Survival Outcomes and Failure Patterns in Patients with Inoperable Non-Metastatic Pancreatic Cancer Treated with Definitive Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
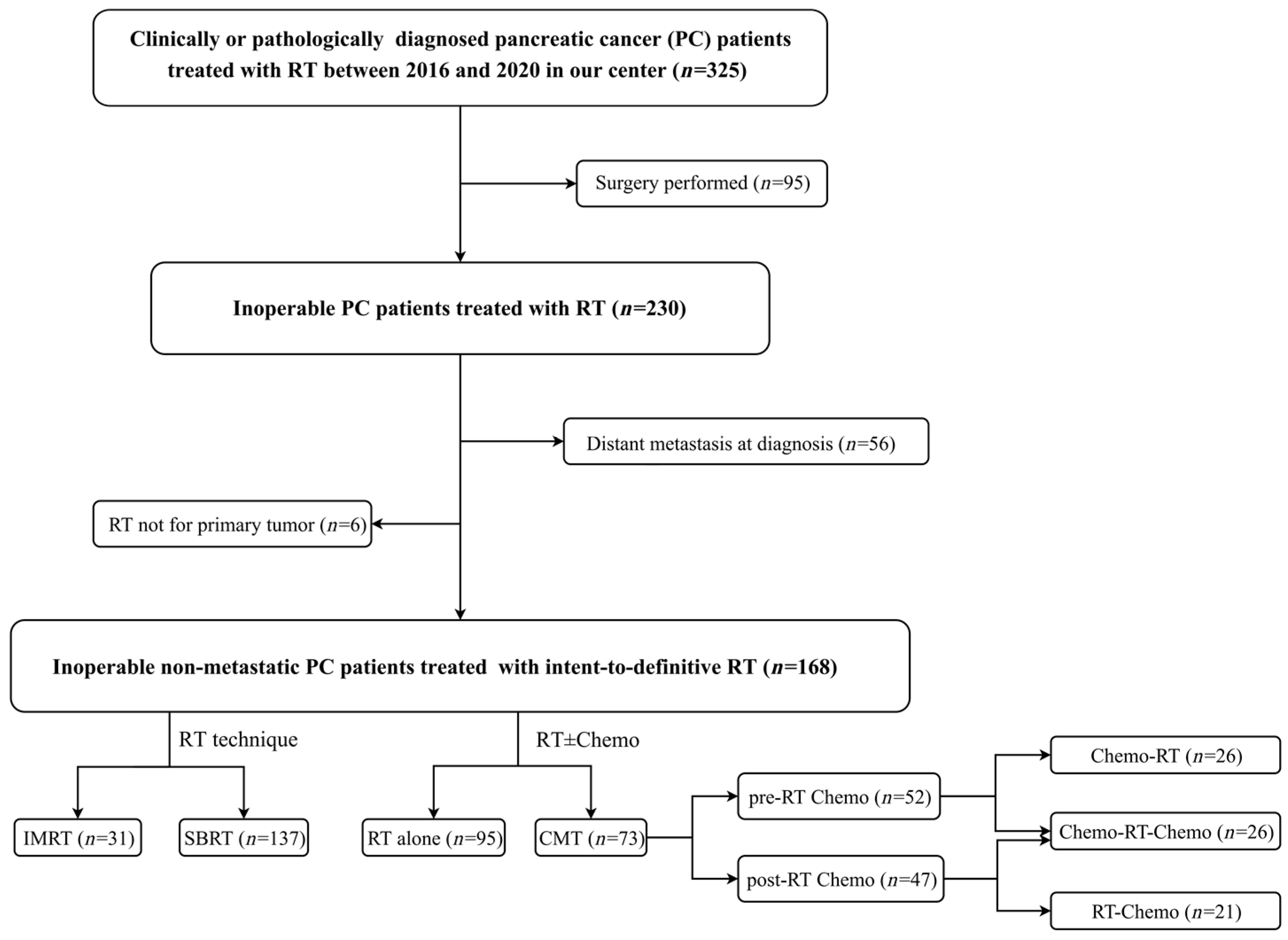
2.1. Patient Selection
2.2. Treatment
2.2.1. IG-IMRT
2.2.2. SBRT
2.3. Chemotherapy
2.4. Follow-Up and Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Overall Survival
3.3. Survival Outcomes According to Definitive RT
3.4. Survival Outcomes According to Treatment Modalities by Stage
3.5. Prognostic Factors
3.6. Treatment-Related Toxicity
3.7. Disease Progression and Failure Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- National Cancer Institude. Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 20 January 2023).
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Zhu, X.F.; Li, F.Q.; Ju, X.P.; Cao, F.; Cao, Y.S.; Fang, F.; Qing, S.W.; Shen, Y.X.; Jia, Z.; Zhang, H.J. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med. 2017, 6, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma, Version 2. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 20 January 2023).
- Kishi, T.; Nakamura, A.; Itasaka, S.; Shibuya, K.; Matsumoto, S.; Kanai, M.; Kodama, Y.; Takaori, K.; Mizowaki, T.; Hiraoka, M. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology 2015, 15, 694–700. [Google Scholar] [CrossRef]
- Hurt, C.N.; Falk, S.; Crosby, T.; McDonald, A.; Ray, R.; Joseph, G.; Staffurth, J.; Abrams, R.A.; Griffiths, G.; Maughan, T.; et al. Long-term results and recurrence patterns from SCALOP: A phase II randomised trial of gemcitabine- or capecitabine-based chemoradiation for locally advanced pancreatic cancer. Br. J. Cancer 2017, 116, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.A.; Keane, F.K.; Voncken, F.E.M.; Thomas, C.R., Jr. Contemporary radiotherapy: Present and future. Lancet 2021, 398, 171–184. [Google Scholar] [CrossRef]
- Reyngold, M.; O’Reilly, E.M.; Varghese, A.M.; Fiasconaro, M.; Zinovoy, M.; Romesser, P.B.; Wu, A.; Hajj, C.; Cuaron, J.J.; Tuli, R.; et al. Association of Ablative Radiation Therapy with Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol. 2021, 7, 735–738. [Google Scholar] [CrossRef]
- Lee, G.; Kim, D.W.; Oladeru, O.T.; Niemierko, A.; Gergelis, K.R.; Haddock, M.G.; Toesca, D.A.S.; Koong, A.J.; Owen, D.; Weekes, C.; et al. Liver Metastasis-Directed Ablative Radiotherapy in Pancreatic Cancer Offers Prolonged Time Off Systemic Therapy in Selected Patients: Data From a Multi-institutional Retrospective Study. Pancreas 2021, 50, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, C.; Rudra, S.; Bommireddy, A.; Hawkins, W.G.; Wang-Gillam, A.; Fields, R.C.; Cai, B.; Park, J.; Green, O.; Roach, M.; et al. Ablative Five-Fraction Stereotactic Body Radiation Therapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Adv. Radiat. Oncol. 2021, 6, 100506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, J.; Xu, S.P.; Xie, C.B.; Gong, H.S.; Wang, X.S.; Qu, B.L. Planning target volume margin definition from image guided hypofractionated radiation therapy for pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E155–E156. [Google Scholar] [CrossRef]
- Timmerman, R. A Story of Hypofractionation and the Table on the Wall. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Jiang, N.; Rosenberg, S.A.; Olsen, J.R.; Roach, M.C.; Wan, L.; Portelance, L.; Mellon, E.A.; Bruynzeel, A.; Lagerwaard, F.; et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019, 8, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Chadha, A.S.; Suh, Y.; Chen, H.C.; Rao, A.; Das, P.; Minsky, B.D.; Mahmood, U.; Delclos, M.E.; Sawakuchi, G.O.; et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 755–765. [Google Scholar] [CrossRef]
- Loehrer, P.J., Sr.; Feng, Y.; Cardenes, H.; Wagner, L.; Brell, J.M.; Cella, D.; Flynn, P.; Ramanathan, R.K.; Crane, C.H.; Alberts, S.R.; et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2011, 29, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Burns, W.R.; Frankel, T.L.; Cho, C.S.; Nathan, H. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann. Surg. Oncol. 2017, 24, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Chu, L.C.; Deig, C.R.; Fishman, E.K.; Hwang, W.L.; Maitra, A.; Marks, D.L.; Mehta, A.; Nabavizadeh, N.; Simeone, D.M.; et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J. Clin. 2020, 70, 375–403. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Higuera, O.; Ghanem, I.; Nasimi, R.; Prieto, I.; Koren, L.; Feliu, J. Management of pancreatic cancer in the elderly. World J. Gastroenterol. 2016, 22, 764–775. [Google Scholar] [CrossRef]
- Treatment of locally unresectable carcinoma of the pancreas: Comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J. Natl. Cancer Inst. 1988, 80, 751–755. [CrossRef]
- Chauffert, B.; Mornex, F.; Bonnetain, F.; Rougier, P.; Mariette, C.; Bouche, O.; Bosset, J.F.; Aparicio, T.; Mineur, L.; Azzedine, A.; et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann. Oncol. 2008, 19, 1592–1599. [Google Scholar] [CrossRef]
- Hammel, P.; Huguet, F.; van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouche, O.; Shannon, J.; Andre, T.; et al. Effect of Chemoradiotherapy vs. Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, D.J.; MacIntyre, J.M.; Catton, G.E.; Engstrom, P.F.; Moertel, C.G. Treatment of locally unresectable cancer of the stomach and pancreas: A randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—An Eastern Cooperative Oncology Group study. J. Clin. Oncol. 1985, 3, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Aref, A.; Berri, R. Role of radiation therapy in the management of locally advanced pancreatic cancer. J Clin Oncol 2012, 30, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- de Geus, S.W.L.; Eskander, M.F.; Kasumova, G.G.; Ng, S.C.; Kent, T.S.; Mancias, J.D.; Callery, M.P.; Mahadevan, A.; Tseng, J.F. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer 2017, 123, 4158–4167. [Google Scholar] [CrossRef]
- Zhong, J.; Switchenko, J.; Behera, M.; Kooby, D.; Maithel, S.K.; McDonald, M.W.; Lin, J.Y.; Cassidy, R.J.; El-Rayes, B.; Landry, J.; et al. Chemotherapy with or Without Definitive Radiation Therapy in Inoperable Pancreatic Cancer. Ann. Surg. Oncol. 2018, 25, 1026–1033. [Google Scholar] [CrossRef]
- Ma, S.J.; Prezzano, K.M.; Hermann, G.M.; Singh, A.K. Dose escalation of radiation therapy with or without induction chemotherapy for unresectable locally advanced pancreatic cancer. Radiat. Oncol. 2018, 13, 214. [Google Scholar] [CrossRef]





| Characteristic | No. (%) |
|---|---|
| Median age at time of RT (range), year | 64 (36–85) |
| Sex | |
| Female | 69 (41.1) |
| Male | 99 (58.9) |
| Tumor location | |
| Head | 111 (66.1) |
| Body/tail | 57 (33.9) |
| ECOG score | |
| 0–1 | 92 (53.6) |
| 2 | 76 (46.4) |
| Diagnosis confirmation | |
| Pathologically | 39 (23.2) |
| Clinically | 129 (76.8) |
| T Stage | |
| T1–T2 | 37 (22.0) |
| T3–T4 | 131(78.0) |
| N Stage | |
| N0 | 104 (61.9) |
| N+ | 64 (38.1) |
| AJCC Stage | |
| I | 26 (15.5) |
| II | 24 (14.3) |
| I–II | 50 (29.8) |
| III | 118 (70.2) |
| Tumor size, median (range), cm | 3.8 (1.3–10.0) |
| Pre-RT CA19–9, median (range), U/mL | 182.8 (0.6–20,000) |
| Chemotherapy | |
| No | 95 (56.5) |
| Yes | 73 (43.5) |
| Sequence of RT and chemotherapy | |
| CT-RT-CT | 26 (35.6) |
| CT-RT | 26 (35.6) |
| RT-CT | 21 (28.8) |
| Pre-RT chemotherapy cycles | |
| ≤6 | 42 (57.5) |
| >6 | 31 (42.5) |
| Total chemotherapy cycles | |
| ≤8 | 43 (58.9) |
| >8 | 30 (41.1) |
| RT technique | |
| IMRT | 31 (18.5) |
| SBRT | 137 (81.5) |
| BED10 | |
| ≤80 Gy | 54 (32.1) |
| >80 Gy | 114 (67.9) |
| Characteristics | mOS (Months) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | ||
| Age at time of RT | 18.0 | 1.008 (0.991–1.025) | 0.363 | ||
| Sex | |||||
| Female | 17.1 | Reference | |||
| Male | 18.0 | 0.935 (0.613–1.426) | 0.755 | ||
| Tumor location | |||||
| Head | 17.0 | Reference | |||
| Body/tail | 21.1 | 0.853 (0.545–1.336) | 0.488 | ||
| ECOG score | |||||
| 0–1 | 18.0 | Reference | |||
| 2 | 16.7 | 1.200 (0.793–1.816) | 0.388 | ||
| Diagnosis confirmation | |||||
| Clinically | 17.1 | Reference | |||
| Pathologically | 18.0 | 0.797 (0.483–1.315) | 0.375 | ||
| Pre-RT CA19–9 | |||||
| ≤130 U/mL | 22.2 | Reference | |||
| >130 U/mL | 16.1 | 1.623 (1.058–2.491) | 0.027 | 1.765 (1.142–2.728) | 0.011 |
| Tumor size | |||||
| ≤4 cm | 21.7 | Reference | |||
| >4 cm | 18.0 | 1.123 (0.737–1.709) | 0.590 | ||
| T stage | |||||
| T1–T2 | 21.7 | Reference | |||
| T3–T4 | 17.6 | 1.276 (0.773–2.104) | 0.340 | ||
| N stage | |||||
| N0 | 18.0 | Reference | |||
| N+ | 17.0 | 0.890 (0.579–1.369) | 0.595 | ||
| AJCC Stage | |||||
| I–II | 21.1 | Reference | |||
| III | 17.0 | 1.525 (0.952–2.443) | 0.079 | 1.726 (1.049–2.840) | 0.032 |
| Chemotherapy | |||||
| No | 16.0 | Reference | |||
| Yes | 19.2 | 0.642 (0.422–0.978) | 0.039 | 0.509 (0.327–0.791) | 0.003 |
| RT technique | |||||
| SBRT | 18.0 | Reference | |||
| IMRT | 16.4 | 1.055 (0.627–1.774) | 0.841 | ||
| BED10 | |||||
| ≤80 Gy | 16.4 | Reference | |||
| >80 Gy | 21.1 | 0.592 (0.373–0.938) | 0.026 | 0.541 (0.332–0.881) | 0.014 |
| Toxicity | Grade 1–2 | Grade 3–4 |
|---|---|---|
| Acute toxicity | ||
| Nausea and vomiting | 122 (72.6%) | 11(6.5%) |
| Neutropenia | 57 (33.9%) | 5 (3.0%) |
| Thrombocytopenia | 13 (7.7%) | 3 (1.8%) |
| Hyperbilirubinemia | 6 (3.6%) | 1(0.6%) |
| Gastrointestinal ulcer | 2 (1.2%) | 0 (0.0%) |
| Late toxicity | ||
| Gastric ulcer | 5 (3.0%) | 0 (0.0%) |
| Duodenal ulcer | 14 (8.3%) | 0 (0.0%) |
| Gastrointestinal obstruction | 12 (7.1%) | 4 (2.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, B.; Zhang, L.; Wu, C.; Liu, X.; Wang, Q.; Tong, F.; Yang, W.; Wang, J. Survival Outcomes and Failure Patterns in Patients with Inoperable Non-Metastatic Pancreatic Cancer Treated with Definitive Radiotherapy. Cancers 2023, 15, 2213. https://doi.org/10.3390/cancers15082213
Cao B, Zhang L, Wu C, Liu X, Wang Q, Tong F, Yang W, Wang J. Survival Outcomes and Failure Patterns in Patients with Inoperable Non-Metastatic Pancreatic Cancer Treated with Definitive Radiotherapy. Cancers. 2023; 15(8):2213. https://doi.org/10.3390/cancers15082213
Chicago/Turabian StyleCao, Biyang, Letian Zhang, Chenchen Wu, Xiaoliang Liu, Qianqian Wang, Fang Tong, Wei Yang, and Jing Wang. 2023. "Survival Outcomes and Failure Patterns in Patients with Inoperable Non-Metastatic Pancreatic Cancer Treated with Definitive Radiotherapy" Cancers 15, no. 8: 2213. https://doi.org/10.3390/cancers15082213
APA StyleCao, B., Zhang, L., Wu, C., Liu, X., Wang, Q., Tong, F., Yang, W., & Wang, J. (2023). Survival Outcomes and Failure Patterns in Patients with Inoperable Non-Metastatic Pancreatic Cancer Treated with Definitive Radiotherapy. Cancers, 15(8), 2213. https://doi.org/10.3390/cancers15082213






