Characteristics of Cancer-Related Fatigue and an Efficient Model to Identify Patients with Gynecological Cancer Seeking Fatigue-Related Management
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Statistics
3. Results
4. Discussion
4.1. Summary of the Main Results
4.2. The Other Predictive Model
4.3. Results in the Context of Published Literature
4.4. Treatment for Cancer-Related Fatigue
4.5. Strengths and Weaknesses
4.6. Implications for Practice and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taiwan Cancer Registry Annual Report 2019. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=14913 (accessed on 20 January 2023).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network [NCCN guideline]. Uterine Neoplasm (Version 1. 2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 20 January 2023).
- National Health Research Institute. Taiwan Cooperative Oncology Group Clinical Practice Guideline of Gynaecologic Oncology. Available online: https://tcog.nhri.org.tw/wp-content/uploads/2020/05/100gogpg.pdf (accessed on 20 January 2023).
- National Comprehensive Cancer Network Clinical Practice Guideline in Oncology. Cervical Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 19 December 2018).
- NCCN Clinical Practice Guideline in Oncology, Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 1.2020—11 March 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 20 January 2023).
- Hofman, M.; Morrow, G.R.; Roscoe, J.A.; Hickok, J.T.; Mustian, K.M.; Moore, D.F.; Wade, J.L.; Fitch, T.R. Cancer patients’ expectations of experiencing treatment-related side effects. Cancer 2004, 101, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Barsevick, A.M.; Cleeland, C.S.; Manning, D.C.; O’Mara, A.M.; Reeve, B.B.; Scott, J.A.; Sloan, J.A. ASCPRO Recommendations for the Assessment of Fatigue as an Outcome in Clinical Trials. J. Pain Symptom Manag. 2010, 39, 1086–1099. [Google Scholar] [CrossRef]
- Minton, O.; Berger, A.; Barsevick, A.; Cramp, F.; Goedendorp, M.; Mitchell, S.A.; Stone, P.C. Cancer-related fatigue and its impact on functioning. Cancer 2013, 119, 2124–2130. [Google Scholar] [CrossRef]
- Sekse, R.J.T.; Hufthammer, K.O.; Vika, M.E. Fatigue and quality of life in women treated for various types of gynaecological cancers: A cross-sectional study. J. Clin. Nurs. 2014, 24, 546–555. [Google Scholar] [CrossRef]
- Hofman, M.; Ryan, J.L.; Figueroa-Moseley, C.D.; Jean-Pierre, P.; Morrow, G.R. Cancer-Related Fatigue: The Scale of the Problem. Oncologist 2007, 12, 4–10. [Google Scholar] [CrossRef]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of Cancer-Related Fatigue on the Lives of Patients: New Findings From the Fatigue Coalition. Oncologist 2000, 5, 353–360. [Google Scholar] [CrossRef]
- Mock, V.; Atkinson, A.; Barsevick, A.; Cella, D.; Cimprich, B.; Cleeland, C.; Donnelly, J.; Eisenberger, M.A.; Escalante, C.; Hinds, P.; et al. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology 2000, 14, 151–161. [Google Scholar]
- Luckett, T.; King, M.; Butow, P.; Friedlander, M.; Paris, T. Assessing Health-Related Quality of Life in Gynecologic Oncology. Int. J. Gynecol. Cancer 2010, 20, 664–684. [Google Scholar] [CrossRef]
- Seyidova-Khoshknabi, D.; Walsh, D.; Davis, M.P. Review Article: A Systematic Review of Cancer-Related Fatigue Measurement Questionnaires. Am. J. Hosp. Palliat. Med. 2010, 28, 119–129. [Google Scholar] [CrossRef]
- Wang, X.S.; Woodruff, J.F. Cancer-related and treatment-related fatigue. Gynecol. Oncol. 2014, 136, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Agarwal, S.; Minhas, V.; Bhatnagar, S.; Mishra, S.; Kumar, V.; Bharati, S.J.; Gupta, N.; Khan, M.A. To assess the prevalence and predictors of cancer-related fatigue and its impact on quality of life in advanced cancer patients receiving palliative care in a tertiary care hospital: A cross-sectional descriptive study. Indian J. Palliat. Care 2020, 26, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.L.; Dalton, S.O.; Christensen, J.; Carlsen, K.; Ross, L.; Johansen, C. Factors correlated with fatigue in breast cancer survivors undergoing a rehabilitation course, Denmark, 2002–2005. Psycho-Oncology 2010, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Weis, J.; Brähler, E.; Härter, M.; Geue, K.; Ernst, J. Fatigue in cancer patients: Comparison with the general population and prognostic factors. Support. Care Cancer 2020, 28, 4517–4526. [Google Scholar] [CrossRef]
- Taiwan Society of Cancer Palliative Medicine. Management of Cancer-Related Fatigue—A Guide for Taiwan. Available online: https://www.wecare.org.tw/wp-content/uploads/2017/11/Cancer-Fatigue-%E7%AC%AC%E4%BA%8C%E5%88%B7%E6%89%8B%E5%86%8A.pdf (accessed on 4 July 2022).
- Mendoza, T.R.; Wang, X.S.; Cleeland, C.S.; Morrissey, M.; Johnson, B.A.; Wendt, J.K.; Huber, S.L. The Rapid Assessment of Fatigue Severity in Cancer Patients: Use of the Brief Fatigue Inventory. Cancer 1999, 85, 1186–1196. [Google Scholar] [CrossRef]
- Yanez, B.; Pearman, T.; Lis, C.; Beaumont, J.; Cella, D. The FACT-G7: A rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann. Oncol. 2013, 24, 1073–1078. [Google Scholar] [CrossRef]
- McCorkle, R.; Young, K. Development of a symptom distress scale. Cancer Nurs. 1978, 1, 373–378. [Google Scholar] [CrossRef]
- Aoun, S.M.; Monterosso, L.; Kristjanson, L.J.; McConigley, R. Measuring Symptom Distress in Palliative Care: Psychometric Properties of the Symptom Assessment Scale (SAS). J. Palliat. Med. 2011, 14, 315–321. [Google Scholar] [CrossRef]
- Chen, H.W.; Lin, I.H.; Chen, Y.J.; Chang, K.H.; Wu, M.H.; Su, W.H.; Huang, G.C.; Lai, Y.L. A novel infusible botanically-derived drug, PG2, for cancer-related fatigue: A phase II double-blind, randomized placebo-controlled study. Clin. Investig. Med. 2012, 35, 1–11. [Google Scholar] [CrossRef]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2020, 73, 572. [Google Scholar] [CrossRef] [PubMed]
- Poort, H.; De Rooij, B.H.; Uno, H.; Weng, S.; Ezendam, N.P.M.; Van De Poll-Franse, L.; Wright, A.A. Patterns and predictors of cancer-related fatigue in ovarian and endometrial cancers: 1-year longitudinal study. Cancer 2020, 126, 3526–3533. [Google Scholar] [CrossRef]
- Haghighat, S.; Akbari, M.E.; Holakouei, K.; Rahimi, A.; Montazeri, A. Factors predicting fatigue in breast cancer patients. Support. Care Cancer 2003, 11, 533–538. [Google Scholar] [CrossRef]
- Von Ah, D.M.; Kang, D.-H.; Carpenter, J.S. Predictors of Cancer-Related Fatigue in Women With Breast Cancer Before, During, and After Adjuvant Therapy. Cancer Nurs. 2008, 31, 134–144. [Google Scholar] [CrossRef]
- Hwang, S.S.; Chang, V.T.; Rue, M.; Kasimis, B. Multidimensional independent predictors of cancer-related fatigue. J. Pain Symptom Manag. 2003, 26, 604–614. [Google Scholar] [CrossRef]
- Jewett, P.I.; Teoh, D.; Petzel, S.; Lee, H.; Messelt, A.; Kendall, J.; Hatsukami, D.; Everson-Rose, S.A.; Blaes, A.H.; Vogel, R.I. Cancer-Related Distress: Revisiting the Utility of the National Comprehensive Cancer Network Distress Thermometer Problem List in Women With Gynecologic Cancers. JCO Oncol. Pr. 2020, 16, e649–e659. [Google Scholar] [CrossRef]
- Liavaag, A.H.; Dorum, A.; Fossa, S.D.; Tropé, C.; Dahl, A.A. Controlled study of fatigue, quality of life, and somatic and mental morbidity in epithelial ovarian cancer survivors: How lucky are the lucky ones? J. Clin. Oncol. 2007, 25, 2049–2056. [Google Scholar] [CrossRef]
- Medysky, M.E.; Temesi, J.; Culos-Reed, S.N.; Millet, G. Exercise, sleep and cancer-related fatigue: Are they related? Neurophysiol. Clin. 2017, 47, 111–122. [Google Scholar] [CrossRef]
- Velthuis, M.J.; Bussche, E.V.D.; May, A.M.; Gijsen, B.C.M.; Nijs, S.; Vlaeyen, J.W.S. Fear of movement in cancer survivors: Validation of the Modified Tampa Scale of Kinesiophobia-Fatigue. Psycho-Oncology 2011, 21, 762–770. [Google Scholar] [CrossRef]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2015, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, CD006145. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.K.; Kohli, S.; Mustian, K.M.; Roscoe, J.A.; Morrow, G.R. Pharmacologic Treatment of Cancer-Related Fatigue. Oncologist 2007, 12, 43–51. [Google Scholar] [CrossRef]
- Minton, O.; Richardson, A.; Sharpe, M.; Hotopf, M.; Stone, P. A Systematic Review and Meta-Analysis of the Pharmacological Treatment of Cancer-Related Fatigue. Gynecol. Oncol. 2008, 100, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.B.; Donovan, K.A.; Vadaparampil, S.T.; Small, B.J. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007, 26, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Poort, H.; Peters, M.; Bleijenberg, G.; Gielissen, M.F.; Goedendorp, M.M.; Jacobsen, P.; Verhagen, S.; Knoop, H. Psychosocial interventions for fatigue during cancer treatment with palliative intent. Cochrane Database Syst. Rev. 2017, 7, CD012030. [Google Scholar] [CrossRef] [PubMed]
| Patients (N = 190) | |
|---|---|
| Age, mean ± SD | 56.87 ± 11.89 |
| Cancer type, n (%) | |
| Endometrium cancer | 76 (40.0) |
| Cervical cancer | 55 (28.9) |
| Ovarian cancer | 59 (31.1) |
| FIGO stage, n (%) | |
| I | 95 (50.0) |
| II | 29 (15.3) |
| III | 45 (23.7) |
| IV | 21 (11.1) |
| ECOG performance status, n (%) | |
| 0 | 55 (28.9) |
| 1 | 122 (64.2) |
| 2 | 12 (6.3) |
| 3 | 1 (0.5) |
| Current disease condition, n (%) | |
| Complete response | 48 (25.3) |
| Partial response | 3 (1.6) |
| Stable disease | 134 (70.5) |
| Progressive disease | 5 (2.6) |
| ICD-10 diagnosed fatigue, n (%) | |
| No fatigue | 90 (47.4) |
| Non-cancer-related fatigue | 81 (42.6) |
| CRF | 19 (10.0) |
| BFI-T questionnaire-based fatigue, n (%) | |
| No: 0 | 93 (48.9) |
| Mild: 1–3 | 61 (32.1) |
| Moderate to severe: ≥4 | 36 (18.9) |
| Fatigue-related management, n (%) | |
| Never | 42 (22.1) |
| Receive limited (≤5) managements | 65 (34.2) |
| Receive multiple (>5) managements | 83 (43.7) |
| Fatigue-Related Management | ||||
|---|---|---|---|---|
| 0 (n = 42) | 1 (n = 65) | 2 (n = 83) | p-Value | |
| Age, years, mean ± SD | 57.96 ± 8.97 | 58.1 ± 12.96 | 55.35 ± 12.25 | 0.3021 |
| ≥60, n (%) | 22 (52.4) | 33 (50.8) | 52 (62.7) | |
| <60, n (%) | 20 (47.6) | 32 (49.2) | 31 (37.3) | 0.2965 |
| Cancer type, n (%) | ||||
| Endometrial cancer | 5 (11.9) | 26 (40.0) | 45 (54.2) | |
| Cervical cancer | 8 (19.0) | 22 (33.8) | 25 (30.1) | |
| Ovarian cancer | 29 (69.0) | 17 (26.2) | 13 (15.7) | <0.0001 |
| FIGO stage, n (%) | ||||
| I | 39 (92.9) | 29 (44.6) | 27 (32.5) | <0.0001 |
| II | 1 (2.4) | 15 (23.1) | 13 (15.7) | |
| III | 1 (2.4) | 15 (23.1) | 29 (34.9) | |
| IV | 1 (2.4) | 6 (9.2) | 14 (16.9) | |
| ECOG, n (%) | ||||
| 0 | 26 (61.9) | 15 (23.1) | 14 (16.9) | <0.0001 |
| 1 | 16 (38.1) | 46 (70.8) | 60 (72.3) | |
| 2 | 0 (0.0) | 3 (4.6) | 9 (10.8) | |
| 3 | 0 (0.0) | 1 (1.5) | 0 (0.0) | |
| Current disease condition, n (%) | ||||
| Complete response + partial response | 26 (61.9) | 11 (16.9) | 14 (16.9) | <0.0001 |
| Stable disease + progressive disease | 16 (38.1) | 54 (83.1) | 69 (83.1) | |
| ICD-10-diagnosed fatigue, n (%) | ||||
| No fatigue | 27 (64.3) | 29 (44.6) | 34 (41.0) | |
| Non-cancer-related fatigue | 13 (31.0) | 27 (41.5) | 41 (49.4) | |
| CRF | 2 (4.8) | 9 (13.8) | 8 (9.6) | 0.1016 |
| BFI-T questionnaire-based fatigue, n (%) | ||||
| No: 0 | 27 (64.3) | 28 (43.1) | 38 (45.8) | |
| Mild: 1–3 | 13 (31.0) | 21 (32.3) | 27 (32.5) | |
| Moderate to severe: ≥4 | 2 (4.8) | 16 (24.6) | 18 (21.7) | 0.0731 |
| FACT-G7, mean ± SD | ||||
| Total score | 24.00 ± 3.13 | 20.28 ± 4.67 | 20.90 ± 5.62 | 0.0004 |
| Physical well-being | 10.38 ± 1.74 | 9.03 ± 2.23 | 9.10 ± 2.63 | 0.0061 |
| Emotional well-being | 3.33 ± 0.69 | 2.58 ± 0.95 | 2.78 ± 1.12 | 0.0006 |
| Functional well-being | 10.29 ± 1.38 | 8.66 ± 2.28 | 9.02 ± 2.63 | 0.0014 |
| Cancer treatment in recent 1 week, n (%) | ||||
| No | 42 (100.0) | 42 (64.6) | 54 (65.1) | |
| Yes | 0 (0.0) | 23 (35.4) | 29 (34.9) | <0.0001 |
| Fatigue-Related Management | 1 vs. 0 | 2 vs. 0 | 2 vs. 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 190) | 0 (n = 42) | 1 (n = 65) | 2 (n = 83) | p-Value * | Difference Ls-Mean (95% CI) | p-Value | Difference Ls-Mean (95% CI) | p-Value | Difference Ls-Mean (95% CI) | p-Value | |
| Cancer-related symptoms, mean ± SD | 13.04 ± 16.17 | 5.74 ± 8.62 | 15.17 ± 14.97 | 15.07 ± 18.85 | 0.0004 | 4.41 (−2.83, 11.64) | 0.2816 | 2.77 (−4.75, 10.28) | 0.5929 | −1.64 (−7.25, 3.96) | 0.7541 |
| Pain | 0.86 ± 2.04 | 0.29 ± 1.29 | 1.03 ± 2.25 | 1.02 ± 2.15 | 0.0471 | 0.29 (−0.65, 1.24) | 0.6812 | 0.07 (−0.91, 1.06) | 0.9765 | −0.22 (−0.96, 0.51) | 0.7412 |
| Fatigue | 2.21 ± 2.63 | 0.83 ± 1.86 | 2.69 ± 2.62 | 2.53 ± 2.76 | 0.0002 | 1.56 (0.36, 2.76) | 0.0082 | 1.35 (0.10, 2.59) | 0.0317 | −0.21 (−1.14, 0.71) | 0.8390 |
| Nausea | 0.88 ± 2.13 | 0.07 ± 0.46 | 0.82 ± 2.04 | 1.35 ± 2.56 | 0.0015 | 0.19 (−0.74, 1.12) | 0.8423 | 0.50 (−0.47, 1.46) | 0.3887 | 0.31 (−0.41, 1.03) | 0.5519 |
| Vomiting | 0.55 ± 1.70 | 0.00 ± 0.00 | 0.46 ± 1.56 | 0.89 ± 2.12 | 0.0086 | 0.11 (−0.65, 0.86) | 0.9158 | 0.37 (−0.42, 1.15) | 0.4506 | 0.26 (−0.33, 0.84) | 0.5337 |
| Depression | 1.41 ± 2.35 | 1.00 ± 1.85 | 1.66 ± 2.66 | 1.41 ± 2.32 | 0.8007 | 0.43 (−0.67, 1.54) | 0.5557 | 0.07 (−1.07, 1.22) | 0.9830 | −0.36 (−1.22, 0.49) | 0.5598 |
| Constipation | 1.01 ± 2.19 | 0.88 ± 2.05 | 1.49 ± 2.68 | 0.69 ± 1.75 | 0.3448 | 0.32 (−0.72, 1.37) | 0.6801 | −0.61 (−1.69, 0.48) | 0.3340 | −0.93 (−1.74, −0.12) | 0.0200 |
| Alopecia | 1.15 ± 2.46 | 0.33 ± 1.22 | 1.88 ± 3.13 | 0.99 ± 2.19 | 0.0194 | 0.94 (−0.19, 2.07) | 0.1156 | −0.01 (−1.18, 1.17) | 0.9998 | −0.95 (−1.82, −0.07) | 0.0317 |
| Diarrhea | 0.53 ± 1.49 | 0.48 ± 1.40 | 0.45 ± 1.24 | 0.63 ± 1.71 | 0.8727 | −0.34 (−1.02, 0.35) | 0.4175 | −0.32 (−1.03, 0.39) | 0.4739 | 0.02 (−0.51, 0.55) | 0.9966 |
| Insomnia | 2.07 ± 2.84 | 1.50 ± 2.42 | 2.46 ± 3.12 | 2.05 ± 2.78 | 0.1922 | 0.65 (−0.71, 2.00) | 0.4359 | 0.19 (−1.22, 1.59) | 0.9282 | −0.46 (−1.51, 0.59) | 0.5348 |
| Shortness of breath | 0.62 ± 1.59 | 0.33 ± 1.18 | 0.45 ± 1.20 | 0.90 ± 1.96 | 0.2743 | −0.03 (−0.78, 0.73) | 0.9934 | 0.46 (−0.32, 1.25) | 0.3013 | 0.49 (−0.09, 1.08) | 0.1146 |
| Anorexia | 0.92 ± 2.16 | 0.02 ± 0.15 | 1.08 ± 2.41 | 1.24 ± 2.38 | 0.0012 | 0.38 (−0.60, 1.37) | 0.5588 | 0.34 (−0.68, 1.36) | 0.6472 | −0.04 (−0.80, 0.72) | 0.9887 |
| Weight loss | 0.41 ± 1.53 | 0.00 ± 0.00 | 0.25 ± 0.83 | 0.73 ± 2.15 | 0.0247 | −0.12 (−0.81, 0.58) | 0.8907 | 0.3 (−0.42, 1.02) | 0.5240 | 0.42 (−0.12, 0.95) | 0.1609 |
| Nutrition imbalance | 0.44 ± 1.65 | 0.00 ± 0.00 | 0.46 ± 1.74 | 0.64 ± 1.95 | 0.0506 | 0.01 (−0.77, 0.79) | 0.9991 | 0.06 (−0.75, 0.87) | 0.9773 | 0.05 (−0.56, 0.65) | 0.9794 |
| FACT-G7, mean ± SD | |||||||||||
| Total score | 21.37 ± 5.03 | 24.00 ± 3.13 | 20.28 ± 4.67 | 20.90 ± 5.62 | 0.0004 | −2.25 (−4.48, −0.03) | 0.0467 | −1.26 (−3.57, 1.06) | 0.3537 | 1.00 (−0.73, 2.72) | 0.3446 |
| Physical well-being | 9.36 ± 2.37 | 10.38 ± 1.74 | 9.03 ± 2.23 | 9.10 ± 2.63 | 0.0061 | −0.67 (−1.77, 0.43) | 0.2848 | −0.41 (−1.56, 0.73) | 0.6035 | 0.25 (−0.60, 1.11) | 0.7465 |
| Emotional well-being | 2.84 ± 1.01 | 3.33 ± 0.69 | 2.58 ± 0.95 | 2.78 ± 1.12 | 0.0006 | −0.62 (−1.07, −0.17) | 0.0053 | −0.38 (−0.85, 0.09) | 0.1210 | 0.23 (−0.12, 0.58) | 0.2451 |
| Functional well-being | 9.18 ± 2.35 | 10.29 ± 1.38 | 8.66 ± 2.28 | 9.02 ± 2.63 | 0.0014 | −0.97 (−2.00, 0.06) | 0.0668 | −0.46 (−1.53, 0.61) | 0.5010 | 0.51 (−0.29, 1.31) | 0.2740 |
| Fatigue-Related Management | 1 vs. 0 | 2 vs. 0 | 1 vs. 0 | 2 vs. 0 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n = 42) | 1 (n = 65) | 2 (n = 83) | p-Value | AUC (95% CI) | AUC (95% CI) | Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Cancer type, n (%) | 0.73 (0.64–0.82) | 0.80 (0.72–0.88) | ||||||||
| Ovarian cancer | 29 (69.0) | 17 (26.2) | 13 (15.7) | <0.0001 | 1.00 | 1.00 | ||||
| Cervical cancer | 8 (19.0) | 22 (33.8) | 25 (30.1) | 2.99 (0.85–10.49) | 0.7327 | 3.64 (1.03–12.94) | 0.9058 | |||
| Endometrial cancer | 5 (11.9) | 26 (40.0) | 45 (54.2) | 5.85 (1.52–22.51) | 0.0610 | 11.49 (3.04–43.48) | 0.0049 | |||
| FIGO Stage, n (%) | 0.74 (0.67–0.81) | 0.80 (0.74–0.87) | ||||||||
| I | 39 (92.9) | 29 (44.6) | 27 (32.5) | 1.00 | 1.00 | |||||
| >I | 3 (7.1) | 36 (55.4) | 56 (67.5) | <0.0001 | 10.92 (2.64–45.16) | 0.0010 | 15.42 (3.80–62.65) | 0.0001 | ||
| ECOG performance status, n (%) | 0.69 (0.60–0.78) | 0.73 (0.64–0.81) | ||||||||
| 0 | 26 (61.9) | 15 (23.1) | 14 (16.9) | 1.00 | 1.00 | |||||
| ≥1 | 16 (38.1) | 50 (76.9) | 69 (83.1) | <0.0001 | 1.00 (0.18–5.50) | 0.9987 | 2.58 (0.44–15.15) | 0.2934 | ||
| Current disease condition | 0.73 (0.64–0.81) | 0.73 (0.65–0.82) | ||||||||
| Complete response + partial response | 26 (61.9) | 11 (16.9) | 14 (16.9) | 1.00 | 1.00 | |||||
| Stable disease + progressive disease | 16 (38.1) | 54 (83.1) | 69 (83.1) | <0.0001 | 4.59 (0.82–25.82) | 0.9844 | 1.89 (0.32–11.17) | 0.9788 | ||
| Total score (FACT-G7) | 0.70 (0.62–0.78) | 0.65 (0.57–0.73) | ||||||||
| ≥22 | 35 (83.3) | 28 (43.1) | 44 (53.0) | 1.00 | 1.00 | |||||
| <22 | 7 (16.7) | 37 (56.9) | 39 (47.0) | 0.0002 | 9.09 (2.82–29.28) | 0.0002 | 5.63 (1.70–18.64) | 0.0047 | ||
| Cancer treatment in recent 1 week | 0.68 (0.62–0.74) | 0.67 (0.62–0.73) | ||||||||
| No | 42 (100.0) | 42 (64.6) | 54 (65.1) | 1.00 | 1.00 | |||||
| Yes * | 0 (0.0) † | 23 (35.4) | 29 (34.9) | <0.0001 | ||||||
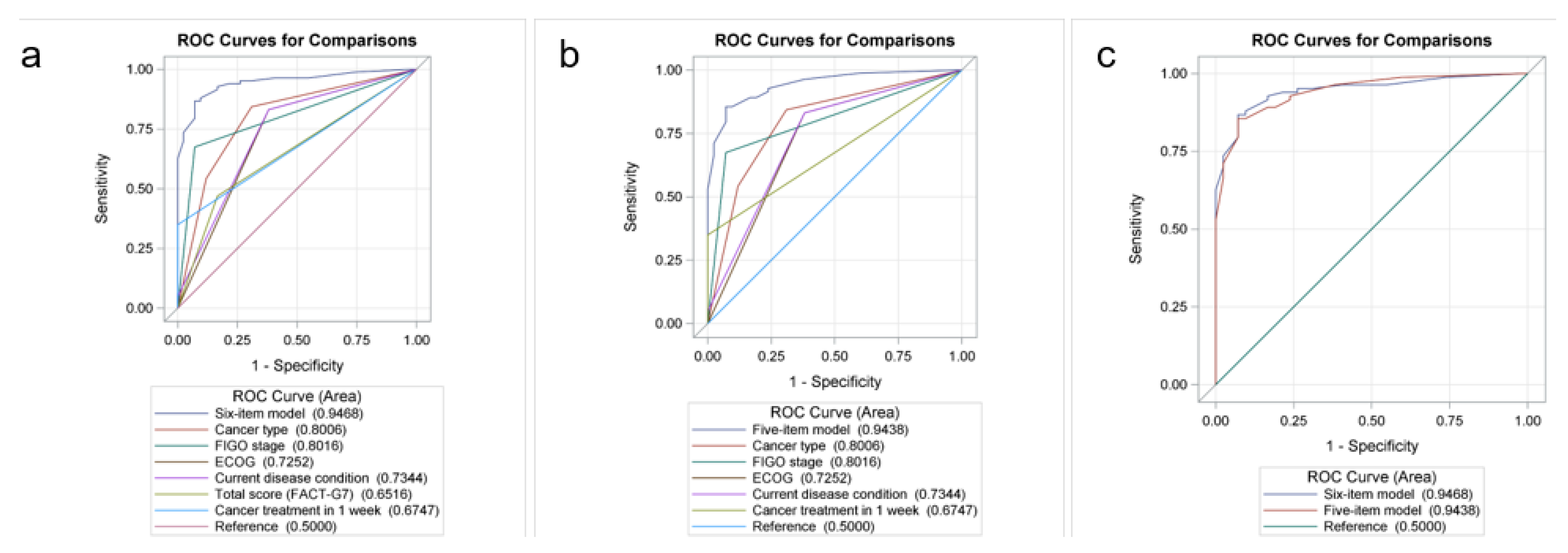
| AUC (95% CI): combined factors ‡ | 0.91 (0.86–0.97) | 0.95 (0.91–0.98) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-W.; Ou, Y.-C.; Lin, H.; Huang, K.-S.; Fu, H.-C.; Wu, C.-H.; Chen, Y.-Y.; Huang, S.-W.; Tu, H.-P.; Tsai, C.-C. Characteristics of Cancer-Related Fatigue and an Efficient Model to Identify Patients with Gynecological Cancer Seeking Fatigue-Related Management. Cancers 2023, 15, 2181. https://doi.org/10.3390/cancers15072181
Wang Y-W, Ou Y-C, Lin H, Huang K-S, Fu H-C, Wu C-H, Chen Y-Y, Huang S-W, Tu H-P, Tsai C-C. Characteristics of Cancer-Related Fatigue and an Efficient Model to Identify Patients with Gynecological Cancer Seeking Fatigue-Related Management. Cancers. 2023; 15(7):2181. https://doi.org/10.3390/cancers15072181
Chicago/Turabian StyleWang, Ying-Wen, Yu-Che Ou, Hao Lin, Kun-Siang Huang, Hung-Chun Fu, Chen-Hsuan Wu, Ying-Yi Chen, Szu-Wei Huang, Hung-Pin Tu, and Ching-Chou Tsai. 2023. "Characteristics of Cancer-Related Fatigue and an Efficient Model to Identify Patients with Gynecological Cancer Seeking Fatigue-Related Management" Cancers 15, no. 7: 2181. https://doi.org/10.3390/cancers15072181
APA StyleWang, Y.-W., Ou, Y.-C., Lin, H., Huang, K.-S., Fu, H.-C., Wu, C.-H., Chen, Y.-Y., Huang, S.-W., Tu, H.-P., & Tsai, C.-C. (2023). Characteristics of Cancer-Related Fatigue and an Efficient Model to Identify Patients with Gynecological Cancer Seeking Fatigue-Related Management. Cancers, 15(7), 2181. https://doi.org/10.3390/cancers15072181






