188Re-SSS Lipiodol Radioembolization in HCC Patients: Results of a Phase 1 Trial (Lip-Re-01 Study)
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Study Design
2.3. Assessment of Adverse Events and Activity-Limiting Toxicities
2.4. Bio-Distribution Assessment
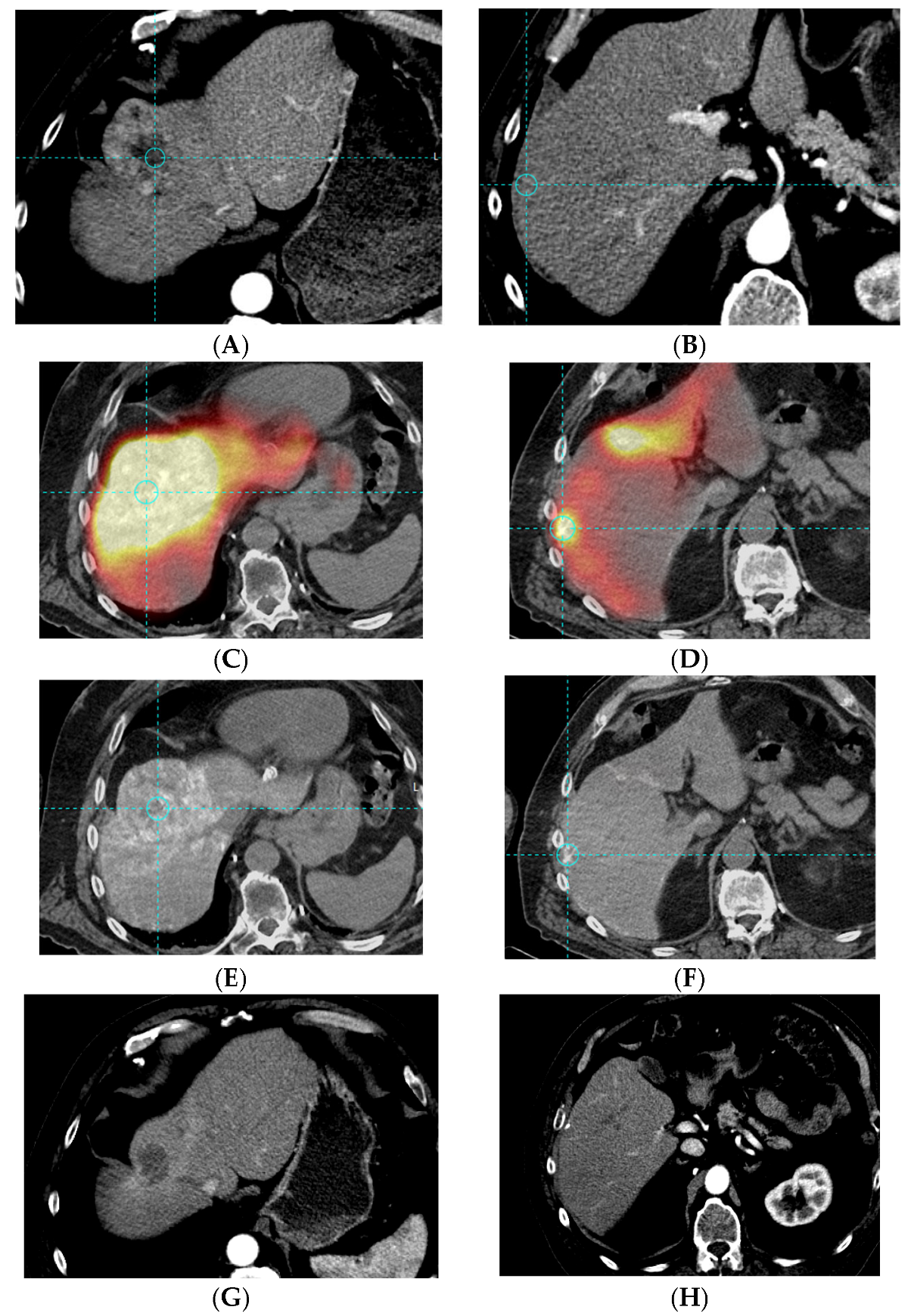
2.4.1. Image Acquisition
2.4.2. Quantitative Analysis
2.4.3. Blood, Urine, and Feces Collection
2.4.4. Dosimetry
2.4.5. Tumor Response Assessment
2.4.6. Statistical Analysis
3. Results
3.1. Safety
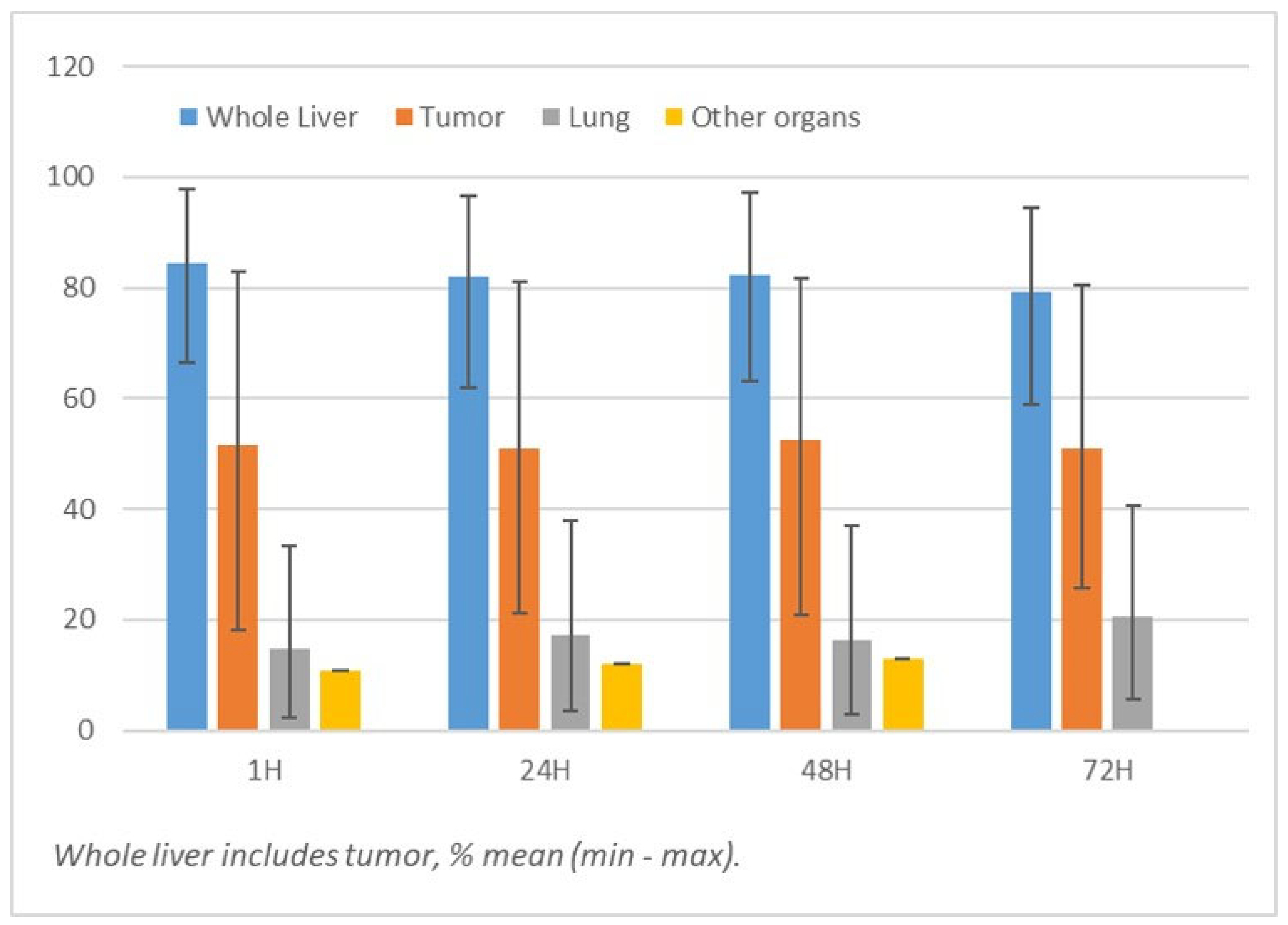
3.2. Bio-Distribution
3.3. Dosimetry
3.4. Tumor Response
3.5. Subsequent Treatment of 188Re-SSS Lipiodol
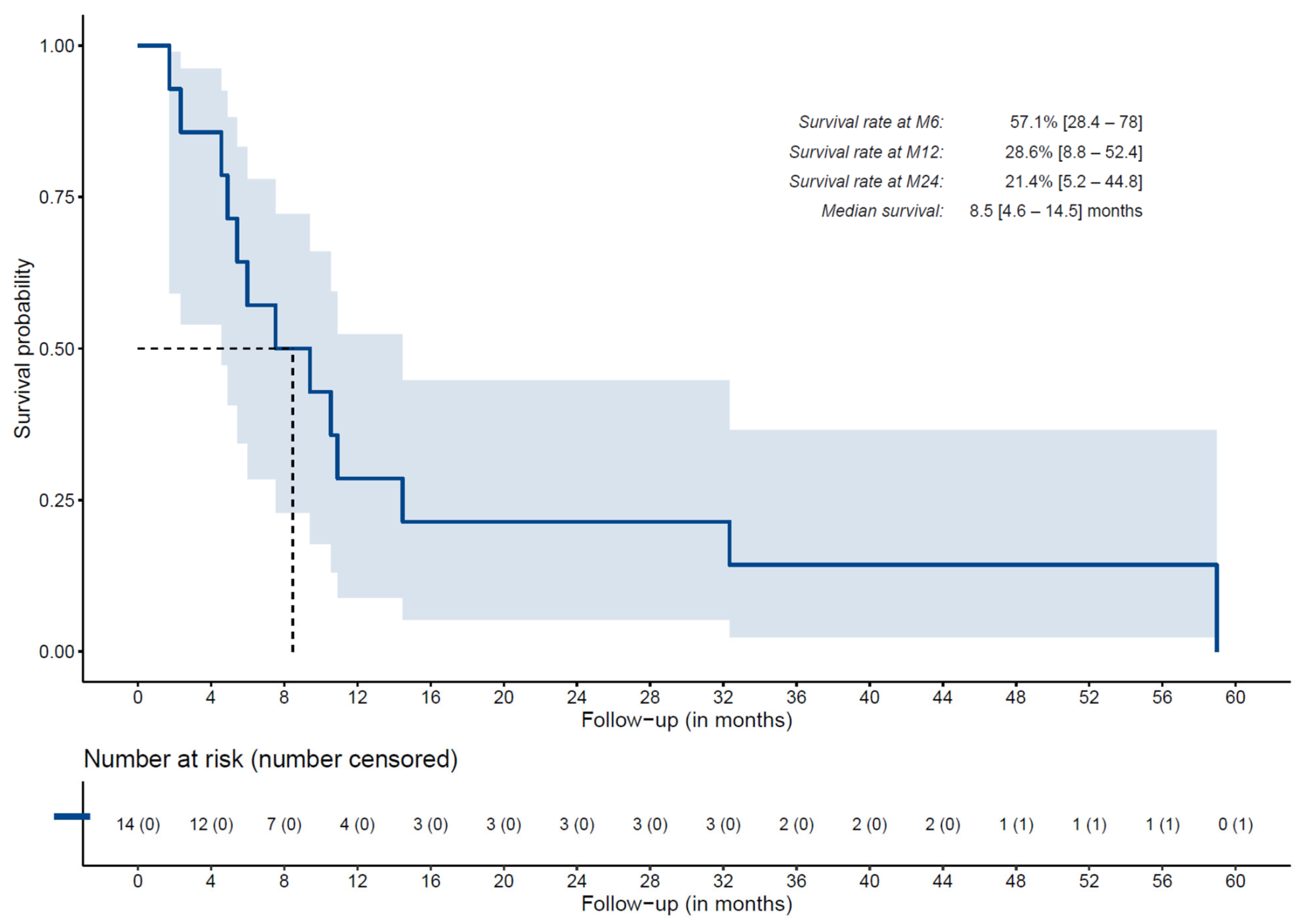
3.6. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, Y.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Selective internal radiation therapy (SIRT) using personalised dosimetry for locally advanced hepatocellular carcinoma (HCC) patients: A multicentre randomised phase 2 study (DOSISPHERE-01 trial). Lancet Gastroenterol. Heptol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.-B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Ricke, J.; Klümpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; de Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.L.; Guyader, D.; Bretagne, J.F.; Duvauferrier, R.; Bourguet, P.; Bekhechi, D.; Deugnier, Y.M.; Gosselin, M. Randomized controlled trial for hepatocellularcarcinoma with portal vein thrombosis: Intra-arterial iodine-131- iodized oil versus medical support. J. Nucl. Med. 1994, 35, 1782–1787. [Google Scholar]
- Lambert, B.; Bacher, K.; Defreyne, L.; Van Vlierberghe, H.; Jeong, J.M.; Wang, R.F.; Van Meerbeeck, J.; Smeets, P.; Troisi, R.; Thierens, H.; et al. (188)re-HDD/lipiodol therapy for hepatocellular carcinoma: An activity escalation study. Eur. J. Nucl. Med. Mol. Imaging. 2006, 33, 344–352. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Duatti, A.; Colamussi, P.; Cittanti, C.; Filice, A.; Rose, A.H.; Martindale, A.A.; Claringbold, P.G.; Kearney, D.; et al. A kit formulation for the preparation of 188Re-lipiodol: Preclinical studies and preliminary therapeutic evaluation in patients with unresectable hepatocellular carcinoma. Nucl. Med. Commun. 2004, 25, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Shinto, A.S.; Karuppusamy, K.K.; Kurup, R.E.R.; Pandiyan, A.; Jayaraj, A.V. Empirical 188Re-HDD/lipiodol intra-arterial therapy based on tumor volume, in patients with solitary inoperable hepatocellular carcinoma. Nucl. Med. Commun. 2021, 42, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Bacher, K.; Defreyne, L.; Gemmel, F.; Van Vlierberghe, H.; Jeong, J.M.; Dierckx, R.A.; Van De Wiele, C.; Thierens, H.; De Vos, F. 188Re-HDD/lipiodol therapy for hepatocellular carcinoma: A phase I clinical trial. J. Nucl. Med. 2005, 46, 60–66. [Google Scholar] [PubMed]
- Garin, E.; Noiret, N.; Malbert, C.-H.; Lepareur, N.; Roucoux, A.; Dazord, L.; Caulet-Maugendre, S.; Turlin, B.; Moisan, A.; Lecloirec, J.; et al. Development of 99mTc labelled lipiodol: Biodistribution following injection into the hepatic artery of the healthy pig. Nucl. Med. Commun. 2004, 25, 291–297. [Google Scholar] [CrossRef]
- Garin, E.; Denizot, B.; Noiret, N.; Lepareur, N.; Roux, J.; Moreau, M.; Herry, J.-Y.; Bourguet, P.; Benoit, J.-P.; Lejeune, J.-J. 188Re-SSS lipiodol: Radiolabelling and biodistribution following injection into the hepatic artery of rats bearing hepatoma. Nucl. Med. Commun. 2004, 25, 1007–1013. [Google Scholar] [CrossRef]
- Lepareur, N.; Ardisson, V.; Noiret, N.; Boucger, E.; Raoul, J.L.; Clement, B.; Garin, E. Automation of the labelling of Lipiodol with high-activity generator-produced 188Re. Appl. Radiat. Isot. 2011, 69, 426–430. [Google Scholar] [CrossRef]
- Delaunay, K.; Edeline, J.; Rolland, Y.; Lepareur, N.; Laffont, S.; Palard, X.; Bouvry, C.; Le Sourd, S.; Pracht, M.; Ardisson, V.; et al. Preliminary results of the Phase 1 Lip-Re I clinical trial: Biodistribution and dosimetry assessments in hepatocellular carcinoma patients treated with 188Re-SSS Lipiodol radioembolization. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Raoul, J.-L.; Stare, J.; Sereegotov, E.; Sundram, F.X.; Kumar, A.; Jeong, J.-M.; Pusuwan, P.; Divgi, C.; Zanzonico, P.; et al. InternationalAtomic EnergyAgency-sponsoredmultination study of intra-arterial rhenium-188-labeled lipiodol in the treatment of inoperable hepatocellular carcinoma: Results with special emphasis on prognostic value of dosimetric study. Semin. Nucl. Med. 2008, 38, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Lam, M.; Chiesa, C.; Konijnenberg, M.; Cremonesi, M.; Flamen, P.; Gnesin, S.; Bodei, L.; Kracmerova, T.; Luster, M.; et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds Writing group. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Riaz, A.; Gates, V.L.; Atassi, B.; Lewandowski, R.J.; Mulcahy, M.F.; Ryu, R.K.; Sato, K.T.; Baker, T.; Kulik, L.; Gupta, R.; et al. Radiation segmentectomy: A novel approach to increase safety and efficacy of radioembolization. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 163–171. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Kan, Z.; Ivancev, K.; Hägerstrand, I.; Chuang, V.P.; Lunderquist, A. In vivo microscopy of the liver after injection of lipiodol into the hepatic artery and portal vein in the rat. Acta Radiol. 1989, 30, 419–425. [Google Scholar]
- Chou, F.I.; Fang, K.C.; Chung, C.; Lui, W.Y.; Chi, C.W.; Liu, R.S.; Chan, W. Lipiodol uptake and retention by human hepatoma cells. Nucl. Med. Biol. 1995, 22, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.Y.; Leung, T.W.; Ho, S.K.; Chan, M.; Machin, D.; Lau, J.; Chan, A.; Yeo, W.; Mok, T.; Yu, S.; et al. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: A prospective randomised trial. Lancet 1999, 353, 797–801. [Google Scholar] [CrossRef]
- Chung, A.Y.F.; Ooi, L.L.P.J.; Machin, D.; Tan, S.B.; Goh, B.K.P.; Wong, S.J.; Chen, Y.M.; Li, P.C.N.; Gandhi, M.; Thng, C.H.; et al. Adjuvant hepatic intra-arterial iodine-131-lipiodol following curative resection of hepatocellular carcinoma: A prospective randomized trial. World J. Surg. 2013, 37, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.; Crawford, M.; Sandroussi, C. Systematic review and meta-analysis of adjuvant i(131) lipiodol after excision of hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
| n = 14 | |
|---|---|
| Age, mean ± SD (years) | 71 ± 4 |
| Gender | |
| Female | 1 (7.1%) |
| Male | 13 (92.9%) |
| Child–Pugh liver function classification | |
| A5 | 9 (64.3%) |
| A6 | 3 (21.4%) |
| B7 | 1 (7.1%) |
| B8 | 1 (7.1%) |
| ECOG performance status | |
| 0 | 9 (64.3%) |
| 1 | 5 (35.7%) |
| BCLC classification | |
| B | 9 (64.3%) |
| C | 5 (35.7%) |
| Portal vein invasion | |
| Present | 5 (35.7%) |
| Absent | 9 (64.3%) |
| Cirrhosis | |
| Present | 13 (92.9%) |
| Absent | 1 (7.1%) |
| Treatment line | |
| Second | 6 (42.9%) |
| Third | 5 (35.7%) |
| Fourth | 3 (21.4%) |
| Tumor distribution | |
| Unifocal | 2 (14.3%) |
| Multifocal | 12 (85.7%) |
| Lobes affected | |
| Unilobar disease | 4 (28.6%) |
| Bilobar disease | 10 (71.4%) |
| Index tumor size, mean ± SD (cm) | 8.9 ± 5.5 |
| AFP level, mean ± SD (kU/L) | 3371.5 ± 12282 |
| Bilirubin level, mean ± SD (μmol/mL) | 19.6 ± 11.6 |
| Events | Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Overall | 1.85 GBq Level | 3.7 GBq Level | 5.5 GBq Level | Overall | 1.85 GBq Level | 3.7 GBq Level | 5.5 GBq Level 3 |
| Grade | ||||||||
| 1 | 76 (46%) | 25 | 43 | 8 | 14 (100%) | 6 | 6 | 2 |
| 2 | 54 (33%) | 17 | 33 | 4 | 12 (86%) | 5 | 6 | 1 |
| 3 | 29 (17%) | 12 | 11 | 6 | 9 (64%) | 4 | 3 | 2 |
| 4 | 2 (1%) | 1 | 1 | 0 | 2 (14%) | 1 | 1 | 0 |
| 5 | 5 (3%) | 2 | 2 | 1 | 5 (36%) | 2 | 2 | 1 |
| Characteristic | Overall, n = 10 1 | 1.85 GBq Level, n = 2 1 | 3.7 GBq Level, n = 5 1 | 5.5 GBq Level, n = 3 1 |
|---|---|---|---|---|
| Preferred Term | ||||
| Acute respiratory failure | 1 (10%) | 0 (0%) | 1 (20%) | 0 (0%) |
| Alanine aminotransferase increased | 1 (10%) | 0 (0%) | 1 (20%) | 0 (0%) |
| Aspartate aminotransferase increased | 3 (30%) | 1 (50%) | 2 (40%) | 0 (0%) |
| Hepatic failure | 1 (10%) | 1 (50%) | 0 (0%) | 0 (0%) |
| Lymphopenia | 4 (40%) | 0 (0%) | 1 (20%) | 3 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garin, E.; Palard, X.; Rolland, Y.; Le Sourd, S.; Lepareur, N.; Ardisson, V.; Bouvry, C.; Laffont, S.; Campillo-Gimenez, B.; Bellissant, E.; et al. 188Re-SSS Lipiodol Radioembolization in HCC Patients: Results of a Phase 1 Trial (Lip-Re-01 Study). Cancers 2023, 15, 2245. https://doi.org/10.3390/cancers15082245
Garin E, Palard X, Rolland Y, Le Sourd S, Lepareur N, Ardisson V, Bouvry C, Laffont S, Campillo-Gimenez B, Bellissant E, et al. 188Re-SSS Lipiodol Radioembolization in HCC Patients: Results of a Phase 1 Trial (Lip-Re-01 Study). Cancers. 2023; 15(8):2245. https://doi.org/10.3390/cancers15082245
Chicago/Turabian StyleGarin, Etienne, Xavier Palard, Yan Rolland, Samuel Le Sourd, Nicolas Lepareur, Valérie Ardisson, Christelle Bouvry, Sophie Laffont, Boris Campillo-Gimenez, Eric Bellissant, and et al. 2023. "188Re-SSS Lipiodol Radioembolization in HCC Patients: Results of a Phase 1 Trial (Lip-Re-01 Study)" Cancers 15, no. 8: 2245. https://doi.org/10.3390/cancers15082245
APA StyleGarin, E., Palard, X., Rolland, Y., Le Sourd, S., Lepareur, N., Ardisson, V., Bouvry, C., Laffont, S., Campillo-Gimenez, B., Bellissant, E., & Edeline, J. (2023). 188Re-SSS Lipiodol Radioembolization in HCC Patients: Results of a Phase 1 Trial (Lip-Re-01 Study). Cancers, 15(8), 2245. https://doi.org/10.3390/cancers15082245








