Pre- and Intraoperative Visualization of GRPR-Expressing Solid Tumors: Preclinical Profiling of Novel Dual-Modality Probes for Nuclear and Fluorescence Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Chemistry and Radiolabeling
2.2. Cell Culture
2.3. In Vitro Cell Uptake Assay
2.4. Animal Model and Experimental Design In Vivo Studies
2.5. Administration of [111In]In-12-15
2.6. In Vivo SPECT/CT/OI for the Biodistribution and Dose Optimization Studies
2.7. Ex Vivo Biodistribution and Optical Imaging
2.8. Ex Vivo Analysis of Xenografts
2.9. In Vitro Dead/Alive Cell Binding Assay
2.10. In Vivo Proof-of-Concept Image-Guided Surgery
2.11. Ex Vivo Autoradiography on Human Cancer Specimens
2.12. Statistics
3. Results
3.1. In Vitro Characterization
3.2. In Vivo Comparison of the Biodistribution Profiles
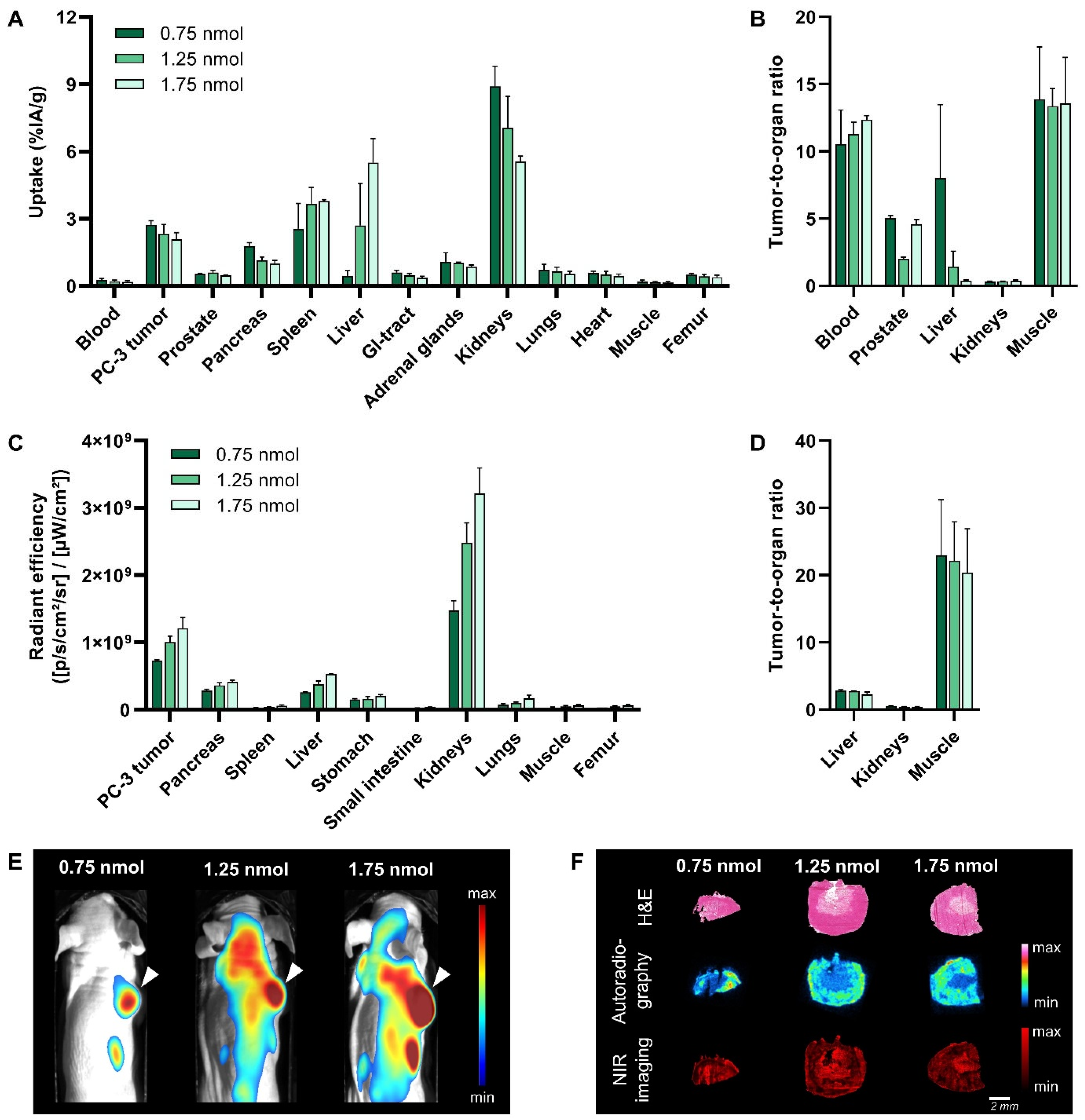
3.3. Dose Optimization
3.4. Translational Applicability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Orosco, R.K.; Tapia, V.J.; Califano, J.A.; Clary, B.; Cohen, E.E.W.; Kane, C.; Lippman, S.M.; Messer, K.; Molinolo, A.; Murphy, J.D.; et al. Positive Surgical Margins in the 10 Most Common Solid Cancers. Sci. Rep. 2018, 8, 5686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, B.; Zha, Z.; Zhao, H.; Jiang, Y.; Yuan, J. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: A meta-analysis from high-quality retrospective cohort studies. World J. Surg. Oncol. 2018, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Osarogiagbon, R.U.; Ray, M.A.; Faris, N.R.; Smeltzer, M.P.; Fehnel, C.; Houston-Harris, C.; Signore, R.S.; McHugh, L.M.; Levy, P.; Wiggins, L.; et al. Prognostic Value of National Comprehensive Cancer Network Lung Cancer Resection Quality Criteria. Ann. Thorac. Surg. 2017, 103, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Macaskill, P.; Luke Marinovich, M.; Morrow, M. The Association of Surgical Margins and Local Recurrence in Women with Early-Stage Invasive Breast Cancer Treated with Breast-Conserving Therapy: A Meta-Analysis. Ann. Surg. Oncol. 2014, 21, 717–730. [Google Scholar] [CrossRef]
- Mondal, S.B.; O’Brien, C.M.; Bishop, K.; Fields, R.C.; Margenthaler, J.A.; Achilefu, S. Repurposing Molecular Imaging and Sensing for Cancer Image-Guided Surgery. J. Nucl. Med. 2020, 61, 1113–1122. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Randall, L.M.; Chambers, S.K.; Butler, K.A.; Winer, I.S.; Langstraat, C.L.; Han, E.S.; Vahrmeijer, A.L.; Chon, H.S.; Morgan, M.A.; et al. A Phase III Study of Pafolacianine Injection (OTL38) for Intraoperative Imaging of Folate Receptor-Positive Ovarian Cancer (Study 006). J. Clin. Oncol. 2023, 41, 276–284. [Google Scholar] [CrossRef]
- Van Oosterom, M.N.; Rietbergen, D.D.D.; Welling, M.M.; Van Der Poel, H.G.; Maurer, T.; Van Leeuwen, F.W.B. Recent advances in nuclear and hybrid detection modalities for image-guided surgery. Expert Rev. Med. Devices 2019, 16, 711–734. [Google Scholar] [CrossRef]
- van Leeuwen, F.W.B.; Schottelius, M.; Brouwer, O.R.; Vidal-Sicart, S.; Achilefu, S.; Klode, J.; Wester, H.J.; Buckle, T. Trending: Radioactive and Fluorescent Bimodal/Hybrid Tracers as Multiplexing Solutions for Surgical Guidance. J. Nucl. Med. 2020, 61, 13–19. [Google Scholar] [CrossRef]
- Baratto, L.; Duan, H.; Mäcke, H.; Iagaru, A. Imaging the Distribution of Gastrin-Releasing Peptide Receptors in Cancer. J. Nucl. Med. 2020, 61, 792–798. [Google Scholar] [CrossRef]
- Mansi, R.; Nock, B.A.; Dalm, S.U.; Busstra, M.B.; van Weerden, W.M.; Maina, T. Radiolabeled Bombesin Analogs. Cancers 2021, 13, 5766. [Google Scholar] [CrossRef]
- Chen, H.; Wan, S.; Zhu, F.; Wang, C.; Cui, S.; Du, C.; Ma, Y.; Gu, Y. A fast tumor-targeting near-infrared fluorescent probe based on bombesin analog for in vivo tumor imaging. Contrast Media Mol. Imaging 2014, 9, 122–134. [Google Scholar] [CrossRef]
- Pagoto, A.; Garello, F.; Marini, G.M.; Tripepi, M.; Arena, F.; Bardini, P.; Stefania, R.; Lanzardo, S.; Valbusa, G.; Porpiglia, F.; et al. Novel Gastrin-Releasing Peptide Receptor Targeted Near-Infrared Fluorescence Dye for Image-Guided Surgery of Prostate Cancer. Mol. Imaging Biol. 2020, 22, 85–93. [Google Scholar] [CrossRef]
- Xu, H.; Bandari, R.P.; Lee, L.; Li, R.; Yu, P.; Smith, C.J.; Ma, L. Design, Synthesis, and in Vitro and in Vivo Evaluation of High Affinity and Specificity Near-Infrared Fluorescent Bombesin Antagonists for Tumor Imaging. J. Med. Chem. 2018, 61, 7657–7670. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Yu, P.; Besch-Williford, C.; Smith, C.J.; Sieckman, G.L.; Hoffman, T.J.; Ma, L. Near-infrared fluorescence imaging of gastrin releasing peptide receptor targeting in prostate cancer lymph node metastases. Prostate 2013, 73, 842–854. [Google Scholar] [CrossRef]
- Dalm, S.U.; Bakker, I.L.; de Blois, E.; Doeswijk, G.N.; Konijnenberg, M.W.; Orlandi, F.; Barbato, D.; Tedesco, M.; Maina, T.; Nock, B.A.; et al. 68Ga/177Lu-NeoBOMB1, a Novel Radiolabeled GRPR Antagonist for Theranostic Use in Oncology. J. Nucl. Med. 2017, 58, 293–299. [Google Scholar] [CrossRef]
- Kaloudi, A.; Lymperis, E.; Giarika, A.; Dalm, S.; Orlandi, F.; Barbato, D.; Tedesco, M.; Maina, T.; de Jong, M.; Nock, B.A. NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [(67)Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice. Molecules 2017, 22, 1950. [Google Scholar] [CrossRef]
- Handula, M.; Verhoeven, M.; Chen, K.-T.; Haeck, J.; de Jong, M.; Dalm, S.U.; Seimbille, Y. Towards Complete Tumor Resection: Novel Dual-Modality Probes for Improved Image-Guided Surgery of GRPR-Expressing Prostate Cancer. Pharmaceutics 2022, 14, 195. [Google Scholar] [CrossRef]
- de Blois, E.; Sze Chan, H.; Konijnenberg, M.; de Zanger, R.; Breeman, W.A.P. Effectiveness of Quenchers to Reduce Radiolysis of 111In- or 177Lu-Labelled Methionine-Containing Regulatory Peptides. Maintaining Radiochemical Purity as Measured by HPLC. Curr. Top. Med. Chem. 2012, 12, 2677–2685. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Baker, J.G.; Middleton, R.; Adams, L.; May, L.T.; Briddon, S.J.; Kellam, B.; Hill, S.J. Influence of fluorophore and linker composition on the pharmacology of fluorescent adenosine A1 receptor ligands. Br. J. Pharmacol. 2010, 159, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Desai, P.; Koike, Y.; Houghton, J.; Carlin, S.; Tandon, N.; Touijer, K.; Weber, W.A. Dual-Modality Imaging of Prostate Cancer with a Fluorescent and Radiogallium-Labeled Gastrin-Releasing Peptide Receptor Antagonist. J. Nucl. Med. 2017, 58, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Baranski, A.C.; Schäfer, M.; Bauder-Wüst, U.; Roscher, M.; Schmidt, J.; Stenau, E.; Simpfendörfer, T.; Teber, D.; Maier-Hein, L.; Hadaschik, B.; et al. PSMA-11-Derived Dual-Labeled PSMA Inhibitors for Preoperative PET Imaging and Precise Fluorescence-Guided Surgery of Prostate Cancer. J. Nucl. Med. 2018, 59, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Nock, B.A.; Kulkarni, H.; Singh, A.; Baum, R.P. Theranostic Prospects of Gastrin-Releasing Peptide Receptor-Radioantagonists in Oncology. PET Clin. 2017, 12, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, E.A.M.; Verhoeven, M.; Konijnenberg, M.W.; de Blois, E.; de Ridder, C.M.A.; Stuurman, D.C.; Bertarione, L.; Rolfo, K.; de Jong, M.; Dalm, S.U. Safety of [177Lu]Lu-NeoB treatment: A preclinical study characterizing absorbed dose and acute, early, and late organ toxicity. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4440–4451. [Google Scholar] [CrossRef]
- Hamann, F.M.; Brehm, R.; Pauli, J.; Grabolle, M.; Frank, W.; Kaiser, W.A.; Fischer, D.; Resch-Genger, U.; Hilger, I. Controlled Modulation of Serum Protein Binding and Biodistribution of Asymmetric Cyanine Dyes by Variation of the Number of Sulfonate Groups. Mol. Imaging 2011, 10, 258–269. [Google Scholar] [CrossRef]
- Chen, X.; Conti, P.S.; Moats, R.A. In vivo Near-Infrared Fluorescence Imaging of Integrin αvβ3 in Brain Tumor Xenografts. Cancer Res. 2004, 64, 8009–8014. [Google Scholar] [CrossRef]
- Bunschoten, A.; van Willigen, D.M.; Buckle, T.; van den Berg, N.S.; Welling, M.M.; Spa, S.J.; Wester, H.J.; van Leeuwen, F.W. Tailoring Fluorescent Dyes To Optimize a Hybrid RGD-Tracer. Bioconj. Chem. 2016, 27, 1253–1258. [Google Scholar] [CrossRef]
- Chen, Y.; Pullambhatla, M.; Banerjee, S.R.; Byun, Y.; Stathis, M.; Rojas, C.; Slusher, B.S.; Mease, R.C.; Pomper, M.G. Synthesis and Biological Evaluation of Low Molecular Weight Fluorescent Imaging Agents for the Prostate-Specific Membrane Antigen. Bioconj. Chem. 2012, 23, 2377–2385. [Google Scholar] [CrossRef]
- Buckle, T.; van Willigen, D.M.; Spa, S.J.; Hensbergen, A.W.; van der Wal, S.; de Korne, C.M.; Welling, M.M.; van der Poel, H.G.; Hardwick, J.C.H.; van Leeuwen, F.W.B. Tracers for Fluorescence-Guided Surgery: How Elongation of the Polymethine Chain in Cyanine Dyes Alters the Pharmacokinetics of a Dual-Modality c[RGDyK] Tracer. J. Nucl. Med. 2018, 59, 986–992. [Google Scholar] [CrossRef]
- Shrivastava, A.; Ding, H.; Kothandaraman, S.; Wang, S.H.; Gong, L.; Williams, M.; Milum, K.; Zhang, S.; Tweedle, M.F. A high-affinity near-infrared fluorescent probe to target bombesin receptors. Mol. Imaging Biol. 2014, 16, 661–669. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Guo, K.; Akers, W.; Livingston, J.; Solomon, M.; Lee, H.; Liang, K.; Agee, A.; Achilefu, S. Rational Approach To Select Small Peptide Molecular Probes Labeled with Fluorescent Cyanine Dyes for in Vivo Optical Imaging. Biochemistry 2011, 50, 2691–2700. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Hernandez Vargas, S.; Rodriguez, M.; Kossatz, S.; Voss, J.; Carmon, K.S.; Reiner, T.; Schonbrunn, A.; Azhdarinia, A. Synthesis of a Fluorescently Labeled (68)Ga-DOTA-TOC Analog for Somatostatin Receptor Targeting. ACS Med. Chem. Lett. 2017, 8, 720–725. [Google Scholar] [CrossRef]
- Stroet, M.C.M.; de Blois, E.; Stuurman, D.C.; de Ridder, C.M.A.; Haeck, J.; Seimbille, Y.; Mezzanotte, L.; de Jong, M.; Löwik, C.W.G.M.; Panth, K.M. In Vivo Evaluation of Indium-111–Labeled 800CW as a Necrosis-Avid Contrast Agent. Mol. Imaging Biol. 2020, 22, 1333–1341. [Google Scholar] [CrossRef]
- Kubeil, M.; Martínez, I.I.S.; Bachmann, M.; Kopka, K.; Tuck, K.L.; Stephan, H. Dual-Labelling Strategies for Nuclear and Fluorescence Molecular Imaging: Current Status and Future Perspectives. Pharmaceuticals 2022, 15, 432. [Google Scholar] [CrossRef]
- Lee, H.J.; McAuley, A.; Schilke, K.F.; McGuire, J. Molecular origins of surfactant-mediated stabilization of protein drugs. Adv. Drug Deliv. Rev. 2011, 63, 1160–1171. [Google Scholar] [CrossRef]
- Xie, B.; Stammes, M.A.; van Driel, P.B.A.A.; Cruz, L.J.; Knol-Blankevoort, V.T.; Löwik, M.A.M.; Mezzanotte, L.; Que, I.; Chan, A.; van den Wijngaard, J.P.H.M.; et al. Necrosis avid near infrared fluorescent cyanines for imaging cell death and their use to monitor therapeutic efficacy in mouse tumor models. Oncotarget 2015, 6, 39036–39049. [Google Scholar] [CrossRef]
- Stroet, M.C.M.; Dijkstra, B.M.; Dulfer, S.E.; Kruijff, S.; den Dunnen, W.F.A.; Kruyt, F.A.E.; Groen, R.J.M.; Seimbille, Y.; Panth, K.M.; Mezzanotte, L.; et al. Necrosis binding of Ac-Lys(0)(IRDye800CW)-Tyr(3)-octreotate: A consequence from cyanine-labeling of small molecules. EJNMMI Res. 2021, 11, 47. [Google Scholar] [CrossRef]
- Richards, C.H.; Mohammed, Z.; Qayyum, T.; Horgan, P.G.; McMillan, D.C. The prognostic value of histological tumor necrosis in solid organ malignant disease: A systematic review. Future Oncol. 2011, 7, 1223–1235. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Chi, C.; Xiao, X.; Wang, J.; Lang, L.; Ali, I.; Niu, G.; Zhang, L.; Tian, J.; et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using (68)Ga-IRDye800CW-BBN. Theranostics 2018, 8, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, R.; Das Saha, N.; Pahwa, M.; Rao, S.; Joshi, D.; Inamdar, M.S.; Sheeba, V.; Agasti, S.S. Synthetic Host–Guest Assembly in Cells and Tissues: Fast, Stable, and Selective Bioorthogonal Imaging via Molecular Recognition. Anal. Chem. 2018, 90, 11305–11314. [Google Scholar] [CrossRef] [PubMed]

 ; right shoulder) and GRPR-negative NCI-H69 (
; right shoulder) and GRPR-negative NCI-H69 ( ; left shoulder) tumor-bearing mice at 24 h post injection of [111In]In-12-15 (20 MBq/1 nmol). SPECT/CT images represent an overlay of a CT slice and the corresponding SPECT slice on which the tumor cross-sections are clearly visible. The arrow heads point to the location of the tumor.
; left shoulder) tumor-bearing mice at 24 h post injection of [111In]In-12-15 (20 MBq/1 nmol). SPECT/CT images represent an overlay of a CT slice and the corresponding SPECT slice on which the tumor cross-sections are clearly visible. The arrow heads point to the location of the tumor.
 ; right shoulder) and GRPR-negative NCI-H69 (
; right shoulder) and GRPR-negative NCI-H69 ( ; left shoulder) tumor-bearing mice at 24 h post injection of [111In]In-12-15 (20 MBq/1 nmol). SPECT/CT images represent an overlay of a CT slice and the corresponding SPECT slice on which the tumor cross-sections are clearly visible. The arrow heads point to the location of the tumor.
; left shoulder) tumor-bearing mice at 24 h post injection of [111In]In-12-15 (20 MBq/1 nmol). SPECT/CT images represent an overlay of a CT slice and the corresponding SPECT slice on which the tumor cross-sections are clearly visible. The arrow heads point to the location of the tumor.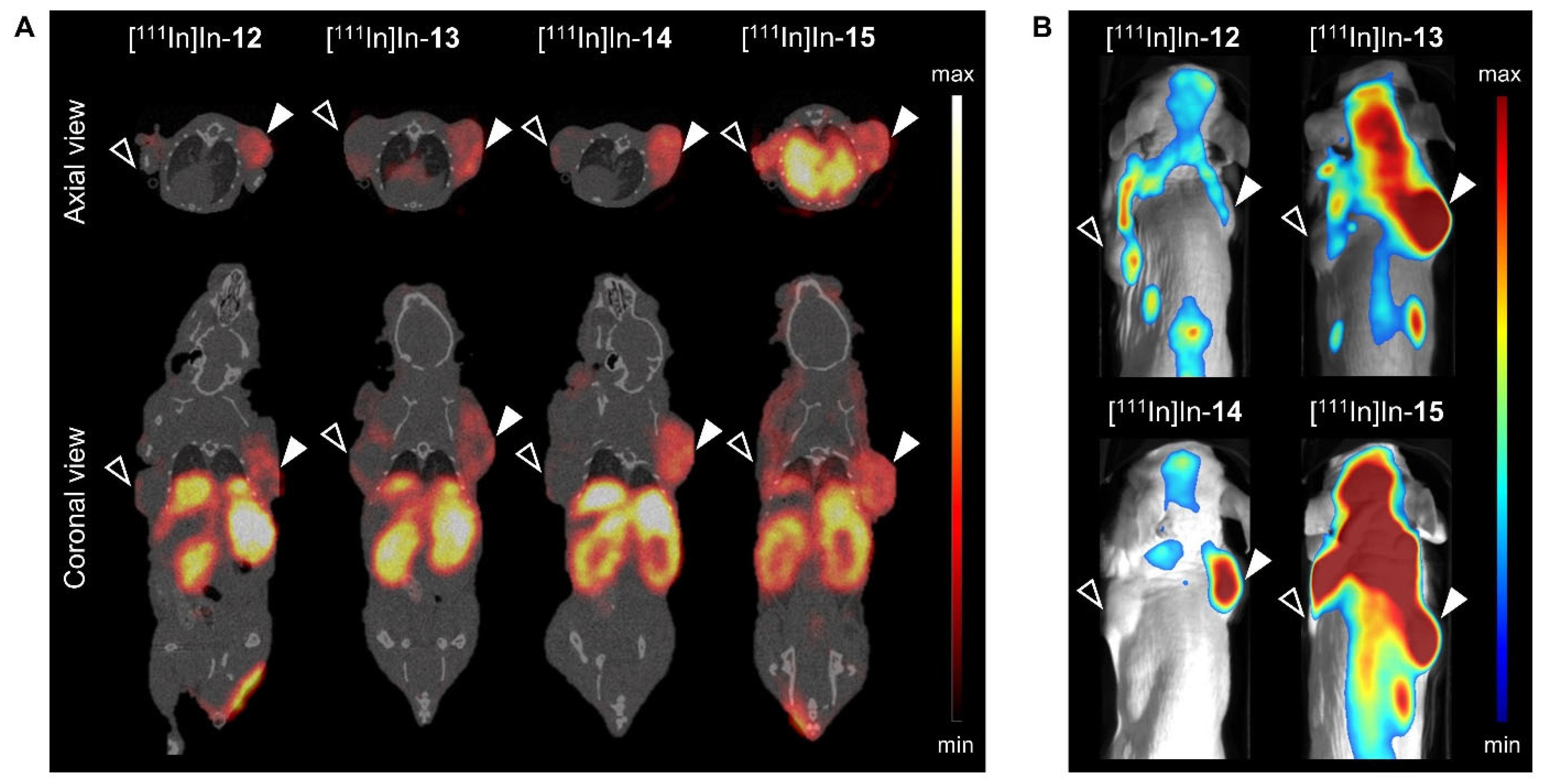
 ; right shoulder) and NCI-H69 (
; right shoulder) and NCI-H69 ( ; left shoulder) tumor-bearing mice at 24 h post injection 20 MBq/0.75 nmol [111In]In-14. Shown are a preoperative SPECT/CT scan (left panel), post-mortem merged photograph and fluorescence images obtained prior to, during and after PC-3 tumor resection (middle panel), plus a final image to check the surgical margins with a more sensitive scale (right panel). The fluorescent signal is displayed as average radiant efficiency in p/sec/cm2/sr per μW/cm2. For the SPECT/CT scan, a maximum intensity projection is shown in combination with an axial SPECT/CT image representing an overlay of a CT slice and the corresponding SPECT slice on which the tumor cross-sections are clearly visible. The arrow heads point to the location of the tumor.
; left shoulder) tumor-bearing mice at 24 h post injection 20 MBq/0.75 nmol [111In]In-14. Shown are a preoperative SPECT/CT scan (left panel), post-mortem merged photograph and fluorescence images obtained prior to, during and after PC-3 tumor resection (middle panel), plus a final image to check the surgical margins with a more sensitive scale (right panel). The fluorescent signal is displayed as average radiant efficiency in p/sec/cm2/sr per μW/cm2. For the SPECT/CT scan, a maximum intensity projection is shown in combination with an axial SPECT/CT image representing an overlay of a CT slice and the corresponding SPECT slice on which the tumor cross-sections are clearly visible. The arrow heads point to the location of the tumor.
 ; right shoulder) and NCI-H69 (
; right shoulder) and NCI-H69 ( ; left shoulder) tumor-bearing mice at 24 h post injection 20 MBq/0.75 nmol [111In]In-14. Shown are a preoperative SPECT/CT scan (left panel), post-mortem merged photograph and fluorescence images obtained prior to, during and after PC-3 tumor resection (middle panel), plus a final image to check the surgical margins with a more sensitive scale (right panel). The fluorescent signal is displayed as average radiant efficiency in p/sec/cm2/sr per μW/cm2. For the SPECT/CT scan, a maximum intensity projection is shown in combination with an axial SPECT/CT image representing an overlay of a CT slice and the corresponding SPECT slice on which the tumor cross-sections are clearly visible. The arrow heads point to the location of the tumor.
; left shoulder) tumor-bearing mice at 24 h post injection 20 MBq/0.75 nmol [111In]In-14. Shown are a preoperative SPECT/CT scan (left panel), post-mortem merged photograph and fluorescence images obtained prior to, during and after PC-3 tumor resection (middle panel), plus a final image to check the surgical margins with a more sensitive scale (right panel). The fluorescent signal is displayed as average radiant efficiency in p/sec/cm2/sr per μW/cm2. For the SPECT/CT scan, a maximum intensity projection is shown in combination with an axial SPECT/CT image representing an overlay of a CT slice and the corresponding SPECT slice on which the tumor cross-sections are clearly visible. The arrow heads point to the location of the tumor.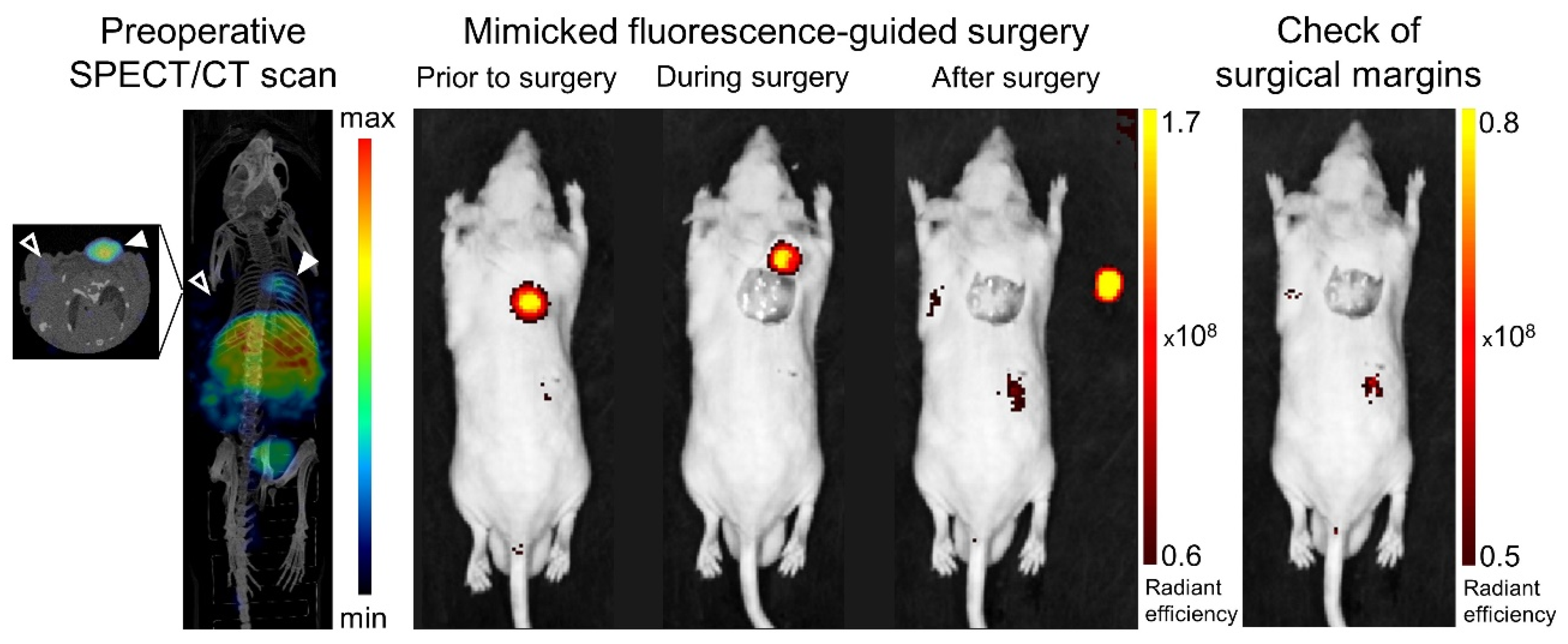

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoeven, M.; Handula, M.; van den Brink, L.; de Ridder, C.M.A.; Stuurman, D.C.; Seimbille, Y.; Dalm, S.U. Pre- and Intraoperative Visualization of GRPR-Expressing Solid Tumors: Preclinical Profiling of Novel Dual-Modality Probes for Nuclear and Fluorescence Imaging. Cancers 2023, 15, 2161. https://doi.org/10.3390/cancers15072161
Verhoeven M, Handula M, van den Brink L, de Ridder CMA, Stuurman DC, Seimbille Y, Dalm SU. Pre- and Intraoperative Visualization of GRPR-Expressing Solid Tumors: Preclinical Profiling of Novel Dual-Modality Probes for Nuclear and Fluorescence Imaging. Cancers. 2023; 15(7):2161. https://doi.org/10.3390/cancers15072161
Chicago/Turabian StyleVerhoeven, Marjolein, Maryana Handula, Lilian van den Brink, Corrina M. A. de Ridder, Debra C. Stuurman, Yann Seimbille, and Simone U. Dalm. 2023. "Pre- and Intraoperative Visualization of GRPR-Expressing Solid Tumors: Preclinical Profiling of Novel Dual-Modality Probes for Nuclear and Fluorescence Imaging" Cancers 15, no. 7: 2161. https://doi.org/10.3390/cancers15072161
APA StyleVerhoeven, M., Handula, M., van den Brink, L., de Ridder, C. M. A., Stuurman, D. C., Seimbille, Y., & Dalm, S. U. (2023). Pre- and Intraoperative Visualization of GRPR-Expressing Solid Tumors: Preclinical Profiling of Novel Dual-Modality Probes for Nuclear and Fluorescence Imaging. Cancers, 15(7), 2161. https://doi.org/10.3390/cancers15072161






