Prognostic Role of the Intrahepatic Lymphatic System in Liver Cancer
Simple Summary
Abstract
1. Introduction
2. Methods
Literature Search and Meta-Analyses
3. Results
3.1. Intrahepatic Lymphatic Vessels
3.2. Intrahepatic Lymphatic Vessels and Liver Cirrhosis
3.3. HCC
3.4. ICC
| Author | Published Year | Study Period | LVD | Patient Number | Tumor Size, cm | Tumor Number | Vascular Invasion | Poor Differential Pathology | Cirrhosis | LNM | 5-year OS | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC | Thelen et al. [53] | 2009 | 1997–1998 | >22.9 vessels (200× view) | 24 | T1/2, 5 (21%) | multiple, 8 (33%) | 11 (46%) | 5 (21%) | 16 (67%) | 2 (3%) | 24% | 0.018 |
| ≤22.9 vessels (200× view) | 36 | T1/2, 12 (33%) | multiple, 11 (31%) | 18 (50%) | 7 (19%) | 8 (22%) | NR | 56% | |||||
| ICC | Thelen at al. [62] | 2010 | NR | >12.66 vessels (200× view) | 46 | >5, 32 (70%) | multiple, 21 (46%) | 13 (28%) | 17 (37%) | NR | 27 (59%) | 6.5% | <0.001 |
| ≤12.66 vessels (200× view) | 68 | >5, 49 (72%) | multiple, 21 (31%) | 17 (25%) | 27 (40%) | 26 (38%) | 31% | ||||||
| Sha et al. [79] | 2019 | 2007–2015 | ≥13 vessels (400× view) | 50 | ≥5, 32 (64.0%) | multiple, 11 (22.0%) | 13 (26.0%) | 27 (54%) | 24 (40%) | 32 (64.0%) | 0% | <0.001 | |
| <13 vessels (400× view) | 56 | ≥5, 29 (51.8%) | multiple, 6 (10.7%) | 15 (26.8%) | 27 (48.2%) | NR | 16 (28.6%) | 48% |
3.5. CRLM
3.6. Association between LVI and LNM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ohtani, O.; Ohtani, Y. Lymph Circulation in the Liver. Anat. Rec. 2008, 291, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Iwakiri, Y. The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Banerjee, P.; Ekser, B.; Bayless, K.; Zawieja, D.; Alpini, G.; Glaser, S.S.; Chakraborty, S. Targeting Lymphangiogenesis and Lymph Node Metastasis in Liver Cancer. Am. J. Pathol. 2021, 191, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Trutmann, M.; Sasse, D. The lymphatics of the liver. Anat. Embryol. 1994, 190, 201–209. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- Altekruse, S.F.; Devesa, S.S.; A Dickie, L.; McGlynn, K.A.; Kleiner, D.E. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J. Regist. Manag. 2011, 38, 201–205. [Google Scholar]
- Aliseda, D.; Martí-Cruchaga, P.; Zozaya, G.; Rodríguez-Fraile, M.; Bilbao, J.I.; Benito-Boillos, A.; De La Cuesta, A.M.; Lopez-Olaondo, L.; Hidalgo, F.; Ponz-Sarvisé, M.; et al. Liver Resection and Transplantation Following Yttrium-90 Radioembolization for Primary Malignant Liver Tumors: A 15-Year Single-Center Experience. Cancers 2023, 15, 733. [Google Scholar] [CrossRef]
- Sherman, M. Recurrence of Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 2045–2047. [Google Scholar] [CrossRef]
- Famularo, S.; Di Sandro, S.; Giani, A.; Lauterio, A.; Sandini, M.; De Carlis, R.; Buscemi, V.; Uggeri, F.; Romano, F.; Gianotti, L.; et al. Recurrence patterns after anatomic or parenchyma-sparing liver resection for hepa-tocarcinoma in a western population of cirrhotic patients. Ann. Surg. Oncol. 2018, 25, 3974–3981. [Google Scholar] [CrossRef] [PubMed]
- Erridge, S.; Pucher, P.H.; Markar, S.R.; Malietzis, G.; Athanasiou, T.; Darzi, A.; Sodergren, M.H.; Jiao, L.R. Meta-analysis of determinants of survival following treatment of recurrent hepato-cellular carcinoma. Br. J. Surg. 2017, 104, 1433–1442. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef]
- Spolverato, G.; Vitale, A.; Cucchetti, A.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Gamblin, T.C.; Maithel, S.K.; Sandroussi, C.; Bauer, T.W.; et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer 2015, 121, 3998–4006. [Google Scholar] [CrossRef]
- Sakamoto, K.; Ogawa, K.; Tohyama, T.; Ueno, Y.; Tamura, K.; Inoue, H.; Nakamura, T.; Watanabe, J.; Takai, A.; Takada, Y. Serosal invasion is a strong prognostic factor for hepatocellular carcinoma after hepatectomy. Hepatol. Res. 2018, 49, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Kamiyama, T.; Yokoo, H.; Orimo, T.; Wakayama, K.; Einama, T.; Kakisaka, T.; Kamachi, H.; Taketomi, A. Clinicopathological Characteristics of Hepatocellular Carcinoma with Microscopic Portal Venous Invasion and the Role of Anatomical Liver Resection in These Cases. World J. Surg. 2017, 41, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Kokudo, N.; Imamura, H.; Matsuyama, Y.; Aoki, T.; Minagawa, M.; Sano, K.; Sugawara, Y.; Takayama, T.; Makuuchi, M. Prognostic Impact of Anatomic Resection for Hepatocellular Carcinoma. Ann. Surg. 2005, 242, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ikai, I.; Hatano, E.; Hasegawa, S.; Fujii, H.; Taura, K.; Uyama, N.; Shimahara, Y. Prognostic Index for Patients with Hepatocellular Carcinoma Combined with Tumor Thrombosis in the Major Portal Vein. J. Am. Coll. Surg. 2006, 202, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Poon, R.T.; Abdalla, E.K.; Ikai, I.; Nagorney, D.M.; Belghiti, J.; Kianmanesh, R.; Ng, I.O.-L.; Curley, S.A.; Yamaoka, Y.; et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein inva-sion: Results of a multicenter study. Surgery 2005, 137, 403–410. [Google Scholar] [CrossRef]
- Izumi, R.; Shimizu, K.; Ii, T.; Yagi, M.; Matsui, O.; Nonomura, A.; Miyazaki, I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994, 106, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, M.; Makuuchi, M.; Takayama, T.; Ohtomo, K. Selection criteria for hepatectomy in patients with hepatocellular car-cinoma and portal vein tumor thrombus. Ann. Surg. 2001, 233, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Nagano, H. Surgical treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus. Hepatol. Res. 2017, 47, 957–962. [Google Scholar] [CrossRef]
- Shindoh, J.; Hasegawa, K.; Inoue, Y.; Ishizawa, T.; Nagata, R.; Aoki, T.; Sakamoto, Y.; Sugawara, Y.; Makuuchi, M.; Kokudo, N. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB 2013, 15, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, F.; Hayashi, M.; Miyamoto, Y.; Asakuma, M.; Shimizu, T.; Komeda, K.; Inoue, Y.; Uchiyama, K. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol. Res. 2013, 44, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, T.; Shirabe, K.; Aishima, S.; Wang, H.; Fujita, N.; Ninomiya, M.; Yamashita, Y.; Ikegami, T.; Uchiyama, H.; Yoshizumi, T.; et al. New pathologic stratification of microvascular invasion in hepatocellular carcinoma: Predicting prognosis after living-donor liver transplantation. Transplantation 2015, 99, 1236–1242. [Google Scholar] [CrossRef]
- Zheng, J.; Seier, K.; Gonen, M.; Balachandran, V.P.; Kingham, T.P.; D’Angelica, M.I.; Allen, P.J.; Jarnagin, W.R.; DeMatteo, R.P. Utility of Serum Inflammatory Markers for Predicting Microvascular Invasion and Survival for Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2017, 24, 3706–3714. [Google Scholar] [CrossRef]
- Yang, P.; Qiu, J.; Li, J.; Wu, D.; Wan, X.; Lau, W.Y.; Yuan, Y.; Shen, F. Nomograms for pre- and postoperative prediction of long-term survival for patients who under-went hepatectomy for multiple hepatocellular carcinomas. Ann Surg 2016, 263, 778–786. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takasaki, K.; Otsubo, T.; Katsuragawa, H.; Katagiri, S. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. J. Hepato-Biliary-Pancreatic Surg. 2001, 8, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Thelen, A.; Benckert, C.; Biskup, W.; Neumann, U.; Rudolph, B.; Lopez-Häänninen, E.; Neuhaus, P. Extended liver resection for intrahepatic cholangiocarcinoma. A comparison of the prognostic accuracy of the fifth and six editions of the TNM classification. Ann. Surg. 2009, 249, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S. Intrahepatic cholangiocarcinoma: Macroscopic type and stage classification. J. Hepato-Biliary-Pancreatic Surg. 2003, 10, 288–291. [Google Scholar] [CrossRef]
- Shimada, M.; Yamashita, Y.; Aishima, S.; Shirabe, K.; Takenaka, K.; Sugimachi, K. Value of lymph node dissection during resection of intrahepatic cholangiocarci-noma. Br. J. Surg. 2001, 88, 1463–1466. [Google Scholar] [CrossRef]
- Choi, S.-B.; Kim, K.-S.; Choi, J.-Y.; Park, S.-W.; Lee, W.-J.; Chung, J.-B. The Prognosis and Survival Outcome of Intrahepatic Cholangiocarcinoma Following Surgical Resection: Association of Lymph Node Metastasis and Lymph Node Dissection with Survival. Ann. Surg. Oncol. 2009, 16, 3048–3056. [Google Scholar] [CrossRef]
- Nanashima, A.; Shibata, K.; Nakayama, T.; Tobinaga, S.; Araki, M.; Kunizaki, M.; Takeshita, H.; Hidaka, S.; Sawai, T.; Nagayasu, T. Relationship between vessel count and postoperative survival in patients with intrahepatic cholangiocarcinoma. Am. Surg. Oncol. 2009, 16, 2123–2129. [Google Scholar] [CrossRef]
- Yamashita, Y.-I.; Taketomi, A.; Morita, K.; Fukuhara, T.; Ueda, S.; Sanefuji, K.; Iguchi, T.; Kayashima, H.; Sugimachi, K.; Maehara, Y. The impact of surgical treatment and poor prognostic factors for patients with intrahepatic cholangiocarcinoma: Retrospective analysis of 60 patients. Anticancer. Res. 2008, 28, 2353–2360. [Google Scholar]
- Shirabe, K.; Mano, Y.; Taketomi, A.; Soejima, Y.; Uchiyama, H.; Aishima, S.; Kayashima, H.; Ninomiya, M.; Maehara, Y. Clinicopathological Prognostic Factors After Hepatectomy for Patients with Mass-Forming Type Intrahepatic Cholangiocarcinoma: Relevance of the Lymphatic Invasion Index. Ann. Surg. Oncol. 2010, 17, 1816–1822. [Google Scholar] [CrossRef]
- De Ridder, J.A.M.; Knijn, N.; Wiering, B.; De Wilt, J.H.W.; Nagtegaal, I.D. Lymphatic Invasion is an Independent Adverse Prognostic Factor in Patients with Colorectal Liver Metastasis. Ann. Surg. Oncol. 2015, 22, 638–645. [Google Scholar] [CrossRef][Green Version]
- Sasaki, A.; Aramaki, M.; Kawano, K.; Yasuda, K.; Inomata, M.; Kitano, S. Prognostic significance of intrahepatic lymphatic invasion in patients with hepatic resection due to metastases from colorectal carcinoma. Cancer 2002, 95, 105–111. [Google Scholar] [CrossRef]
- Lupinacci, R.M.; Mello, E.S.; Pinheiro, R.S.; Marques, G.; Coelho, F.F.; Kruger, J.A.P.; Perini, M.V.; Herman, P. Intrahepatic Lymphatic Invasion but not Vascular Invasion is a Major Prognostic Factor after Resection of Colorectal Cancer Liver Metastases. World J. Surg. 2014, 38, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Korita, P.V.; Wakai, T.; Shirai, Y.; Sakata, J.; Takizawa, K.; Cruz, P.V.; Ajioka, Y.; Hatakeyama, K. Intrahepatic Lymphatic Invasion Independently Predicts Poor Survival and Recurrences after Hepatectomy in Patients with Colorectal Carcinoma Liver Metastases. Ann. Surg. Oncol. 2007, 14, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.B.; Patel, S.H.; Kooby, D.A.; Weber, S.; Bloomston, M.; Cho, C.; Hatzaras, I.; Schmidt, C.; Winslow, E.; Staley, C.A.; et al. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: A multi-institution analysis. HPB 2012, 14, 514–522. [Google Scholar] [CrossRef]
- Cornwell, L.B.; Mcmasters, K.M.; Chagpar, A.B. The Impact of Lymphovascular Invasion on Lymph Node Status in Patients with Breast Cancer. Am. Surg. 2011, 77, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Shin, S.H.; Cho, J.S.; Park, M.H.; Yoon, J.H.; Jegal, Y.J. The role of lymphovascular invasion as a prognostic factor in pa-tients with lymph node-positive operable invasive breast cancer. J. Breast Cancer 2011, 14, 198–203. [Google Scholar] [CrossRef]
- Royston, D.; Jackson, D.G. Mechanisms of lymphatic metastasis in human colorectal adenocarcinoma. J. Pathol. 2009, 217, 608–619. [Google Scholar] [CrossRef]
- Gondo, T.; Nakashima, J.; Ozu, C.; Ohno, Y.; Horiguchi, Y.; Namiki, K.; Yoshioka, K.; Ohori, M.; Hatano, T.; Tachibana, M. Risk stratification of survival by lymphovascular invasion, pathological stage, and surgical margin in patients with bladder cancer treated with radical cystectomy. Int. J. Clin. Oncol. 2011, 17, 456–461. [Google Scholar] [CrossRef]
- Brunocilla, E.; Pernetti, R.; Martorana, G. The prognostic role of lymphovascular invasion in urothelial-cell carcinoma of upper and lower urinary tract. Anticancer. Res. 2011, 31, 3503–3506. [Google Scholar]
- Amini, N.; Ejaz, A.; Spolverato, G.; Maithel, S.K.; Kim, Y.; Pawlik, T.M. Management of Lymph Nodes During Resection of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: A Systematic Review. J. Gastrointest. Surg. 2014, 18, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wu, L.; Lau, W.-Y.; Li, G.; Huan, H.; Qian, C.; Ma, K.; Bie, P. Positive Lymph Node Metastasis Has a Marked Impact on the Long-Term Survival of Patients with Hepatocellular Carcinoma with Extrahepatic Metastasis. PLoS ONE 2014, 9, e95889. [Google Scholar] [CrossRef]
- Khan, S.A.; Davidson, B.R.; Goldin, R.; Heaton, N.; Karani, J.; Pereira, S.; Rosenberg, W.; Tait, P.; Taylor-Robinson, S.D.; Thillainayagam, A.V.; et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012, 61, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.M.; Ribero, D.; O’Reilly, E.M.; Kokudo, N.; Miyazaki, M.; Pawlik, T.M. Intrahepatic Cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Morine, Y.; Shimada, M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J. Gastroenterol. 2015, 50, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.; Klümpen, H.; Malka, D.; Primrose, J.; Rimassa, L.; Stenzinger, A.; Valle, J.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Makuuchi, M.; Kokudo, N.; Izumi, N.; Ichida, T.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; Matsui, O.; et al. Impact of histologically confirmed lymph node metastases on patient survival after surgical resection for hepatocellular carcinoma: Report of a Japanese nationwide survey. Ann. Surg. 2014, 259, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Xue, F.; Dong, D.-H.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann. Surg. 2020, 274, e1187–e1195. [Google Scholar] [CrossRef] [PubMed]
- Sugino, T.; Yamaguchi, T.; Hoshi, N.; Kusakabe, T.; Ogura, G.; Goodison, S.; Suzuki, T. Sinusoidal tumor angiogenesis is a key component in hepatocellular carcinoma metastasis. Clin. Exp. Metastasis 2008, 25, 835–841. [Google Scholar] [CrossRef]
- Thelen, A.; Jonas, S.; Benckert, C.; Weichert, W.; Schott, E.; Bötcher, C.; Dietz, E.; Wiedenmann, B.; Neuhaus, P.; Scholz, A. Tumor-Associated Lymphangiogenesis Correlates with Prognosis after Resection of Human Hepatocellular Carcinoma. Ann. Surg. Oncol. 2009, 16, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiology 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Inoue, K.; Makuuchi, M.; Takayama, T.; Torzilli, G.; Yamamoto, J.; Shimada, K.; Kosuge, T.; Yamasaki, S.; Konishi, M.; Kinoshita, T.; et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery 2000, 127, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell. Oncol. 2016, 39, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.A.; Cochrane Working Group On Meta-Analysis Using Individual Patient Data Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat. Med. 1995, 14, 2057–2079. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Yokomori, H.; Oda, M.; Kaneko, F.; Kawachi, S.; Tanabe, M.; Yoshimura, K.; Kitagawa, Y.; Hibi, T. Lymphatic marker podoplanin/D2-40 in human advanced cirrhotic liver- Re-evaluations of microlymphatic abnormalities. BMC Gastroenterol. 2010, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Aishima, S.; Nishihara, Y.; Iguchi, T.; Taguchi, K.; Taketomi, A.; Maehara, Y.; Tsuneyoshi, M. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod. Pathol. 2008, 21, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Thelen, A.; Scholz, A.; Weichert, W.; Wiedenmann, B.; Neuhaus, P.; Geßner, R.; Benckert, C.; Jonas, S. Tumor-Associated Angiogenesis and Lymphangiogenesis Correlate With Progression of Intrahepatic Cholangiocarcinoma. Am. J. Gastroenterol. 2010, 105, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, G.; Molmenti, E.; Fouzas, I.; Sgourakis, G.; Radtke, A.; Malagó, M.; Lang, H. Liver Transplantation for Hepatocellular Carcinoma With Intrahepatic Lymphatic Invasion: Case Reports. Transplant. Proc. 2008, 40, 3213–3214. [Google Scholar] [CrossRef]
- Cioca, A.; Ceausu, A.R.; Marin, I.; Raica, M.; Cimpean, A.M. The multifaceted role of podoplanin expression in hepatocellular carcinoma. Eur. J. Histochem. 2017, 61, 2707. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, Y.; Liu, X.; Cai, Y.; Luo, B. Innovative signature establishment using lymphangiogenesis-related lncRNA pairs to predict prognosis of hepatocellular carcinoma. Heliyon 2022, 8, e10215. [Google Scholar] [CrossRef]
- Thelen, A.; Scholz, A.; Benckert, C.; von Marschall, Z.; Schröder, M.; Wiedenmann, B.; Neuhaus, P.; Rosewicz, S.; Jonas, S. VEGF-D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int. J. Cancer 2008, 122, 2471–2481. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, J.; Wu, M.; Wang, H.-Y.; Arbuthnot, P.; Kew, M.; Feitelson, M.A. Hepatitis B x antigen up-regulates vascular endothelial growth factor receptor 3 in hepatocarcinogenesis. Hepatology 2007, 45, 1390–1399. [Google Scholar] [CrossRef]
- Yu, S.; Lv, H.; Zhang, H.; Jiang, Y.; Hong, Y.; Xia, R.; Zhang, Q.; Ju, W.; Jiang, L.; Ou, G.; et al. Heparanase-1-induced shedding of heparan sulfate from syndecan-1 in hepatocarcinoma cell facilitates lymphatic endothelial cell proliferation via VEGF-C/ERK pathway. Biochem. Biophys. Res. Commun. 2017, 485, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Geis, T.; Popp, R.; Hu, J.; Fleming, I.; Henke, N.; Dehne, N.; Brüne, B. HIF-2α attenuates lymphangiogenesis by up-regulating IGFBP1 in hepatocellular carcinoma. Biol. Cell 2015, 107, 175–188. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, X.; Xue, X.; Sun, D.; Cai, P.; Song, Q.; Zhang, B.; Qin, L. HANR promotes lymphangiogenesis of hepatocellular carcinoma via secreting miR-296 exosome and regulating EAG1/VEGFA signaling in HDLEC cells. J. Cell. Biochem. 2019, 120, 17699–17708. [Google Scholar] [CrossRef] [PubMed]
- Carreira, C.M.; Nasser, S.M.; di Tomaso, E.; Padera, T.P.; Boucher, Y.; Tomarev, S.I.; Jain, R.K. LYVE-1 is not restricted to the lymph vessels: Expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001, 61, 8079–8084. [Google Scholar]
- Lurje, G.; Bednarsch, J.; Czigany, Z.; Lurje, I.; Schlebusch, I.K.; Boecker, J.; Meister, F.A.; Tacke, F.; Roderburg, C.; Dulk, M.D.; et al. The prognostic role of lymphovascular invasion and lymph node metastasis in perihilar and intrahepatic cholangiocarcinoma. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 1468–1478. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.-J.; Kim, S.H.; Han, S.-S.; Kim, Y.-K.; Lee, K.-W.; Lee, S.-A.; Hong, E.K.; Lee, W.J.; Woo, S.M. Survival Analysis of Intrahepatic Cholangiocarcinoma After Resection. Ann. Surg. Oncol. 2010, 17, 1823–1830. [Google Scholar] [CrossRef]
- Lang, H.; Sotiropoulos, G.C.; Sgourakis, G.; Schmitz, K.J.; Paul, A.; Hilgard, P.; Zöpf, T.; Trarbach, T.; Malagó, M.; Baba, H.A.; et al. Operations for Intrahepatic Cholangiocarcinoma: Single-Institution Experience of 158 Patients. J. Am. Coll. Surg. 2009, 208, 218–228. [Google Scholar] [CrossRef]
- Nakajima, T.; Kondo, Y.; Miyazaki, M.; Okui, K. A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: Histologic classification and modes of spreading. Hum. Pathol. 1988, 19, 1228–1234. [Google Scholar] [CrossRef]
- Patel, S.H.; Kooby, D.A.; Staley, C.A.; Sarmiento, J.M.; Maithel, S.K. The prognostic importance of lymphovascular invasion in cholangiocarcinoma above the cystic duct: A new selection criterion for adjuvant therapy? HPB 2011, 13, 605–611. [Google Scholar] [CrossRef]
- Hong, J.C.; Petrowsky, H.; Kaldas, F.M.; Farmer, D.G.; Durazo, F.A.; Finn, R.S.; Saab, S.; Han, S.-H.; Lee, P.; Markovic, D.; et al. Predictive Index for Tumor Recurrence after Liver Transplantation for Locally Advanced Intrahepatic and Hilar Cholangiocarcinoma. J. Am. Coll. Surg. 2011, 212, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2007, 98, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Jeong, S.; Wang, X.; Tong, Y.; Cao, J.; Sun, H.-Y.; Xia, L.; Xu, N.; Xi, Z.-F.; Zhang, J.-J.; et al. Tumor-associated lymphangiogenesis predicts unfavorable prognosis of intrahepatic cholangiocarcinoma. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Sha, M.; Jeong, S.; Chen, X.-S.; Tong, Y.; Cao, J.; Sun, H.-Y.; Xia, L.; Xu, N.; Wang, X.; Han, L.-Z.; et al. Expression of VEGFR-3 in intrahepatic cholangiocarcinoma correlates with unfavorable prognosis through lymphangiogenesis. Int. J. Biol. Sci. 2018, 14, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, M.; Romanzi, A.; Guido, M.; Sarcognato, S.; Cillo, U.; Gringeri, E.; Zanus, G.; Strazzabosco, M.; Simioni, P.; Villa, E.; et al. Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma. J. Pers. Med. 2022, 12, 1086. [Google Scholar] [CrossRef]
- Fabris, L.; Perugorria, M.J.; Mertens, J.; Björkström, N.K.; Cramer, T.; Lleo, A.; Solinas, A.; Sänger, H.; Lukacs-Kornek, V.; Moncsek, A.; et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 63–78. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, M.; Brivio, S.; Mertens, J.; Vismara, M.; Moncsek, A.; Milani, C.; Fingas, C.; Malerba, M.C.; Nardo, G.; Dall’Olmo, L.; et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J. Hepatol. 2018, 70, 700–709. [Google Scholar] [CrossRef]
- Carpino, G.; Cardinale, V.; Di Giamberardino, A.; Overi, D.; Donsante, S.; Colasanti, T.; Amato, G.; Mennini, G.; Franchitto, M.; Conti, F.; et al. Thrombospondin 1 and 2 along with PEDF inhibit angiogenesis and promote lymphangiogenesis in intrahepatic cholangiocarcinoma. J. Hepatol. 2021, 75, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, W.; Bai, Y.; Wan, L.; Sun, X.; Liu, Y.; Xiong, W.; Zhang, Y.-Y.; Zhou, L. Oxyresveratrol prevents murine H22 hepatocellular carcinoma growth and lymph node metastasis via inhibiting tumor angiogenesis and lymphangiogenesis. J. Nat. Med. 2018, 72, 481–492. [Google Scholar] [CrossRef]
- Thelen, A.; Scholz, A.; Benckert, C.; Weichert, W.; Dietz, E.; Wiedenmann, B.; Neuhaus, P.; Jonas, S. Tumor-Associated Lymphangiogenesis Correlates with Lymph Node Metastases and Prognosis in Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2008, 15, 791–799. [Google Scholar] [CrossRef]
- Schoppmann, A.; Tamandl, D.; Herberger, B.; Längle, F.; Birner, P.; Geleff, S.; Gruenberger, T.; Schoppmann, S.F. Comparison of lymphangiogenesis between primary colorectal cancer and corresponding liver metastases. Anticancer. Res. 2011, 31, 4605–4611. [Google Scholar] [PubMed]
- Nathanson, S.D. Insights into the mechanisms of lymph node metastasis. Cancer 2003, 98, 413–423. [Google Scholar] [CrossRef]
- Lin, M.; Lin, H.-Z.; Ma, S.-P.; Ji, P.; Xie, D.; Yu, J.-X. Vascular endothelial growth factor-A and -C: Expression and correlations with lymphatic metastasis and prognosis in colorectal cancer. Med. Oncol. 2010, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Vellinga, T.T.; Kranenburg, O.; Frenkel, N.; Ubink, I.; Marvin, D.; Govaert, K.; van Schelven, S.; Hagendoorn, J.; Rinkes, I.H.B. Lymphangiogenic Gene Expression Is Associated With Lymph Node Recurrence and Poor Prognosis After Partial Hepatectomy for Colorectal Liver Metastasis. Ann. Surg. 2017, 266, 765–771. [Google Scholar] [CrossRef]
- Sonohara, F.; Nomoto, S.; Inokawa, Y.; Kanda, M.; Yamada, S.; Fujii, T.; Sugimoto, H.; Kodera, Y. Serosal invasion strongly associated with recurrence after curative hepatic resection of hepatocellular carcinoma: A retrospective study of 214 consecutive cases. Medicine 2015, 94, e602. [Google Scholar] [CrossRef]
- Kato, Y.; Okamura, Y.; Sugiura, T.; Ito, T.; Yamamoto, Y.; Ashida, R.; Uesaka, K. The Impact of Serosal Invasion on Prognosis after Curative Hepatectomy for Hepatocellular Carcinoma: Invasion to Adjacent Organs and Rupture of Tumor Were Crucial Tumor-Related Prognostic Factors Needed for Survival. Dig. Surg. 2017, 35, 155–163. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 14th ed.; Tokyo Kanehara and Co.: Kanehara, Japan, 2010. [Google Scholar]
- Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma, 8th ed.; Tokyo Kanehara and Co.: Kanehara, Japan, 2013. [Google Scholar]
- Japanese Society of Hepato-Biliary-Pancreatic Surgery. General Rules for the Clinical and Pathological Studies on Cancer of the Biliary Tract, 6th ed.; Tokyo Kanehara and Co.: Kanehara, Japan, 2013. [Google Scholar]
- Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 6th ed.; Tokyo Kanehara and Co.: Kanehara, Japan, 2015. [Google Scholar]
- Lee, C.-W.; Chan, K.-M.; Lee, C.-F.; Yu, M.-C.; Lee, W.-C.; Wu, T.-J.; Chen, M.-F. Hepatic Resection for Hepatocellular Carcinoma With Lymph Node Metastasis: Clinicopathological Analysis and Survival Outcome. Asian J. Surg. 2011, 34, 53–62. [Google Scholar] [CrossRef]
- Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treat-ment. Ann. Surg. 1990, 211, 277–287. [Google Scholar]
- Vauthey, J.-N.; Lauwers, G.Y.; Esnaola, N.F.; Do, K.-A.; Belghiti, J.; Mirza, N.; Curley, S.A.; Ellis, L.M.; Regimbeau, J.-M.; Rashid, A.; et al. Simplified Staging for Hepatocellular Carcinoma. J. Clin. Oncol. 2002, 20, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Changchien, C.-S.; Chen, C.-L.; Yen, Y.-H.; Wang, J.-H.; Hu, T.-H.; Lee, C.-M.; Wang, C.-C.; Cheng, Y.-F.; Huang, Y.-J.; Lin, C.-Y.; et al. Analysis of 6381 hepatocellular carcinoma patients in southern Taiwan: Prognostic features, treatment outcome, and survival. J. Gastroenterol. 2008, 43, 159–170. [Google Scholar] [CrossRef]
- Sun, H.-C.; Zhuang, P.-Y.; Qin, L.-X.; Ye, Q.-H.; Wang, L.; Ren, N.; Zhang, J.-B.; Qian, Y.-B.; Lu, L.; Fan, J.; et al. Incidence and prognostic values of lymph node metastasis in operable hepatocellular carcinoma and evaluation of routine complete lymphadenectomy. J. Surg. Oncol. 2007, 96, 37–45. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Wang, L.; Gonen, M.; Fong, Y.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Schwartz, L.; Blumgart, L.H.; Jarnagin, W.R. Perihepatic Lymph Node Assessment in Patients Undergoing Partial Hepatectomy for Malignancy. Ann. Surg. 2006, 244, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ercolani, G.; Grazi, G.L.; Ravaioli, M.; Grigioni, W.F.; Cescon, M.; Gardini, A.; Del Gaudio, M.; Cavallari, A. The role of lymphadenectomy for liver tumors: Further considerations on the appropriateness of treatment strategy. Ann. Surg. 2004, 239, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Xiaohong, S.; Huikai, L.; Feng, W.; Ti, Z.; Yunlong, C.; Qiang, L. Clinical Significance of Lymph Node Metastasis in Patients Undergoing Partial Hepatectomy for Hepatocellular Carcinoma. World J. Surg. 2010, 34, 1028–1033. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, H.; Cai, J. The role of lymph node dissection and a new N-staging system for intrahepatic cholangiocarcinoma: A study from the SEER database. J. Int. Med. Res. 2021, 49, 3000605211012209. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, D.; Li, W.; Tan, W.; Zhu, S.; Chen, X.; Min, J.; Shang, C.; Chen, Y. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB 2019, 21, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.G.; Lee, J.H.; Kang, I.; Rho, S.Y.; Choi, G.H.; Han, D.H.; Kim, K.S.; Choi, J.S. Prognostic significance of and risk prediction model for lymph node metastasis in resectable intrahepatic cholangiocarcinoma: Do all require lymph node dissection? HPB 2020, 22, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, T.; Kobayashi, S.; Hanaki, T.; Iwagami, Y.; Tomimaru, Y.; Akita, H.; Noda, T.; Gotoh, K.; Takeda, Y.; Tanemura, M.; et al. Clinical significance of preoperative CA19-9 and lymph node metastasis in intrahepatic cholangiocarcinoma. Surg. Today 2020, 50, 1176–1186. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Sahara, K.; Paredes, A.Z.; Moro, A.; Mehta, R.; Moris, D.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; et al. Predicting Lymph Node Metastasis in Intrahepatic Cholangiocarcinoma. J. Gastrointest. Surg. 2020, 25, 1156–1163. [Google Scholar] [CrossRef]
- Jolissaint, J.S.; Soares, K.C.; Seier, K.P.; Kundra, R.; Gönen, M.; Shin, P.J.; Boerner, T.; Sigel, C.; Madupuri, R.; Vakiani, E.; et al. Intrahepatic Cholangiocarcinoma with Lymph Node Metastasis: Treatment-Related Outcomes and the Role of Tumor Genomics in Patient Selection. Clin. Cancer Res. 2021, 27, 4101–4108. [Google Scholar] [CrossRef]
- Yoh, T.; Hatano, E.; Seo, S.; Terajima, H.; Uchida, Y.; Taura, K.; Yasuchika, K.; Uemoto, S. Preoperative criterion identifying a low-risk group for lymph node metastasis in intrahepatic cholangiocarcinoma. J. Hepato-Biliary-Pancreatic Sci. 2018, 25, 299–307. [Google Scholar] [CrossRef]
- Enquist, I.B.; Good, Z.; Jubb, A.M.; Fuh, G.; Wang, X.; Junttila, M.R.; Jackson, E.L.; Leong, K.G. Lymph node-independent liver metastasis in a model of metastatic colorectal cancer. Nat. Commun. 2014, 5, 3530. [Google Scholar] [CrossRef] [PubMed]
- Bacac, M.; Stamenkovic, I. Metastatic Cancer Cell. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 221–247. [Google Scholar] [CrossRef]
- Derwinger, K.; Gustavsson, B. A study of lymph node ratio in stage IV colorectal cancer. World J. Surg. Oncol. 2008, 6, 127. [Google Scholar] [CrossRef]
- Saad, R.S.; Kordunsky, L.; Liu, Y.L.; Denning, K.L.; Kandil, H.A.; Silverman, J.F. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod. Pathol. 2006, 19, 1317–1323. [Google Scholar] [CrossRef]
- Saharinen, P.; Tammela, T.; Karkkainen, M.J.; Alitalo, K. Lymphatic vasculature: Development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004, 25, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Togashi, K.; Nokubi, M.; Koinuma, K.; Miyakura, Y.; Horie, H.; Lefor, A.T.; Yasuda, Y. Evaluation of Venous Invasion by Elastica van Gieson Stain and Tumor Budding Predicts Local and Distant Metastases in Patients With T1 Stage Colorectal Cancer. Am. J. Surg. Pathol. 2009, 33, 1601–1607. [Google Scholar] [CrossRef]
- Sakamoto, K.; Beppu, T.; Honda, G.; Kotake, K.; Yamamoto, M.; Takahashi, K.; Endo, I.; Hasegawa, K.; Itabashi, M.; Hashiguchi, Y.; et al. Comprehensive data of 4502 patients newly diagnosed with colorectal liver metastasis between 2015 and 2017, and prognostic data of 2427 patients newly diagnosed with colorectal liver metastasis in 2013 and 2014: Third report of a nationwide survey in Japan. J. Hepato-Biliary-Pancreatic Sci. 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshida, M.; Furuse, J.; Sano, K.; Ohtsuka, M.; Yamashita, S.; Beppu, T.; Iwashita, Y.; Wada, K.; Nakajima, T.E.; et al. Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J. Hepato-Biliary-Pancreatic Sci. 2020, 28, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Honda, G.; Beppu, T.; Kotake, K.; Yamamoto, M.; Takahashi, K.; Endo, I.; Hasegawa, K.; Itabashi, M.; Hashiguchi, Y.; et al. Comprehensive data of 3525 patients newly diagnosed with colorectal liver metastasis between 2013 and 2014: 2nd report of a nationwide survey in Japan. J. Hepato-Biliary-Pancreatic Sci. 2020, 27, 555–562. [Google Scholar] [CrossRef]
- Sakamoto, K.; Honda, G.; Beppu, T.; Kotake, K.; Yamamoto, M.; Takahashi, K.; Endo, I.; Hasegawa, K.; Itabashi, M.; Hashiguchi, Y.; et al. Comprehensive data of 3820 patients newly diagnosed with colorectal liver metastasis between 2005 and 2007: Report of a nationwide survey in Japan. J. Hepato-Biliary-Pancreatic Sci. 2017, 25, 115–123. [Google Scholar] [CrossRef] [PubMed]
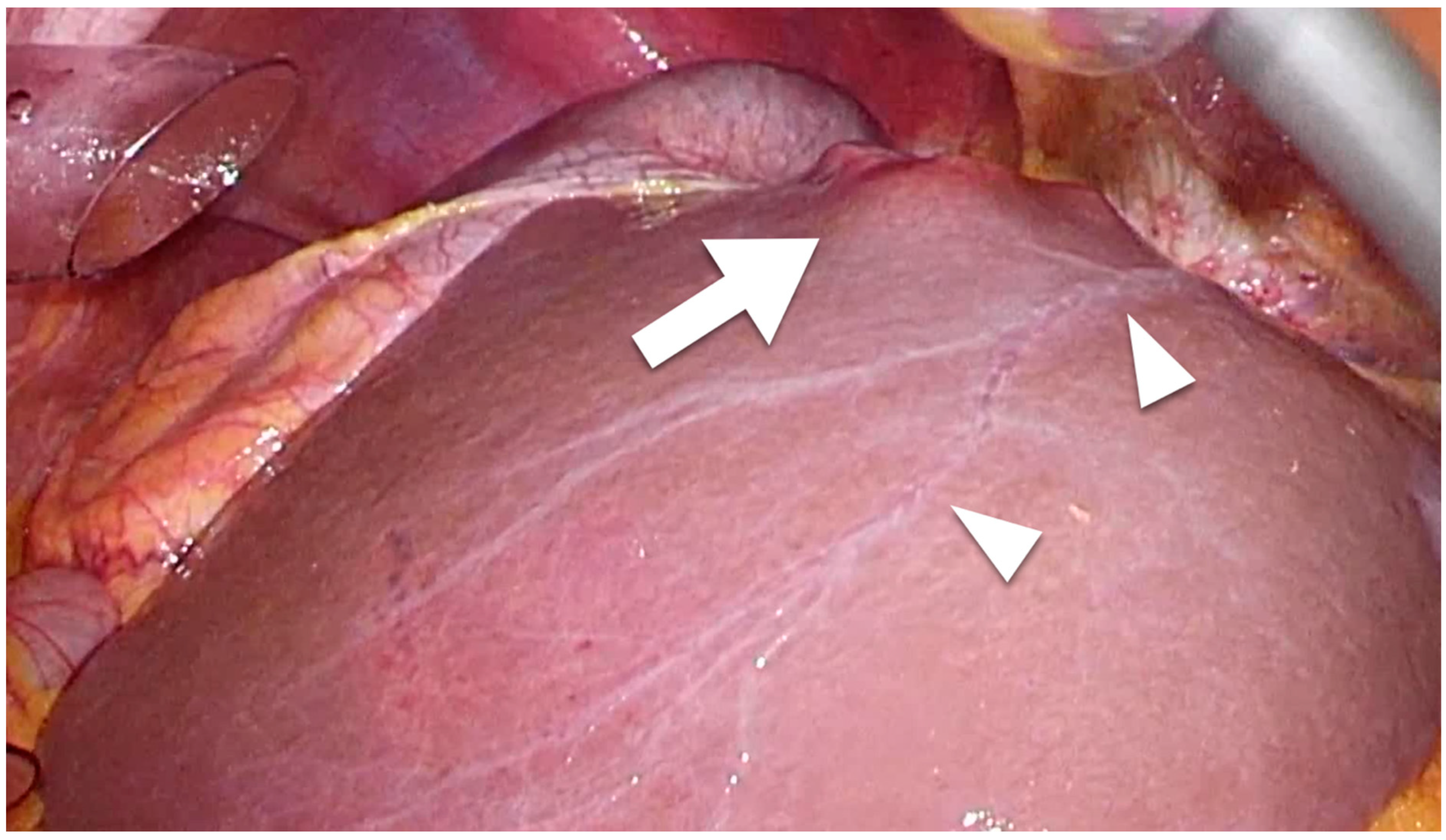
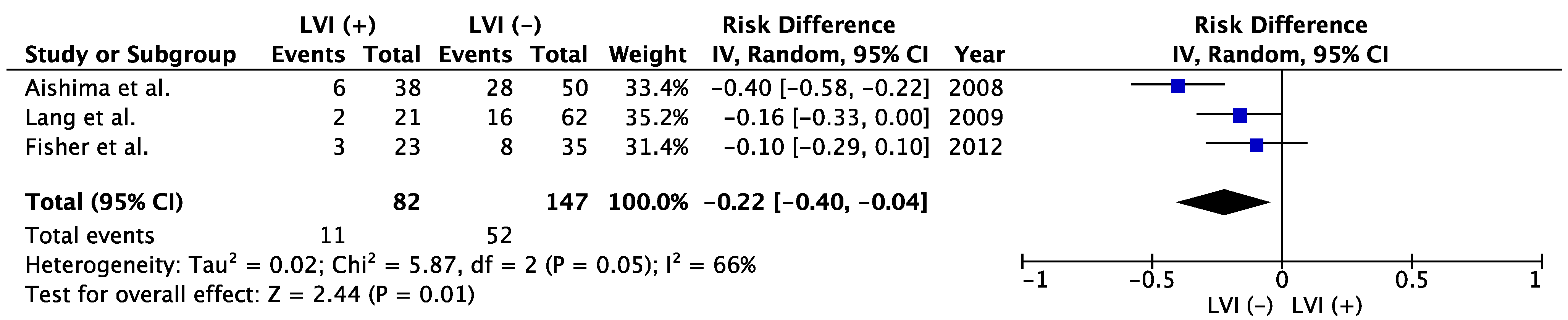
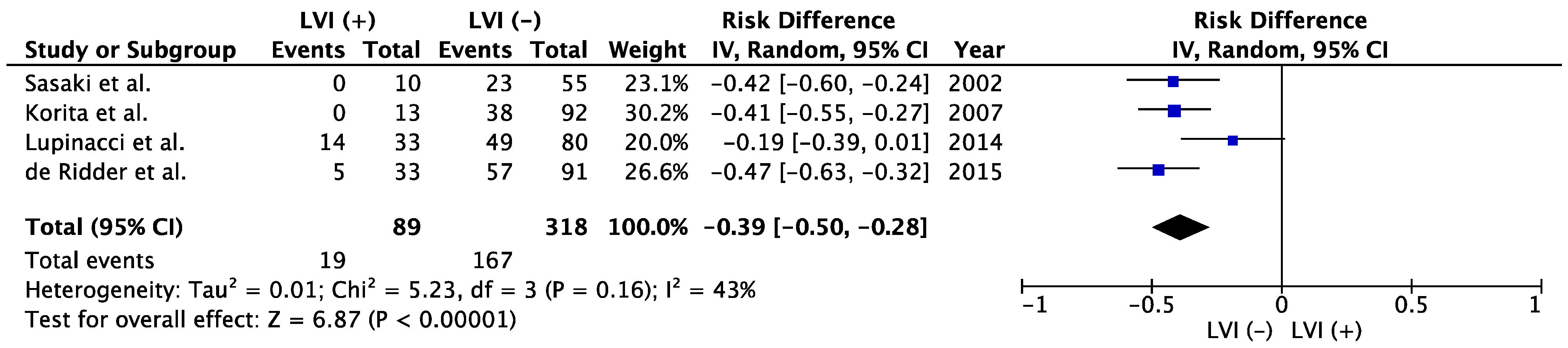
| Author | Published Year | Period | LVI | Patient Number | Tumor Size, cm | Tumor Number | Poor Histological Type | Vascular Invasion | Lymphadenectomy | LNM | 5-Year OS | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICC | Aishima et al. [61] | 2008 | 1986–2005 | positive | 38 | >4 cm, 21 (23.9%) | NR | 28 (31.8%) | NR | NR | 27 (30.1%) | 16% | <0.0001 |
| negative | 50 | 55% | |||||||||||
| Lang et al. [74] | 2009 | 1998–2006 | positive | 21 | >5 cm, 70 (84.3%) | multiple, 36 (43.4%) | 24 (28.9%) | 35 (42.2%) | NR | 28 (33.7%) | 8% | 0.006 | |
| negative | 62 | 26% | |||||||||||
| Cho et al. [73] | 2010 | 2001–2007 | positive | 22 | >5 cm, 36 (57.1%) | multiple, 4 (6.3%) | 32 (53.3%) | 30 (47.6%) | 44 (69.8%) | 13 (29.5%) | MST 9 | 0.008 | |
| negative | 41 | MST 23 | |||||||||||
| Fisher et al. [38] | 2012 | 2000–2010 | positive | 23 | 6.5 (1.3–21) | multiple, 12 (21%) | 19 (33%) | PNI, 22 (38%) | 38 (66%) | 13 (22%) | 14.3% | 0.02 | |
| negative | 35 | 22.2% | |||||||||||
| Lurje et al. [72] | 2019 | 2011–2016 | positive | 13 | ≥T2, 17 (28.3%) | multiple, 20 (28.2%) | 14 (23.7%) | 18 (30.5%) | 71 (100%) | 24 (40.0%) | MST 4 | 0.003 | |
| negative | 58 | MST 40 | |||||||||||
| CRLM | Sasaki et al. [35] | 2002 | 1982–2000 | positive | 10 | ≥5, 18 (26.9%) | ≥4, 7 (10.4%) | non-well, 28 (41.8%) | PVI, 15 (23.1%) | NR | primary lesion, 3 (30.0%) | 0% | <0.01 |
| negative | 55 | primary lesion, 36 (65.5%) | 42.3% | ||||||||||
| Korita et al. [37] | 2007 | 1990–2004 | positive | 13 | NR | NR | NR | PVI, 38 (36%) | 17 (16.2%) | hepatic node, 3 (23%) | 0% | <0.0001 | |
| negative | 92 | hepatic node, 4 (4%) | 41% | ||||||||||
| Lupinacci et al. [36] | 2014 | 2000–2010 | positive | 33 | ≥5, 45 (40.0%) | multiple, 65 (58.0%) | NR | 49 (43%) | NR | primary lesion, 66 (58.0%) | 42% | 0.015 | |
| negative | 80 | 61% | |||||||||||
| de Ridder et al. [34] | 2015 | 1992–2011 | positive | 33 | >5, 31 (25%) | all solitary | NR | 46 (37.1%) | NR | primary lesion, 70 (56.5%) | 14% | 0.013 | |
| negative | 91 | 62.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, K.; Ogawa, K.; Tamura, K.; Honjo, M.; Funamizu, N.; Takada, Y. Prognostic Role of the Intrahepatic Lymphatic System in Liver Cancer. Cancers 2023, 15, 2142. https://doi.org/10.3390/cancers15072142
Sakamoto K, Ogawa K, Tamura K, Honjo M, Funamizu N, Takada Y. Prognostic Role of the Intrahepatic Lymphatic System in Liver Cancer. Cancers. 2023; 15(7):2142. https://doi.org/10.3390/cancers15072142
Chicago/Turabian StyleSakamoto, Katsunori, Kohei Ogawa, Kei Tamura, Masahiko Honjo, Naotake Funamizu, and Yasutsugu Takada. 2023. "Prognostic Role of the Intrahepatic Lymphatic System in Liver Cancer" Cancers 15, no. 7: 2142. https://doi.org/10.3390/cancers15072142
APA StyleSakamoto, K., Ogawa, K., Tamura, K., Honjo, M., Funamizu, N., & Takada, Y. (2023). Prognostic Role of the Intrahepatic Lymphatic System in Liver Cancer. Cancers, 15(7), 2142. https://doi.org/10.3390/cancers15072142







