Clinical Outcomes of Thymic Carcinoma: The Role of Radiotherapy Combined with Multimodal Treatments
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Treatment Characteristics
2.2.1. Surgery
2.2.2. Radiotherapy
2.2.3. Chemotherapy
2.3. Endpoints and Statistical Analyses
3. Results
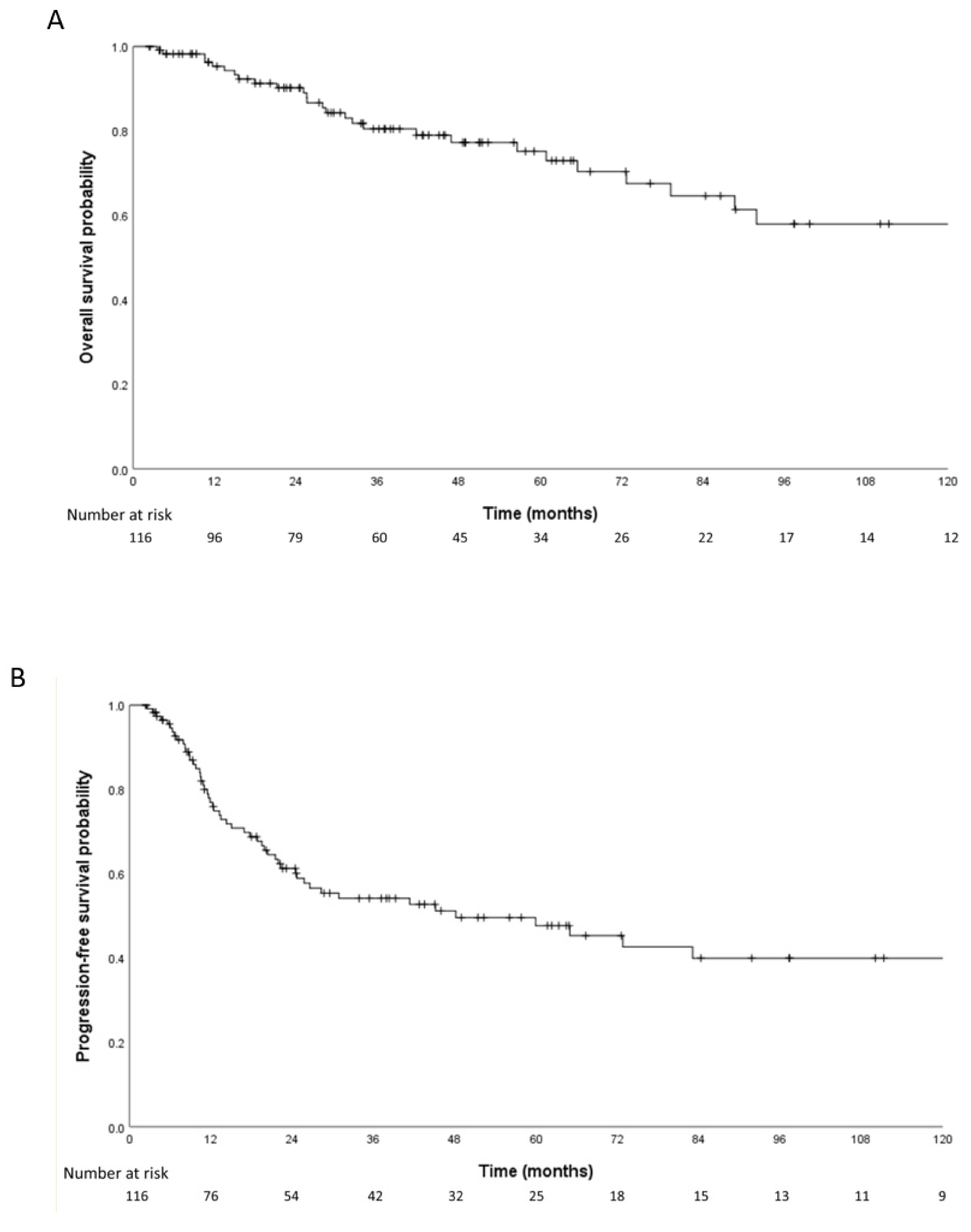
3.1. Survival Outcomes
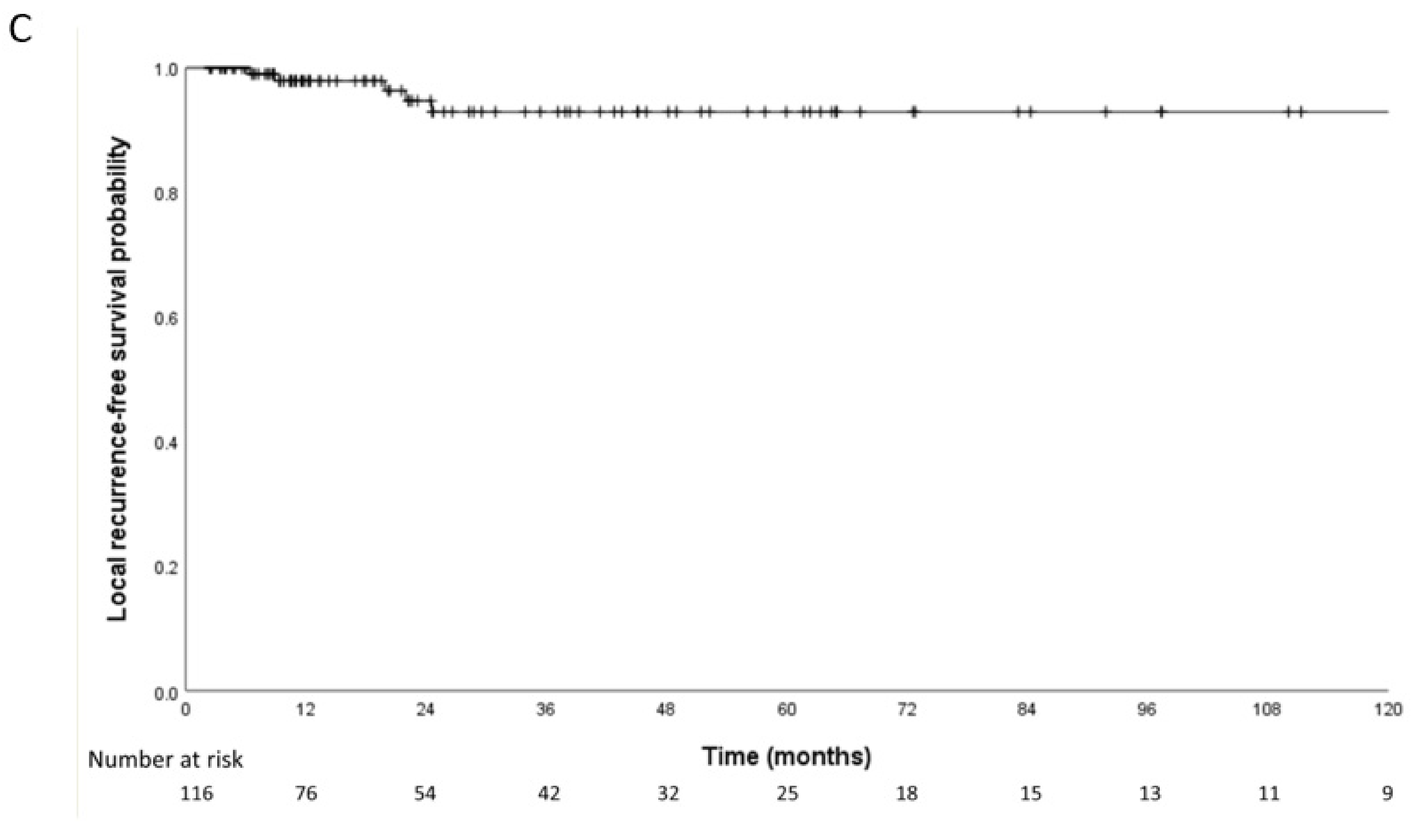
3.2. Patterns of Failure in Nodal Metastasis
3.3. Patterns of Failure
3.4. Prognostic Factors
3.5. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D-CRT | three-dimensional conformal radiotherapy |
| ECOG | European Cooperative Oncology Group |
| IMRT | intensity-modulated radiotherapy |
| ITMIG | International Thymic Malignancy Interest Group |
| ITV | internal target volume |
| LRFS | local recurrence-free survival |
| OS | overall survival |
| PD-1 | programmed death 1 |
| PD-L1 | programmed death ligand 1 |
| PFS | progression-free survival |
| PTV | planning target volume |
| RT | radiotherapy |
References
- Eng, T.Y.; Fuller, C.D.; Jagirdar, J.; Bains, Y.; Thomas, C.R., Jr. Thymic carcinoma: State of the art review. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Süveg, K.; Putora, P.M.; Joerger, M.; Iseli, T.; Fischer, G.F.; Ammann, K.; Glatzer, M. Radiotherapy for thymic epithelial tumours: A review. Transl. Lung Cancer Res. 2021, 10, 2088–2100. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.; Riely, G.; Detterbeck, F.; Simone, C.B., 2nd; Ahmad, U.; Huang, J.; Korst, R.; Rajan, A.; Rimner, A. Thymic Carcinoma Management Patterns among International Thymic Malignancy Interest Group (ITMIG) Physicians with Consensus from the Thymic Carcinoma Working Group. J. Thorac. Oncol. 2017, 12, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.; Rimner, A. The expanding role of radiation therapy for thymic malignancies. J. Thorac. Dis. 2018, 10 (Suppl. 21), S2555–S2564. [Google Scholar] [CrossRef] [PubMed]
- Drevet, G.; Collaud, S.; Tronc, F.; Girard, N.; Maury, J.M. Optimal management of thymic malignancies: Current perspectives. Cancer Manag. Res. 2019, 11, 6803–6814. [Google Scholar] [CrossRef]
- Wei, Y.; Gu, Z.; Shen, Y.; Fu, J.; Tan, L.; Zhang, P.; Han, Y.; Chen, C.; Zhang, R.; Li, Y.; et al. Preoperative Induction Therapy for Locally Advanced Thymic Tumors: A Retrospective Analysis Using the ChART Database. Zhongguo Fei Ai Za Zhi 2016, 19, 445–452. [Google Scholar] [CrossRef]
- Wang, C.L.; Gao, L.T.; Lv, C.X.; Zhu, L.; Fang, W.T. Outcome of nonsurgical treatment for locally advanced thymic tumors. J. Thorac. Dis. 2016, 8, 705–710. [Google Scholar] [CrossRef]
- Lee, K.H.; Noh, J.M.; Ahn, Y.C.; Oh, D.; Kim, J.; Shim, Y.M.; Han, J.H. Patterns of Failure Following Postoperative Radiation Therapy Based on “Tumor Bed with Margin” for Stage II to IV Type C Thymic Epithelial Tumor. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1505–1513. [Google Scholar] [CrossRef]
- Chapet, O.; Kong, F.M.; Quint, L.E.; Chang, A.C.; Ten Haken, R.K.; Eisbruch, A.; Hayman, J.A. CT-based definition of thoracic lymph node stations: An atlas from the University of Michigan. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 170–178. [Google Scholar] [CrossRef]
- Nestle, U.; Schimek-Jasch, T.; Kremp, S.; Schaefer-Schuler, A.; Mix, M.; Küsters, A.; Tosch, M.; Hehr, T.; Eschmann, S.M.; Bultel, Y.P.; et al. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): A multicentre, open-label, randomised, controlled trial. Lancet Oncol. 2020, 21, 581–592. [Google Scholar] [CrossRef]
- Mountain, C.F.; Dresler, C.M. Regional lymph node classification for lung cancer staging. Chest 1997, 111, 1718–1723. [Google Scholar] [CrossRef]
- Huang, J.; Detterbeck, F.C.; Wang, Z.; Loehrer, P.J., Sr. Standard outcome measures for thymic malignancies. J. Thorac. Oncol. 2010, 5, 2017–2023. [Google Scholar] [CrossRef]
- Gomez, D.; Komaki, R.; Yu, J.; Ikushima, H.; Bezjak, A. Radiation therapy definitions and reporting guidelines for thymic malignancies. J. Thorac. Oncol. 2011, 6 (Suppl. 3), S1743–S1748. [Google Scholar] [CrossRef]
- Kondo, K.; Monden, Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann. Thorac. Surg. 2003, 76, 1859–1864; discussion 1864–1865. [Google Scholar] [CrossRef]
- Omasa, M.; Date, H.; Sozu, T.; Sato, T.; Nagai, K.; Yokoi, K.; Okamoto, T.; Ikeda, N.; Tanaka, F.; Maniwa, Y. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: The Japanese Association for Research on the Thymus Database Study. Cancer 2015, 121, 1008–1016. [Google Scholar] [CrossRef]
- Hishida, T.; Nomura, S.; Yano, M.; Asamura, H.; Yamashita, M.; Ohde, Y.; Kondo, K.; Date, H.; Okumura, M.; Nagai, K. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: Results of 306 cases from a Japanese Nationwide Database Study. Eur. J. Cardiothorac. Surg. 2016, 49, 835–841. [Google Scholar] [CrossRef]
- Jackson, M.W.; Palma, D.A.; Camidge, D.R.; Jones, B.L.; Robin, T.P.; Sher, D.J.; Koshy, M.; Kavanagh, B.D.; Gaspar, L.E.; Rusthoven, C.G. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2017, 12, 734–744. [Google Scholar] [CrossRef]
- Lim, Y.J.; Song, C.; Kim, J.S. Improved survival with postoperative radiotherapy in thymic carcinoma: A propensity-matched analysis of Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2017, 108, 161–167. [Google Scholar] [CrossRef]
- Huang, J.; Rizk, N.P.; Travis, W.D.; Riely, G.J.; Park, B.J.; Bains, M.S.; Dycoco, J.; Flores, R.M.; Downey, R.J.; Rusch, V.W. Comparison of patterns of relapse in thymic carcinoma and thymoma. J. Thorac. Cardiovasc. Surg. 2009, 138, 26–31. [Google Scholar] [CrossRef]
- Owen, D.; Chu, B.; Lehman, A.M.; Annamalai, L.; Yearley, J.H.; Shilo, K.; Otterson, G.A. Expression Patterns, Prognostic Value, and Intratumoral Heterogeneity of PD-L1 and PD-1 in Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2018, 13, 1204–1212. [Google Scholar] [CrossRef]
- Fang, W.; Wang, Y.; Pang, L.; Gu, Z.; Wei, Y.; Liu, Y.; Zhang, P.; Chen, C.; Zhou, X.; Liu, Y.; et al. Lymph node metastasis in thymic malignancies: A Chinese multicenter prospective observational study. J. Thorac. Cardiovasc. Surg. 2018, 156, 824–833.e1. [Google Scholar] [CrossRef] [PubMed]
- Zucali, P.A.; De Pas, T.; Palmieri, G.; Favaretto, A.; Chella, A.; Tiseo, M.; Caruso, M.; Simonelli, M.; Perrino, M.; De Vincenzo, F.; et al. Phase II Study of Everolimus in Patients with Thymoma and Thymic Carcinoma Previously Treated with Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2018, 36, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Naidoo, J.; Steele, K.E.; Ni, A.; Moreira, A.L.; Rekhtman, N.; Robbins, P.B.; Karakunnel, J.; Rimner, A.; Huang, J.; et al. Expression of PD-L1 and other immunotherapeutic targets in thymic epithelial tumors. PLoS ONE 2017, 12, e0182665. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Imai, H.; Kagamu, H. Perspective of Immune Checkpoint Inhibitors in Thymic Carcinoma. Cancers 2021, 13, 1065. [Google Scholar] [CrossRef]
- Ahmad, U.; Yao, X.; Detterbeck, F.; Huang, J.; Antonicelli, A.; Filosso, P.L.; Ruffini, E.; Travis, W.; Jones, D.R.; Zhan, Y.; et al. Thymic carcinoma outcomes and prognosis: Results of an international analysis. J. Thorac. Cardiovasc. Surg. 2015, 149, 95–101.e2. [Google Scholar] [CrossRef]
- Kondo, K.; Monden, Y. Therapy for thymic epithelial tumors: A clinical study of 1320 patients from Japan. Ann. Thorac. Surg. 2003, 76, 878–884; discussion 884–885. [Google Scholar] [CrossRef]
- Weksler, B.; Dhupar, R.; Parikh, V.; Nason, K.S.; Pennathur, A.; Ferson, P.F. Thymic carcinoma: A multivariate analysis of factors predictive of survival in 290 patients. Ann. Thorac. Surg. 2013, 95, 299–303. [Google Scholar] [CrossRef]
- Lee, C.Y.; Bae, M.K.; Park, I.K.; Kim, D.J.; Lee, J.G.; Chung, K.Y. Early Masaoka stage and complete resection is important for prognosis of thymic carcinoma: A 20-year experience at a single institution. Eur. J. Cardiothorac. Surg. 2009, 36, 159–162; discussion 163. [Google Scholar] [CrossRef]
- Okereke, I.C.; Kesler, K.A.; Freeman, R.K.; Rieger, K.M.; Birdas, T.J.; Ascioti, A.J.; Badve, S.; Nelson, R.P.; Loehrer, P.J. Thymic carcinoma: Outcomes after surgical resection. Ann. Thorac. Surg. 2012, 93, 1668–1672; discussion 1672–1673. [Google Scholar] [CrossRef]
- Ogawa, K.; Toita, T.; Uno, T.; Fuwa, N.; Kakinohana, Y.; Kamata, M.; Koja, K.; Kinjo, T.; Adachi, G.; Murayama, S. Treatment and prognosis of thymic carcinoma: A retrospective analysis of 40 cases. Cancer 2002, 94, 3115–3119. [Google Scholar] [CrossRef]
- Hosaka, Y.; Tsuchida, M.; Toyabe, S.; Umezu, H.; Eimoto, T.; Hayashi, J. Masaoka stage and histologic grade predict prognosis in patients with thymic carcinoma. Ann. Thorac. Surg. 2010, 89, 912–917. [Google Scholar] [CrossRef]
- Lucchi, M.; Ambrogi, M.C.; Duranti, L.; Basolo, F.; Fontanini, G.; Angeletti, C.A.; Mussi, A. Advanced stage thymomas and thymic carcinomas: Results of multimodality treatments. Ann. Thorac. Surg. 2005, 79, 1840–1844. [Google Scholar] [CrossRef]
- Okuma, Y.; Hosomi, Y.; Takagi, Y.; Sasaki, E.; Hishima, T.; Maeda, Y.; Shibuya, M.; Okamura, T. Clinical outcomes with chemotherapy for advanced thymic carcinoma. Lung Cancer 2013, 80, 75–80. [Google Scholar] [CrossRef]
- Louie, A.V.; Granton, P.V.; Fairchild, A.; Bezjak, A.; Gopaul, D.; Mulroy, L.; Brade, A.; Warner, A.; Debenham, B.; Bowes, D.; et al. Palliative Radiation for Advanced Central Lung Tumors with Intentional Avoidance of the Esophagus (PROACTIVE): A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1–7. [Google Scholar] [CrossRef]
- Filosso, P.L.; Yao, X.; Ruffini, E.; Ahmad, U.; Antonicelli, A.; Huang, J.; Guerrera, F.; Venuta, F.; van Raemdonck, D.; Travis, W.; et al. Comparison of outcomes between neuroendocrine thymic tumours and other subtypes of thymic carcinomas: A joint analysis of the European Society of Thoracic Surgeons and the International Thymic Malignancy Interest Group. Eur. J. Cardiothorac. Surg. 2016, 50, 766–771. [Google Scholar] [CrossRef]
- Kim, S.; Bull, D.A.; Hsu, C.H.; Hsu, C.C. The Role of Adjuvant Therapy in Advanced Thymic Carcinoma: A National Cancer Database Analysis. Ann. Thorac. Surg. 2020, 109, 1095–1103. [Google Scholar] [CrossRef]

| Variables | n | % |
|---|---|---|
| Age, median (IQR), y | 59 (48.3–67.0) | |
| Sex | ||
| Male | 78 | 67.2% |
| Female | 38 | 32.8% |
| ECOG | ||
| 0 | 29 | 25.0% |
| 1 | 82 | 70.7% |
| 2 | 5 | 4.3% |
| Masaoka–Koga stage | ||
| II | 49 | 42.2% |
| III | 23 | 19.8% |
| IVA | 14 | 12.1% |
| IVB | 30 | 25.9% |
| Greatest tumor dimension, median (IQR), cm | 5.7 (4.0–7.6) | |
| Surgical status | ||
| No surgery | 23 | 19.8% |
| R0 | 65 | 56.0% |
| R1 | 19 | 16.4% |
| R2 | 7 | 6.0% |
| Surgery but unknown | 2 | 1.7% |
| RT aim | ||
| Postoperative | 79 | 68.1% |
| Preoperative | 17 | 14.7% |
| Definitive | 11 | 9.5% |
| Palliative | 9 | 7.8% |
| RT modality | ||
| 3D-CRT | 32 | 27.6% |
| IMRT | 83 | 71.6% |
| Unknown | 1 | 0.9% |
| Chemotherapy | ||
| No | 59 | 50.9% |
| Yes | 57 | 49.1% |
| Chemotherapy regimen | ||
| No chemotherapy | 59 | 50.9% |
| Cisplatin/Adriamycin/Cyclophosphamide | 8 | 6.9% |
| Etoposide/Cisplatin | 42 | 36.2% |
| Others | 7 | 6.0% |
| Stages | Stage II | Stage III | Stage IVA | Stage IVB | ||
|---|---|---|---|---|---|---|
| Treatment Modalities | n (%) | 49 (42.2) | 23 (19.8) | 14 (12.1) | 30 (25.9) | |
| Surgery + Post-op RT | 46 (93.8) | 17 (73.9) | 6 (42.9) | 10 (33.3) | ||
| Chemotherapy | Yes | 3 (6.1) | 8 (34.7) | 4 (28.6) | 7 (23.3) | |
| No | 43 (87.8) | 9 (39.1) | 2 (14.3) | 3 (10.0) | ||
| Pre-op RT + Surgery | 3 (6.1) | 5 (21.7) | 1 (7.1) | 8 (26.7) | ||
| Chemotherapy | Yes | 3 (6.1) | 5 (21.7) | 1 (7.1) | 8 (26.7) | |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Definitive RT | 0 (0.0) | 1 (4.3) | 4 (28.6) | 6 (20.0) | ||
| Chemotherapy | Yes | 0 (0.0) | 1 (4.3) | 4 (28.6) | 6 (20.0) | |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Palliative RT | 0 (0.0) | 0 (0.0) | 3 (21.4) | 6 (20.0) | ||
| Chemotherapy | Yes | 0 (0.0) | 0 (0.0) | 3 (21.4) | 6 (20.0) | |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Local | Local +Regional | Regional | Regional +Distant | Distant | Total | |
|---|---|---|---|---|---|---|
| Failure pattern, n | ||||||
| Infield | 0 | 0 | 0 | 0 | 0 | 0 |
| Infield + outfield | 0 | 1 | 0 | 0 | 0 | 1 |
| Marginal | 0 | 0 | 0 | 0 | 0 | 0 |
| Marginal + outfield | 0 | 0 | 0 | 5 | 0 | 5 |
| Outfield | 0 | 0 | 11 | 4 | 32 | 47 |
| Total, n | 0 | 1 | 11 | 9 | 32 | 53 |
| Variables | OS | PFS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | p | |
| Age (per 1y) | 1.00 (0.97–1.03) | 0.733 | 1.00 (0.97–1.01) | 0.308 |
| Female sex (vs. male) | 0.83 (0.37–1.87) | 0.648 | 1.28 (0.73–2.23) | 0.384 |
| ECOG ≥ 2 (vs. 0–1) | 2.20 (0.68–12.53) | 0.150 | 1.63 (0.51–5.24) | 0.414 |
| Masaoka–Koga stage III, IV (vs. IIA, IIB) | 1.41 (0.56–3.59) | 0.465 | 0.99 (0.48–2.06) | 0.975 |
| Greatest tumor dimension ≥5.7 cm (<5.7 cm) | 3.01 (1.25–7.26) | 0.014 | 1.37 (0.77–2.45) | 0.289 |
| Initial surgery yes (vs. no) | 0.62 (0.16–2.41) | 0.493 | 0.81 (0.32–2.05) | 0.662 |
| Surgical status R1, R2, No surgery (vs. R0) | 0.95 (0.34–2.61) | 0.937 | 2.55 (1.38–4.71) | 0.003 |
| Chemotherapy yes (vs. no) | 2.41 (0.76–7.64) | 0.135 | 1.68 (0.72–3.90) | 0.226 |
| Radiotherapy aim Postoperative (vs. others) | 0.20 (0.08–0.52) | 0.001 | 0.34 (0.18–0.64) | 0.001 |
| IMRT (vs. 3D-CRT) | 1.45 (0.62–3.38) | 0.394 | 0.76 (0.32–1.78) | 0.527 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Lee, C.Y.; Kim, E.Y.; Lee, C.G.; Hong, M.H.; Park, B.J.; Yoon, H.I.; Kim, K.H.; Lee, S.H.; Byun, H.K.; et al. Clinical Outcomes of Thymic Carcinoma: The Role of Radiotherapy Combined with Multimodal Treatments. Cancers 2023, 15, 2262. https://doi.org/10.3390/cancers15082262
Yang G, Lee CY, Kim EY, Lee CG, Hong MH, Park BJ, Yoon HI, Kim KH, Lee SH, Byun HK, et al. Clinical Outcomes of Thymic Carcinoma: The Role of Radiotherapy Combined with Multimodal Treatments. Cancers. 2023; 15(8):2262. https://doi.org/10.3390/cancers15082262
Chicago/Turabian StyleYang, Gowoon, Chang Young Lee, Eun Young Kim, Chang Geol Lee, Min Hee Hong, Byung Jo Park, Hong In Yoon, Kyung Hwan Kim, Sang Hoon Lee, Hwa Kyung Byun, and et al. 2023. "Clinical Outcomes of Thymic Carcinoma: The Role of Radiotherapy Combined with Multimodal Treatments" Cancers 15, no. 8: 2262. https://doi.org/10.3390/cancers15082262
APA StyleYang, G., Lee, C. Y., Kim, E. Y., Lee, C. G., Hong, M. H., Park, B. J., Yoon, H. I., Kim, K. H., Lee, S. H., Byun, H. K., & Cho, J. (2023). Clinical Outcomes of Thymic Carcinoma: The Role of Radiotherapy Combined with Multimodal Treatments. Cancers, 15(8), 2262. https://doi.org/10.3390/cancers15082262






