; Remue, C.; Bellemans, V.; Kartheuser, A. Chronic post-surgical pain after colon surgery in patients included in an enhanced recovery program. Acta Anaesthesiol. Scand. 2019, 63, 931–938. [Google Scholar] [CrossRef]
- Gerbershagen, H.J.; Özgür, E.; Dagtekin, O.; Straub, K.; Hahn, M.; Heidenreich, A.; Sabatowski, R.; Petzke, F. Preoperative pain as a risk factor for chronic post-surgical pain—Six month follow-up after radical prostatectomy. Eur. J. Pain 2009, 13, 1054–1061. [Google Scholar] [CrossRef]
- Bruce, J.; Quinlan, J. Chronic Post Surgical Pain. Rev. Pain 2011, 5, 23–29. [Google Scholar] [CrossRef]
- Fregoso, G.; Wang, A.; Tseng, K.; Wang, J. Transition from Acute to Chronic Pain: Evaluating Risk for Chronic Postsurgical Pain. Pain Physician. 2019, 22, 479–488. [Google Scholar]
- Jankowski, M.; Las-Jankowska, M.; Rutkowski, A.; Bała, D.; Wiśniewski, D.; Tkaczyński, K.; Kowalski, W.; Głowacka-Mrotek, I.; Zegarski, W. Clinical Reality and Treatment for Local Recurrence of Rectal Cancer: A Single-Center Retrospective Study. Medicina 2021, 57, 286. [Google Scholar] [CrossRef]
- Duineveld, L.A.M.; van Asselt, K.M.; Bemelman, W.A.; Smits, A.B.; Tanis, P.J.; van Weert, H.C.P.M.; Wind, J. Symptomatic and Asymptomatic Colon Cancer Recurrence: A Multicenter Cohort Study. Ann. Fam. Med. 2016, 14, 215–220. [Google Scholar] [CrossRef]
- Choi, B.C.K. Computer assisted telephone interviewing (CATI) for health surveys in public health surveillance: Methodological issues and challenges ahead. Chronic. Dis. Can. 2004, 25, 21–27. [Google Scholar]
| Scale | Group | Mean | S.D. | ANOVA ** |
|---|---|---|---|---|
| Age | APR | 63.2 | 7.7 | p = 0.3781 |
| AR | 64.5 | 6.6 | ||
| LAR | 63.9 | 11.4 | ||
| HART | 68.1 | 5.9 | ||
| Weight | APR | 76.4 | 14.8 | p = 0.2340 |
| AR | 83.2 | 16.2 | ||
| LAR | 76.2 | 14.3 | ||
| HART | 76.6 | 17.0 | ||
| Height | APR | 170.6 | 8.8 | p = 0.6031 |
| AR | 170.3 | 10.5 | ||
| LAR | 168.4 | 10.3 | ||
| HART | 166.5 | 11.1 | ||
| BMI | APR | 26.4 | 6.0 | p = 0.2748 |
| AR | 28.6 | 4.7 | ||
| LAR | 26.7 | 3.8 | ||
| HART | 27.3 | 3.7 | ||
| Postoperative hospitalization time ** | APR | 10.0 | 6.0 | p = 0.1020 |
| AR | 9.2 | 6.3 | ||
| LAR | 7.4 | 4.2 | ||
| HART | 9.0 | 4.9 |
| Type of Surgical Procedure | 1 Chi-Squared Test 2 Exact Fisher’s Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| APR | AR | LAR | HART | |||||||
| N | % | N | % | N | % | N | % | |||
| Sex | Female | 7 | 31.8 | 11 | 29.7 | 9 | 37.5 | 3 | 23.0 | 1p = 0.8185 |
| Male | 15 | 68.2 | 26 | 70.3 | 15 | 62.5 | 10 | 77.0 | ||
| Type of preoperative treatment | none | 1 | 4.5 | 10 | 27.0 | 9 | 37.5 | 0 | 0 | 2p = 0.0072 |
| chemotherapy | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7.7 | ||
| radiochemotherapy | 13 | 59.1 | 19 | 51.3 | 6 | 25 | 6 | 46.1 | ||
| radiotherapy | 8 | 36.4 | 8 | 21.6 | 9 | 37.5 | 6 | 46.1 | ||
| Type of postoperative treatment | none | 16 | 72.7 | 25 | 67.6 | 15 | 65.2 | 9 | 69.2 | 2p = 0.6871 |
| chemotherapy | 6 | 27.3 | 12 | 32.4 | 7 | 30.4 | 3 | 23.1 | ||
| radiochemotherapy | 0 | 0 | 0 | 0 | 1 | 4.3 | 1 | 7.7 | ||
| radiotherapy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Cancer stagingp TNM/ypTNM | 0 | 0 | 0 | 4 | 10.8 | 1 | 4.2 | 1 | 7.7 | 2p = 0.2308 |
| I | 11 | 52.4 | 7 | 18.9 | 9 | 37.5 | 4 | 30.8 | ||
| IIA | 3 | 14.3 | 14 | 37.8 | 4 | 16.7 | 5 | 38.5 | ||
| IIB | 1 | 4.8 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| IIIA | 1 | 4.8 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| IIIB | 3 | 14.3 | 6 | 16.2 | 7 | 29.2 | 2 | 15.4 | ||
| IIIC | 2 | 9.5 | 6 | 16.2 | 3 | 12.5 | 1 | 7.7 | ||
| Group | Term | Friedman Test | ||||||
|---|---|---|---|---|---|---|---|---|
| I Examination | II Examination | III Examination | ||||||
| Arithmetic Mean | Median | Arithmetic Mean | Median | Arithmetic Mean | Median | |||
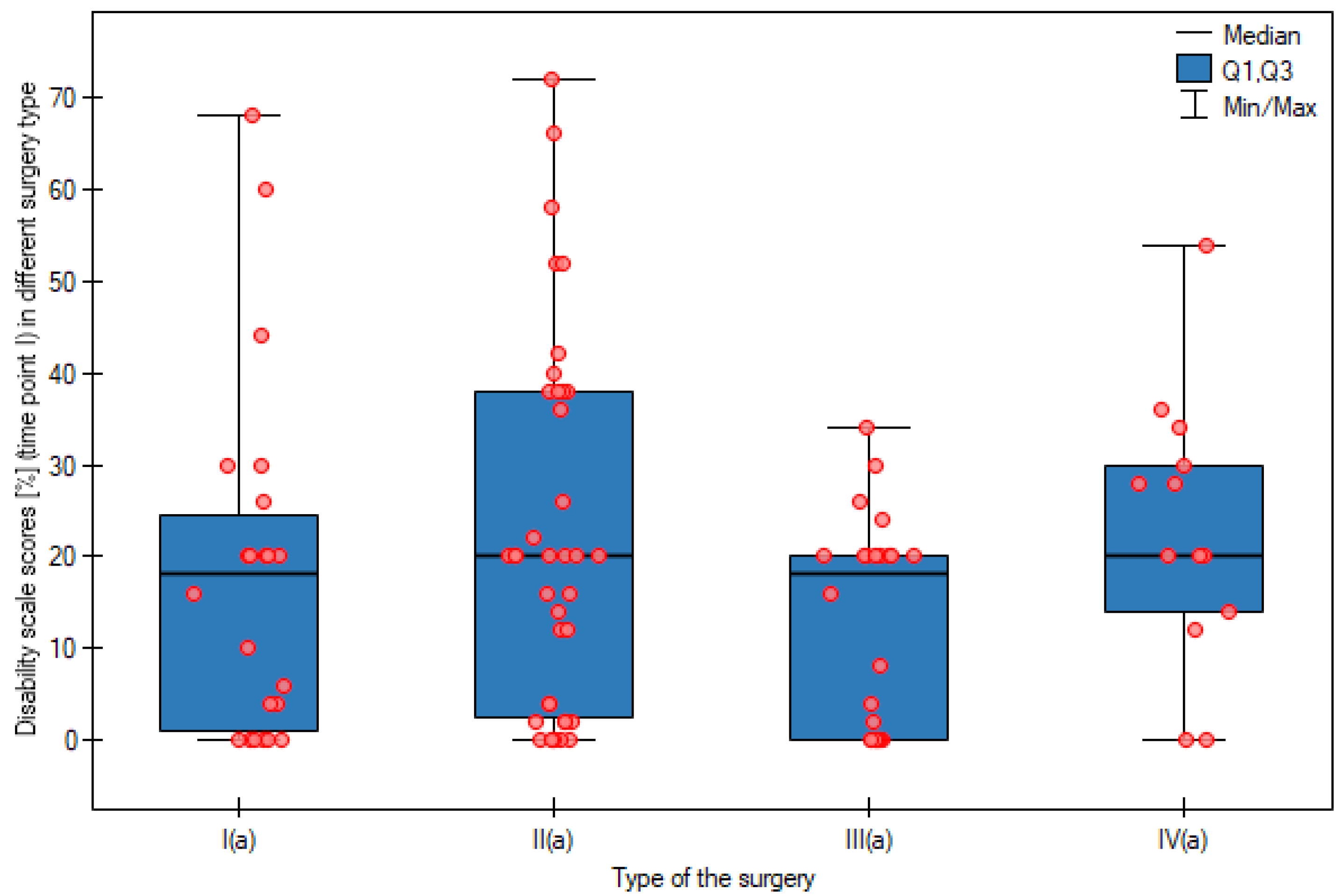
| Pain intensity | APR | 0.86 | 1.00 a a | 2.09 | 2.00 b b | 3.05 | 3.00 c c | p < 0.0001 |
| AR | 1.49 | 1.00 a a | 1.89 | 2.00ab ab | 2.27 | 2.00 b b | p < 0.0001 | |
| LAR | 0.75 | 1.00 a a | 1.42 | 1.00 a ab | 1.50 | 1.00 a b | p = 0.0001 | |
| HART | 1.62 | 1.00 a a | 2.08 | 2.00ab ab | 2.62 | 2.00 bc b | p = 0.0080 | |
| Kruskal–Wallis test | p = 0.0411 | p = 0.0413 | p < 0.0001 | - | ||||
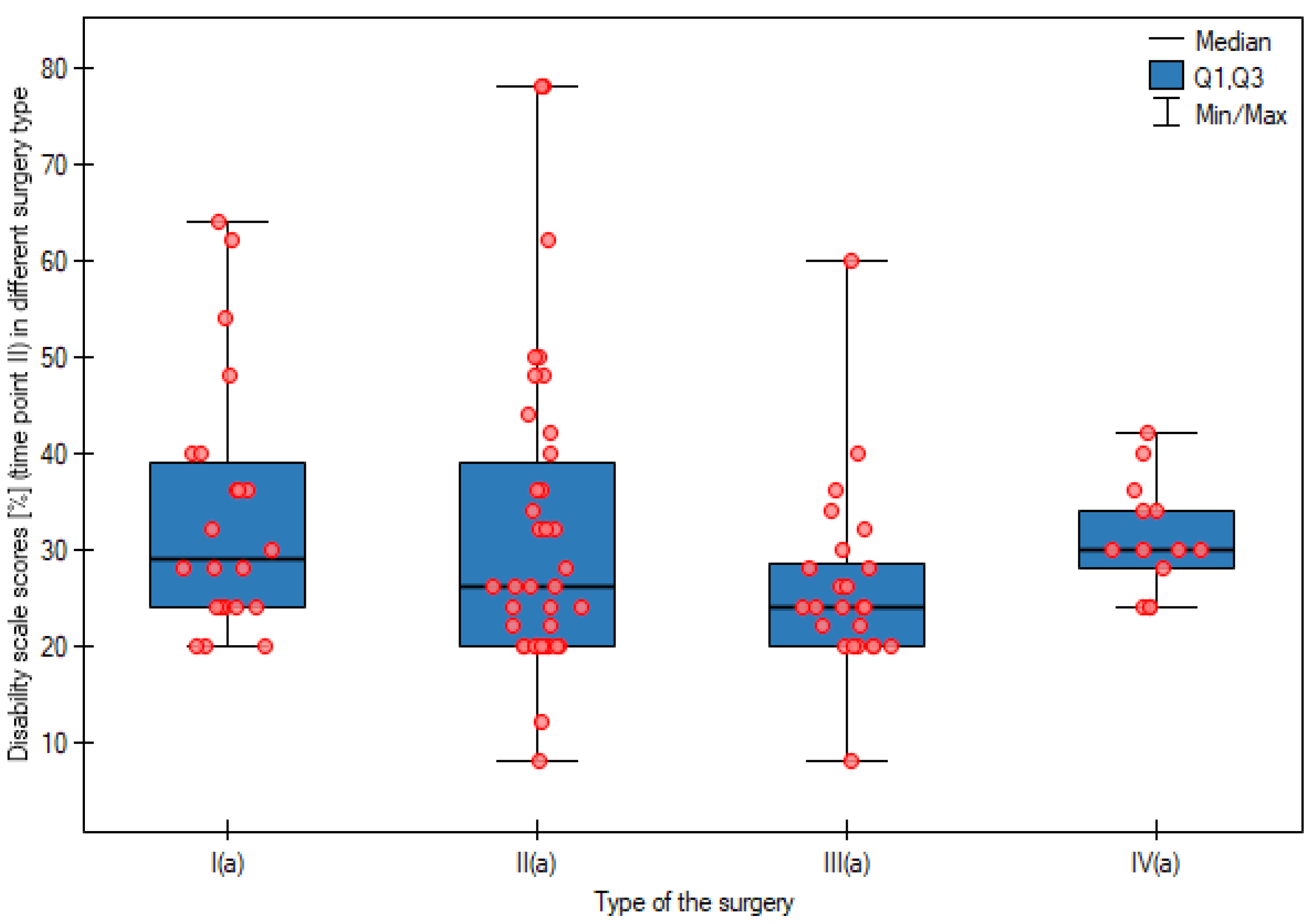
| Personal care | APR | 0.73 | 1.00 a a | 1.23 | 1.00 a a | 1.82 | 2.00 a b | p < 0.0001 |
| AR | 0.89 | 1.00 a a | 1.32 | 1.00 a b | 1.54 | 1.00 a b | p < 0.0001 | |
| LAR | 0.54 | 0.50 a a | 1.04 | 1.00 a ab | 1.29 | 1.00 a b | p < 0.0001 | |
| HART | 0.77 | 1.00aa | 1.38 | 1.00aab | 1.85 | 2.00ab | p = 0.0024 | |
| Kruskal–Wallis test | p = 0.6899 | p = 0.2232 | p = 0.0277 | - | ||||
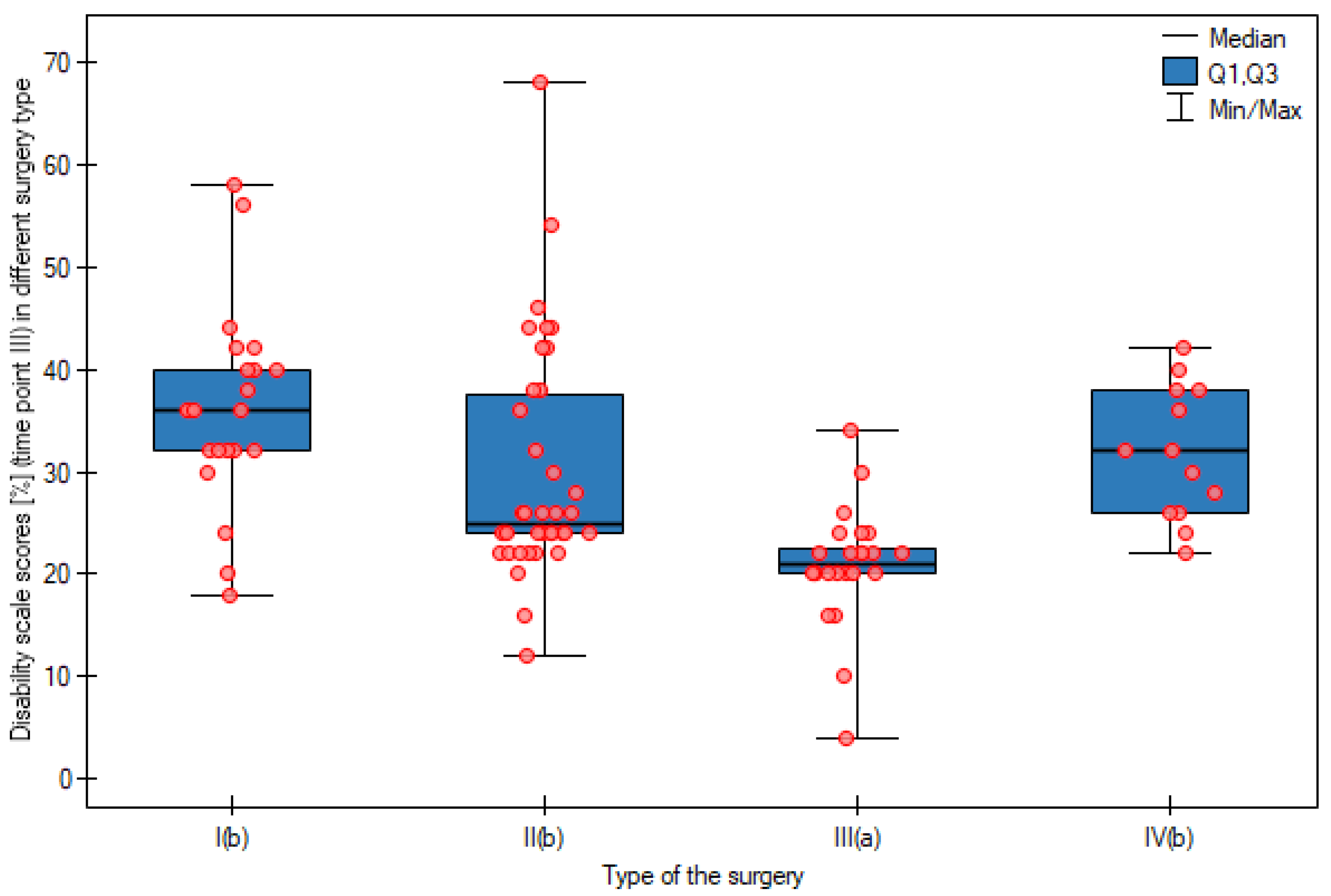
| Lifting | APR | 0.95 | 1.00 a a | 1.95 | 2.00 a b | 3.18 | 3.00 b b | p < 0.0001 |
| AR | 1.32 | 1.00 a a | 2.00 | 2.00 a b | 2.30 | 2.00 b b | p < 0.0001 | |
| LAR | 0.58 | 1.00 a a | 1.58 | 1.00 a b | 1.33 | 1.00 a b | p < 0.0001 | |
| HART | 1.31 | 1.00 a a | 2.62 | 2.00 a ab | 3.00 | 3.00 b b | p = 0.0013 | |
| Kruskal–Wallis test | p = 0.1782 | p = 0.1174 | p = 0.0001 | - | ||||
| Walking | APR | 0.64 | 0.50 a a | 1.68 | 1.00 a b | 1.45 | 1.00 b b | p < 0.0001 |
| AR | 0.86 | 1.00 a a | 1.57 | 1.00 a b | 1.22 | 1.00 ab ab | p < 0.0001 | |
| LAR | 0.54 | 1.00 a a | 1.50 | 1.00 a b | 0.92 | 1.00 a a | p < 0.0001 | |
| HART | 0.69 | 1.00 a a | 1.31 | 1.00 a a | 1.31 | 1.00 ab a | p = 0.0043 | |
| Kruskal–Wallis test | p = 0.4671 | p = 0.7906 | p = 0.0254 | - | ||||
| Sitting | APR | 1.27 | 1.00 a ab | 2.36 | 2.00 a b | 0.95 | 1.00 a a | p = 0.0020 |
| AR | 1.19 | 1.00 a a | 1.89 | 2.00 a b | 1.22 | 1.00 a ab | p = 0.0001 | |
| LAR | 0.75 | 1.00 a a | 1.67 | 2.00 a b | 0.92 | 1.00 a ab | p = 0.0001 | |
| HART | 1.31 | 1.00 a a | 2.15 | 2.00 a a | 1.23 | 1.00 a a | p = 0.0043 | |
| Kruskal–Wallis test | p = 0.5476 | p = 0.5076 | p = 0.3011 | - | ||||
| Standing | APR | 1.27 | 1.00 a a | 1.55 | 1.00 a a | 1.32 | 1.00 b a | p = 0.1381 |
| AR | 1.38 | 1.00 a a | 1.59 | 1.00 a a | 1.32 | 1.00 ab a | p = 0.0529 | |
| LAR | 0.75 | 1.00 a a | 1.50 | 1.00 a b | 0.83 | 1.00 a ab | p = 0.0002 | |
| HART | 1.38 | 1.00 a a | 1.46 | 1.00 a a | 1.15 | 1.00 ab a | p = 0.5866 | |
| Kruskal–Wallis test | p = 0.3333 | p = 0.9917 | p = 0.0266 | - | ||||
| Sleeping | APR | 0.73 | 1.00 a a | 1.55 | 1.00 a b | 1.18 | 1.00 a ab | p = 0.0003 |
| AR | 1.14 | 1.00 a a | 1.51 | 1.00 a a | 1.38 | 1.00 a a | p = 0.0142 | |
| LAR | 0.63 | 1.00 a a | 1.13 | 1.00 a a | 0.92 | 1.00 a a | p = 0.0039 | |
| HART | 1.15 | 1.00 a a | 1.15 | 1.00 a a | 1.31 | 1.00 a a | p = 0.3114 | |
| Kruskal–Wallis test | p = 0.2426 | p = 0.1754 | p = 0.0925 | - | ||||
| Social life | APR | 0.50 | 0.00 a a | 1.55 | 1.00 a b | 1.50 | 1.00 b b | p < 0.0001 |
| AR | 0.89 | 1.00 a a | 1.32 | 1.00 a a | 1.19 | 1.00 ab a | p = 0.0082 | |
| LAR | 0.63 | 1.00 a a | 1.04 | 1.00 a a | 1.00 | 1.00 a a | p = 0.0024 | |
| HART | 1.00 | 1.00 a a | 1.15 | 1.00 a a | 1.08 | 1.00 ab a | p = 0.3679 | |
| Kruskal–Wallis test | p = 0.4439 | p = 0.1965 | p = 0.0336 | - | ||||
| Traveling | APR | 1.05 | 1.00 a a | 1.50 | 1.00 a ab | 1.86 | 2.00 b b | p = 0.0030 |
| AR | 1.14 | 1.00 a a | 1.38 | 1.00 a a | 1.30 | 1.00 a a | p = 0.2040 | |
| LAR | 0.67 | 1.00 a a | 1.13 | 1.00 a a | 0.92 | 1.00 a a | p = 0.0305 | |
| HART | 1.23 | 1.00 a a | 1.00 | 1.00 a a | 1.00 | 1.00 a a | p = 0.2765 | |
| Kruskal–Wallis test | p = 0.1709 | p = 0.1604 | p < 0.0001 | - | ||||
| Pain intensity changes | APR | 1.05 | 1.00 a a | 1.41 | 1.00 a a | 1.77 | 1.50 b a | p = 0.0097 |
| AR | 1.11 | 1.00 a a | 1.59 | 1.00 a b | 1.38 | 1.00 b ab | p = 0.0011 | |
| LAR | 0.50 | 0.00 a a | 1.17 | 1.00 a b | 0.79 | 1.00 a ab | p = 0.0006 | |
| HART | 0.92 | 1.00 a a | 1.31 | 1.00 a a | 1.38 | 1.00 b a | p = 0.1561 | |
| Kruskal–Wallis test | p = 0.0738 | p = 0.2768 | p = 0.0003 | - | ||||
| Overall results of the Oswestry questionnaire | APR | 9.05 | 9.00 a a | 16.86 | 14.50 a b | 18.09 | 18.00 b b | p < 0.0001 |
| AR | 11.41 | 10.00 a a | 16.08 | 13.00 a b | 15.05 | 13.00 b b | p = 0.0002 | |
| LAR | 6.33 | 9.00 a a | 13.17 | 12.00 a b | 10.42 | 10.50 a a | p < 0.0001 | |
| HART | 11.38 | 10.00 a a | 15.62 | 15.00 a a | 15.92 | 16.00 b a | p = 0.0518 | |
| Kruskal–Wallis test | p = 0.1712 | p = 0.0999 | p < 0.0001 | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowacka-Mrotek, I.; Jankowski, M.; Skonieczny, B.; Tarkowska, M.; Ratuszek-Sadowska, D.; Lewandowska, A.; Nowikiewicz, T.; Ogurkowski, K.; Zegarski, W.; Mackiewicz-Milewska, M. The Prevalence of Back Pain in Patients Operated on Due to Colorectal Cancer Depending on the Type of Surgical Procedure Performed. Cancers 2023, 15, 2298. https://doi.org/10.3390/cancers15082298
Głowacka-Mrotek I, Jankowski M, Skonieczny B, Tarkowska M, Ratuszek-Sadowska D, Lewandowska A, Nowikiewicz T, Ogurkowski K, Zegarski W, Mackiewicz-Milewska M. The Prevalence of Back Pain in Patients Operated on Due to Colorectal Cancer Depending on the Type of Surgical Procedure Performed. Cancers. 2023; 15(8):2298. https://doi.org/10.3390/cancers15082298
Chicago/Turabian StyleGłowacka-Mrotek, Iwona, Michał Jankowski, Bartosz Skonieczny, Magdalena Tarkowska, Dorota Ratuszek-Sadowska, Anna Lewandowska, Tomasz Nowikiewicz, Karol Ogurkowski, Wojciech Zegarski, and Magdalena Mackiewicz-Milewska. 2023. "The Prevalence of Back Pain in Patients Operated on Due to Colorectal Cancer Depending on the Type of Surgical Procedure Performed" Cancers 15, no. 8: 2298. https://doi.org/10.3390/cancers15082298
APA StyleGłowacka-Mrotek, I., Jankowski, M., Skonieczny, B., Tarkowska, M., Ratuszek-Sadowska, D., Lewandowska, A., Nowikiewicz, T., Ogurkowski, K., Zegarski, W., & Mackiewicz-Milewska, M. (2023). The Prevalence of Back Pain in Patients Operated on Due to Colorectal Cancer Depending on the Type of Surgical Procedure Performed. Cancers, 15(8), 2298. https://doi.org/10.3390/cancers15082298






