Arginase Inhibition Mitigates Bortezomib-Exacerbated Cardiotoxicity in Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
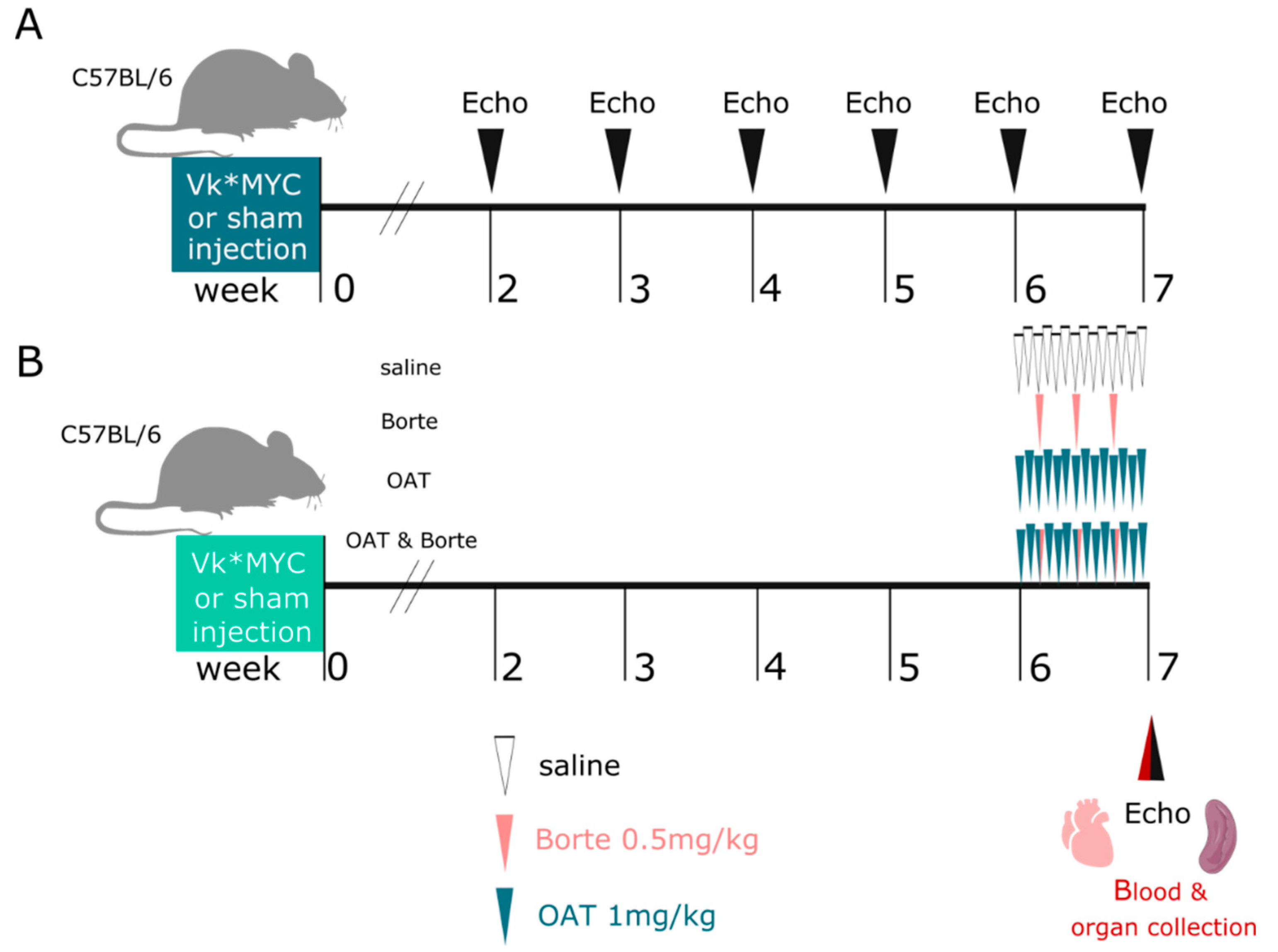
2.1. Mice
2.2. Vκ*MYC MM Model
2.3. Treatments
2.4. Experimental Design
2.5. Endothelial Function Studies
2.6. Nitric Oxide (NO) Measurement in Plasma and Effluent from Isolated Hearts
2.7. Echocardiography Imaging
2.8. Statistical Analysis
3. Results
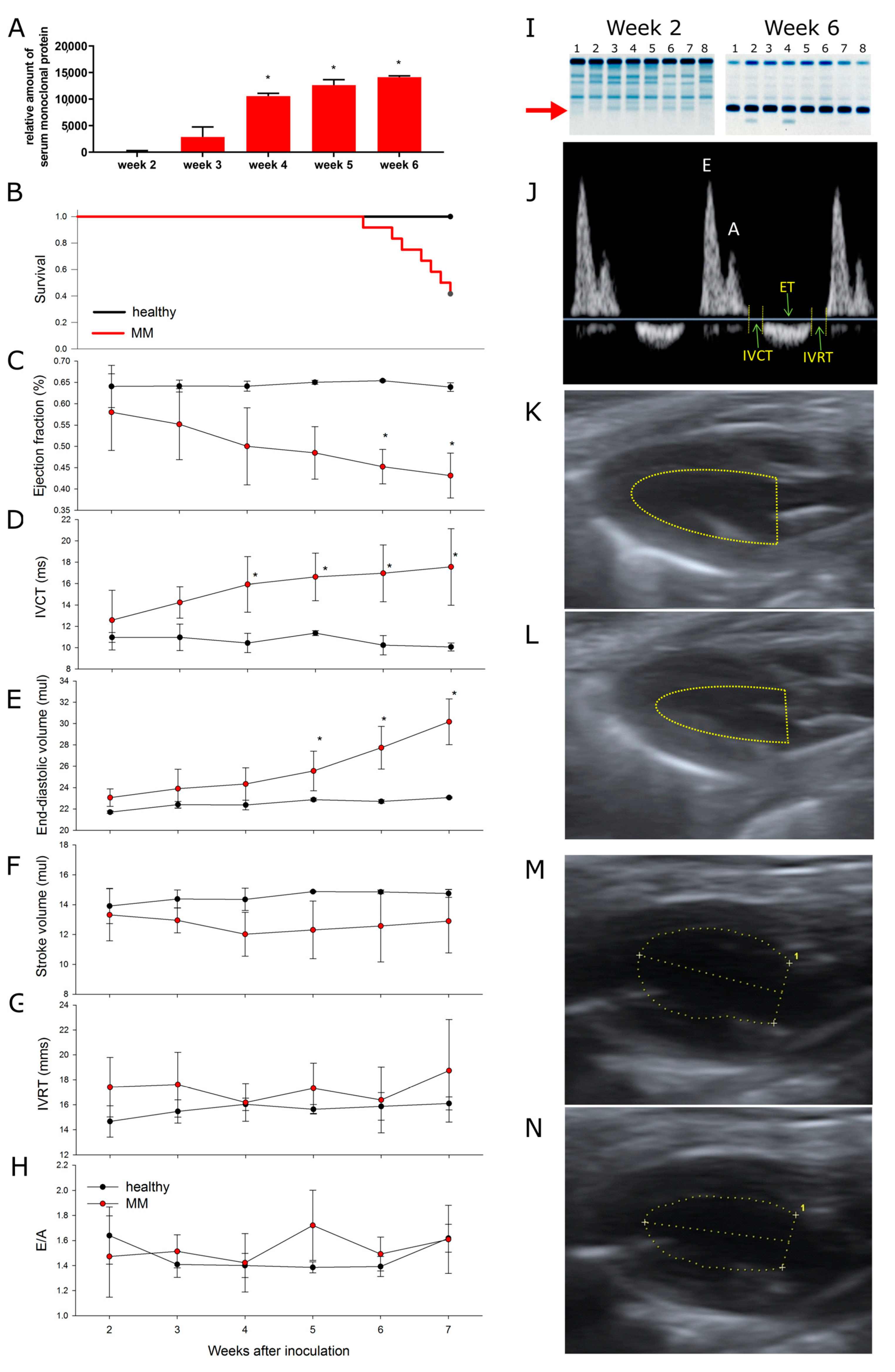
3.1. The Model
3.2. Multiple Myeloma Progression Is Associated with Progressive Cardiotoxicity
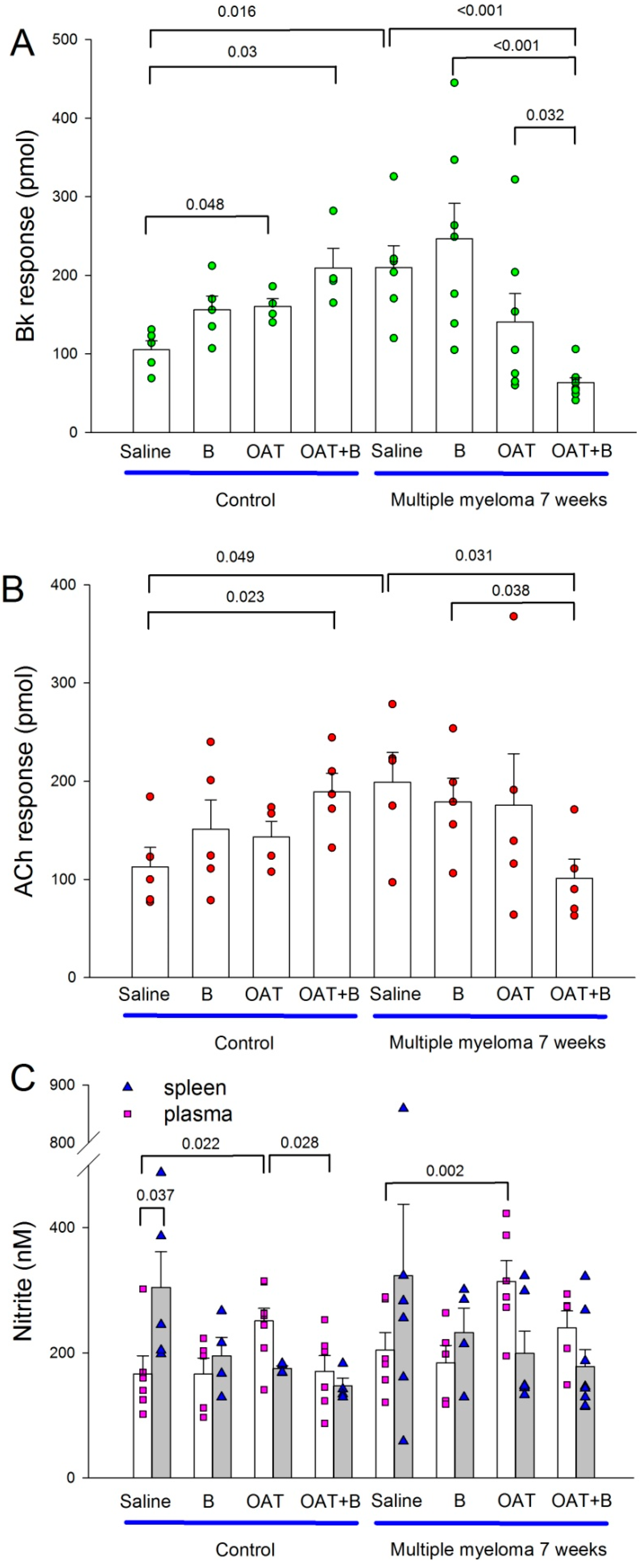
3.3. Bortezomib Exacerbates MM-Associated Cardiotoxicity, While an Arginase Inhibitor Prevents It
3.4. Endothelial Function and Nitric Oxide Metabolites in the Plasma and Spleen
4. Discussion
4.1. Cardiotoxicity Caused by Multiple Myeloma
4.2. Cardiotoxicity of Bortezomib: Summation of Adverse Cardiac Effects of MM and Bortezomib
4.3. Endothelial Function and Nitric Oxide Availability in Multiple Myeloma and under Bortezomib Therapy
4.4. An Arginase Inhibitor Protects the Heart from Multiple Myeloma- and Bortezomib-Induced Toxicity
4.5. Study Limitations
5. Conclusions
Clinical Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Pancheri, E.; Guglielmi, V.; Wilczynski, G.M.; Malatesta, M.; Tonin, P.; Tomelleri, G.; Nowis, D.; Vattemi, G. Non-hematologic toxicity of bortezomib in multiple myeloma: The neuromuscular and cardiovascular adverse effects. Cancers 2020, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Fan, F.; Zhang, B.; Hu, Y.; Sun, C. Cardiovascular-specific mortality among multiple myeloma patients: A population-based study. Ther. Adv. Hematol. 2022, 13, 20406207221086755. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Schou, M.; Ruberg, F.L.; Rucker, D.; Choi, J.; Siddiqi, O.; Monahan, K.; Køber, L.; Gislason, G.; Torp-Pedersen, C.; et al. Cardiovascular morbidity in monoclonal gammopathy of undetermined significance. JACC CardioOncol. 2022, 4, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. Cardiac amyloidosis: Evolving diagnosis and management: A scientific statement from the american heart association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef]
- Gertz, M.A. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am. J. Hematol. 2022, 97, 818–829. [Google Scholar] [CrossRef]
- Yi, J.E.; Lee, S.E.; Jung, H.O.; Min, C.K.; Youn, H.J. Association between left ventricular function and paraprotein type in patients with multiple myeloma. Korean J. Intern. Med. 2017, 32, 459–468. [Google Scholar] [CrossRef]
- Mehta, J.; Singhal, S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin. Thromb. Hemost. 2003, 29, 467–471. [Google Scholar]
- Carlisi, M.; Mancuso, S.; Lo, P.R.; Siragusa, S.; Caimi, G. High output heart failure in multiple myeloma: Pathogenetic con,siderations. Cancers 2022, 14, 610. [Google Scholar] [CrossRef]
- Tsai, A.G.; Acero, C.; Nance, P.R.; Cabrales, P.; Frangos, J.A.; Buerk, D.G.; Intaglietta, M. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1730–H1739. [Google Scholar] [CrossRef]
- Cabrales, P.; Martini, J.; Intaglietta, M.; Tsai, A.G. Blood viscosity maintains microvascular conditions during normovolemic anemia independent of blood oxygen-carrying capacity. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H581–H590. [Google Scholar] [CrossRef] [PubMed]
- Ramji, K.; Grzywa, T.M.; Sosnowska, A.; Paterek, A.; Okninska, M.; Pilch, Z.; Barankiewicz, J.; Garbicz, F.; Borg, K.; Bany-Laszewicz, U.; et al. Targeting arginase-1 exerts antitumor effects in multiple myeloma and mitigates bortezomib-induced cardiotoxicity. Sci. Rep. 2022, 12, 19660. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.J.; Li, J. Proteasome inhibitors: Harnessing proteostasis to combat disease. Molecules 2020, 25, 671. [Google Scholar] [CrossRef]
- Adams, J. The proteasome: A suitable antineoplastic target. Nat. Rev. Cancer 2004, 4, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.F.; Ky, B.; Weiss, B.M.; Dahm, C.N.; Gupta, D.K.; Du, L.; Carver, J.R.; Cohen, A.D.; Engelhardt, B.G.; Garfall, A.L.; et al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J. Clin. Oncol. 2019, 37, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Nowis, D.; Maczewski, M.; Mackiewicz, U.; Kujawa, M.; Ratajska, A.; Wieckowski, M.R.; Wilczyński, G.M.; Malinowska, M.; Bil, J.; Salwa, P.; et al. Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am. J. Pathol. 2010, 176, 2658–2668. [Google Scholar] [CrossRef]
- Wei, Q.; Xia, Y. Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J.Biol. Chem. 2006, 281, 21652–21659. [Google Scholar] [CrossRef]
- Chesi, M.; Robbiani, D.F.; Sebag, M.; Chng, W.J.; Affer, M.; Tiedemann, R.; Valdez, R.; Palmer, S.E.; Haas, S.S.; Stewart, A.K.; et al. Aid-dependent activation of a myc transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell 2008, 13, 167–180. [Google Scholar] [CrossRef]
- Chesi, M.; Matthews, G.M.; Garbitt, V.M.; Palmer, S.E.; Shortt, J.; Lefebure, M.; Stewart, A.K.; Johnstone, R.W.; Bergsagel, P.L. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood 2012, 120, 376–385. [Google Scholar] [CrossRef]
- Grzybowski, M.M.; Stańczak, P.S.; Pomper, P.; Błaszczyk, R.; Borek, B.; Gzik, A.; Nowicka, J.; Jędrzejczak, K.; Brzezińska, J.; Rejczak, T.; et al. Oatd-02 validates the benefits of pharmacological inhibition of arginase 1 and 2 in cancer. Cancers 2022, 14, 3967. [Google Scholar] [CrossRef]
- Maczewski, M.; Beresewicz, A. Role of nitric oxide and free radicals in cardioprotection by blocking na+/h+ and na+/ca2+ exchange in rat heart. Eur. J. Pharmacol. 2003, 461, 139–147. [Google Scholar] [CrossRef]
- Gui, L.; Wang, F.; Shi, J.; Chen, B. The significance of inflammatory markers in the prognosis of newly diagnosed multiple myeloma patients. Blood 2020, 136, 15. [Google Scholar] [CrossRef]
- Chilian, W.M.; Eastham, C.L.; Marcus, M.L. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am. J. Physiol. 1986, 251, H779–H788. [Google Scholar] [CrossRef]
- Rim, S.-J.; Leong-Poi, H.; Lindner, J.R.; Wei, K.; Fisher, N.G.; Kaul, S. Decrease in coronary blood flow reserve during hyperlipidemia is secondary to an increase in blood viscosity. Circulation 2001, 104, 2704–2709. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac energy metabolism in heart failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Otmani, K.; Naimi, D.; Mentaverri, R.; Objois, T.; Nadiabouderssa, N.; Marolleau, J.P. Quantification of nitric oxide in multiple myeloma algerian patients using r & d and arbor assays kits. Biomed. Pharmacol. J. 2018, 11, 1051–1059. [Google Scholar]
- Gavazzoni, M.; Gorga, E.; Vizzardi, E.; Sciatti, E.; Metra, M.; Raddino, R. Cardiology Department UoB, Spedali Civili of Brescia Piazzale Spedali Civili n.1, Italy. P572single centre pilot study on mechanism of vascular effects of carfilzomib in patients with multiple myeloma: Preliminary results on endothelial dysfunction and vascular stiffness. Eur. Heart J. 2017, 38, ehx501.P572. [Google Scholar]
- Gavazzoni, M.; Lombardi, C.M.; Vizzardi, E.; Gorga, E.; Sciatti, E.; Rossi, L.; Belotti, A.; Rossi, G.; Metra, M.; Raddino, R. Irreversible proteasome inhibition with carfilzomib as first line therapy in patients with newly diagnosed multiple myeloma: Early in vivo cardiovascular effects. Eur. J. Pharmacol. 2018, 838, 85–90. [Google Scholar] [CrossRef]
- Vivarelli, S.; Falzone, L.; Basile, M.S.; Candido, S.; Libra, M. Nitric oxide in hematological cancers: Partner or rival? Antioxid. Redox Signal. 2021, 34, 383–401. [Google Scholar] [CrossRef]
- Pernow, J.; Jung, C. Arginase as a potential target in the treatment of cardiovascular disease: Reversal of arginine steal? Cardiovasc. Res. 2013, 98, 334–343. [Google Scholar] [CrossRef]
- Toya, T.; Hakuno, D.; Shiraishi, Y.; Kujiraoka, T.; Adachi, T. Arginase inhibition augments nitric oxide production and facilitates left ventricular systolic function in doxorubicin-induced cardiomyopathy in mice. Physiol. Rep. 2014, 2, e12130. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Han, Y.S.; Jensen, C.; Sieck, G. Effects of arginase inhibition on myocardial ca(2+) and contractile responses. Physiol. Rep. 2022, 10, e15396. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yu, Y.; Montani, J.P.; Yang, Z.; Ming, X.F. Arginase-ii induces vascular smooth muscle cell senescence and apoptosis through p66shc and p53 independently of its l-arginine ureahydrolase activity: Implications for atherosclerotic plaque vulnerability. J. Am. Heart Assoc. 2013, 2, e000096. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterek, A.; Oknińska, M.; Pilch, Z.; Sosnowska, A.; Ramji, K.; Mackiewicz, U.; Golab, J.; Nowis, D.; Mączewski, M. Arginase Inhibition Mitigates Bortezomib-Exacerbated Cardiotoxicity in Multiple Myeloma. Cancers 2023, 15, 2191. https://doi.org/10.3390/cancers15072191
Paterek A, Oknińska M, Pilch Z, Sosnowska A, Ramji K, Mackiewicz U, Golab J, Nowis D, Mączewski M. Arginase Inhibition Mitigates Bortezomib-Exacerbated Cardiotoxicity in Multiple Myeloma. Cancers. 2023; 15(7):2191. https://doi.org/10.3390/cancers15072191
Chicago/Turabian StylePaterek, Aleksandra, Marta Oknińska, Zofia Pilch, Anna Sosnowska, Kavita Ramji, Urszula Mackiewicz, Jakub Golab, Dominika Nowis, and Michał Mączewski. 2023. "Arginase Inhibition Mitigates Bortezomib-Exacerbated Cardiotoxicity in Multiple Myeloma" Cancers 15, no. 7: 2191. https://doi.org/10.3390/cancers15072191
APA StylePaterek, A., Oknińska, M., Pilch, Z., Sosnowska, A., Ramji, K., Mackiewicz, U., Golab, J., Nowis, D., & Mączewski, M. (2023). Arginase Inhibition Mitigates Bortezomib-Exacerbated Cardiotoxicity in Multiple Myeloma. Cancers, 15(7), 2191. https://doi.org/10.3390/cancers15072191