Inflammation Control and Tumor Growth Inhibition of Ovarian Cancer by Targeting Adhesion Molecules of E-Selectin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of E-Selectin-Binding Peptide Modified PTX-Loaded Bovine Serum Albumin Polymerized Nanoparticles (ESBP-BSANPs-PTX)
2.1.1. Preparation of ESBP-BSANPs-PTX
2.1.2. Characterization of Nanoparticle Size, Potential, Morphology, Stability, Encapsulation Efficiency and Drug Load
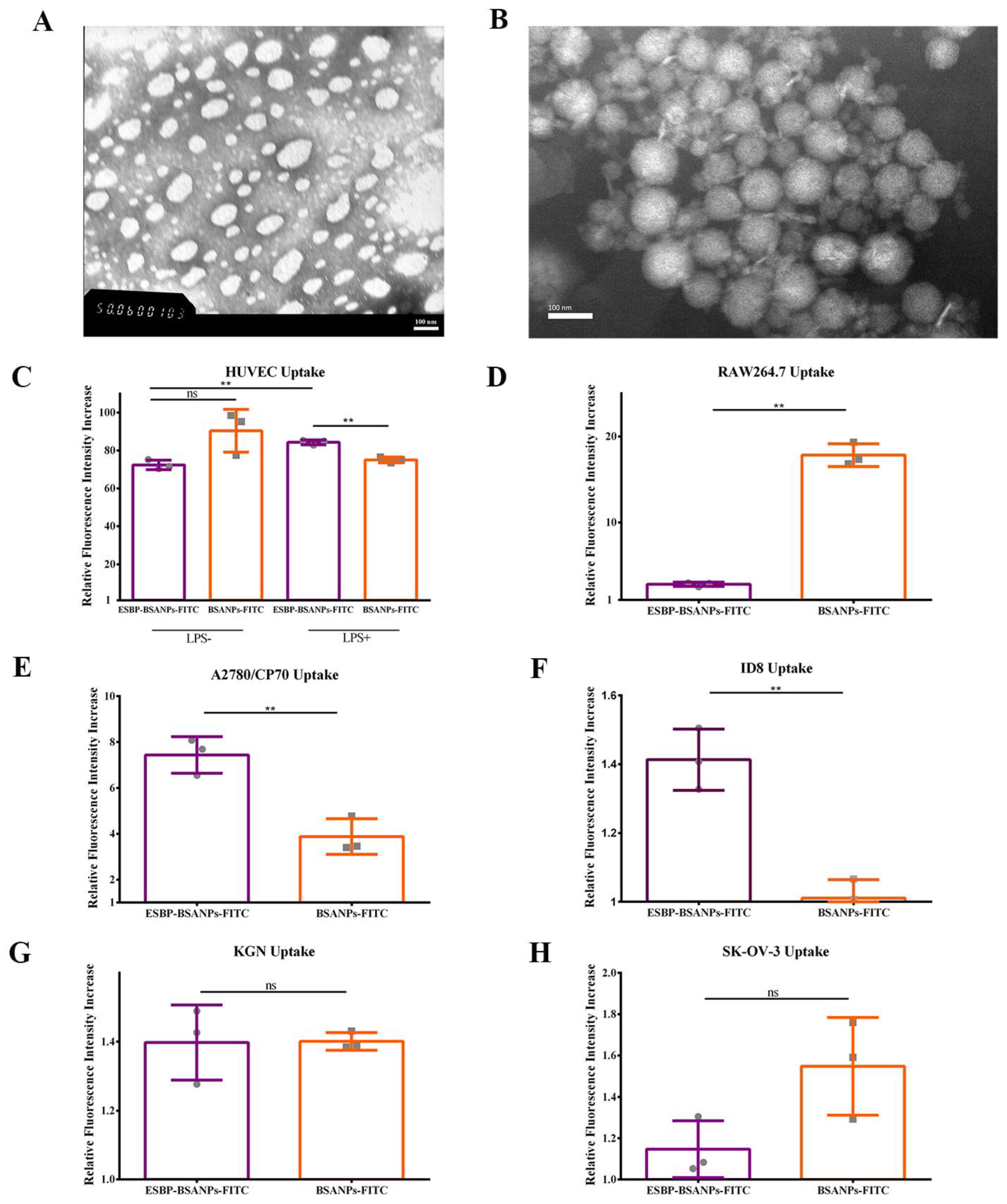
2.1.3. Morphology
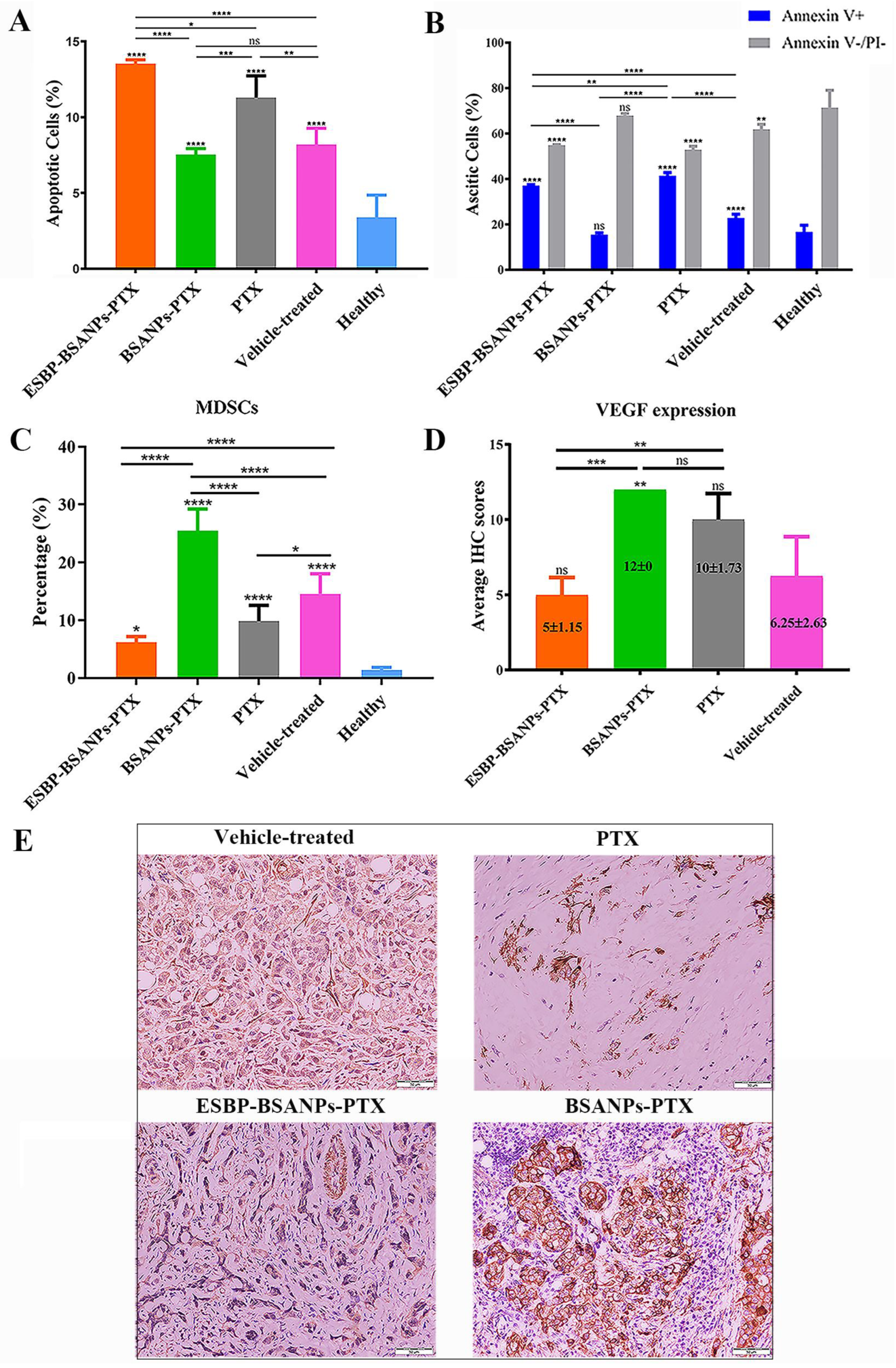
2.2. Immunohistochemistry (IHC) and Haematoxylin and Eosin (H&E) Staining
2.3. Cell Culture
2.4. Determination of Cell Uptake
2.5. Cytotoxicity
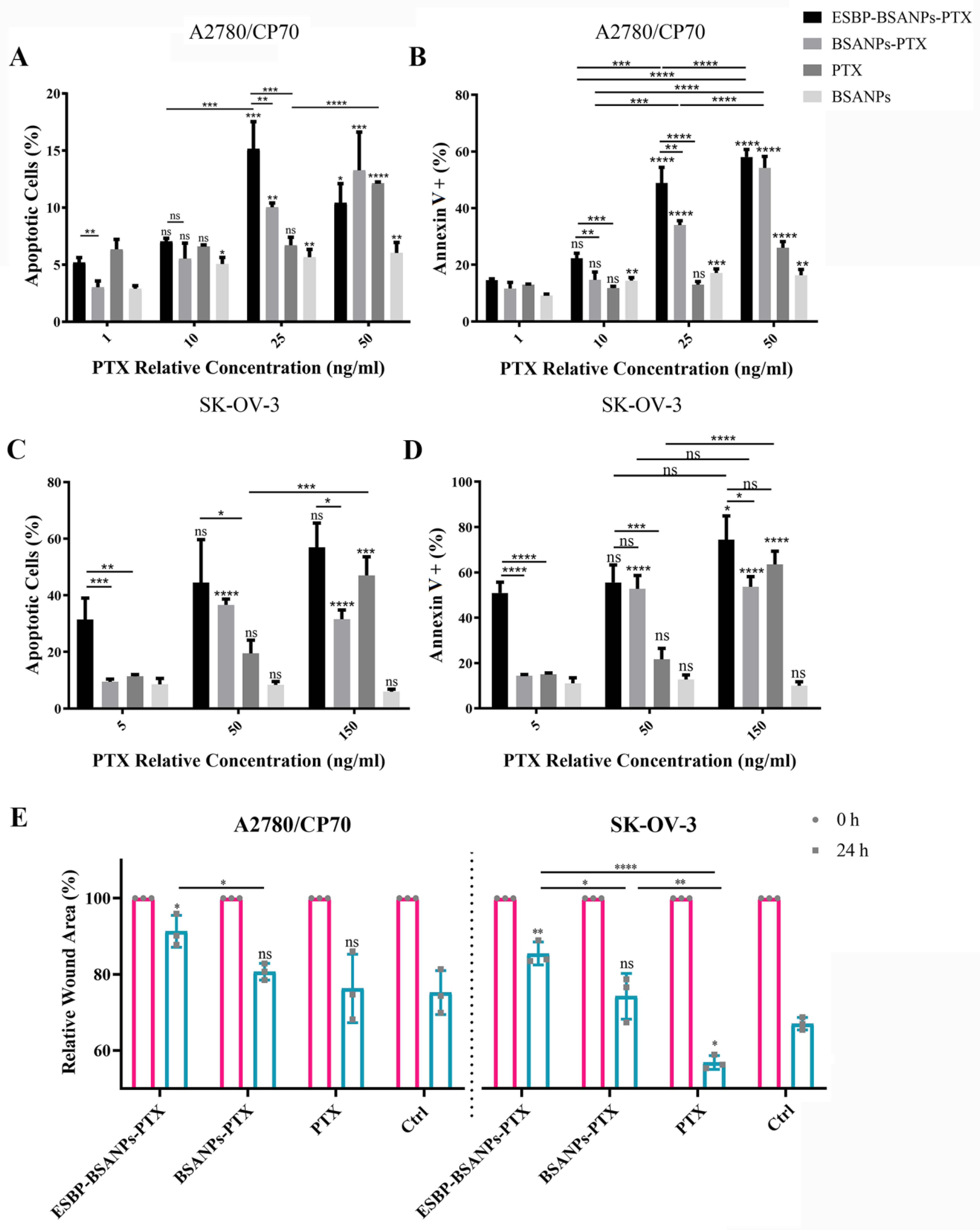
2.6. In Vitro Annexin V/PI Apoptosis Detection
2.7. Wound Healing Assay
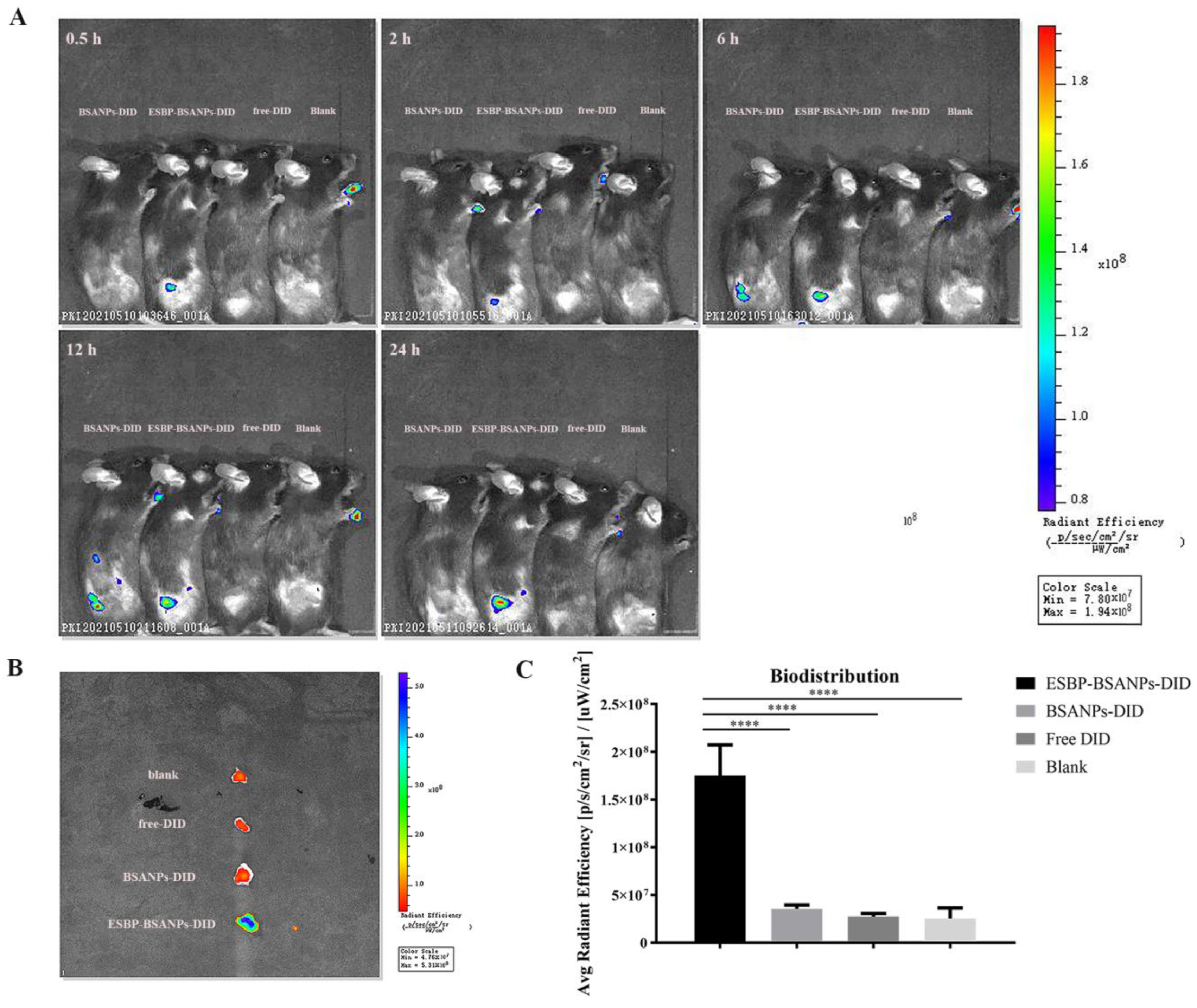
2.8. In Vivo Targeting Examination
2.9. In Vivo Antitumor Assessment
2.9.1. In Vivo Toxicity Test
2.9.2. In Vivo Drug Effects
2.9.3. Survival Curve
2.10. Statistical Analysis
3. Results
3.1. Preparation and Characterization of ESBP-BSANPs-PTX
3.1.1. Characterization of ESBP-BSANPs-PTX
3.1.2. Successful In Vitro Drug-Delivery
3.2. Higher Cell Cytotoxicity of ESBP-BSANPs-PTX than Free PTX
3.3. ESBP-BSANPs-PTX Induced Apoptosis and Inhibited Cell Migration in Ovarian Cancer Cells
3.4. ESBP-BSANPs Sucessfully Targeted Tumor Sites In Vivo
3.5. ESBP-BSANPs-PTX Induced Apoptosis in Ascites and Reduced MDSCs
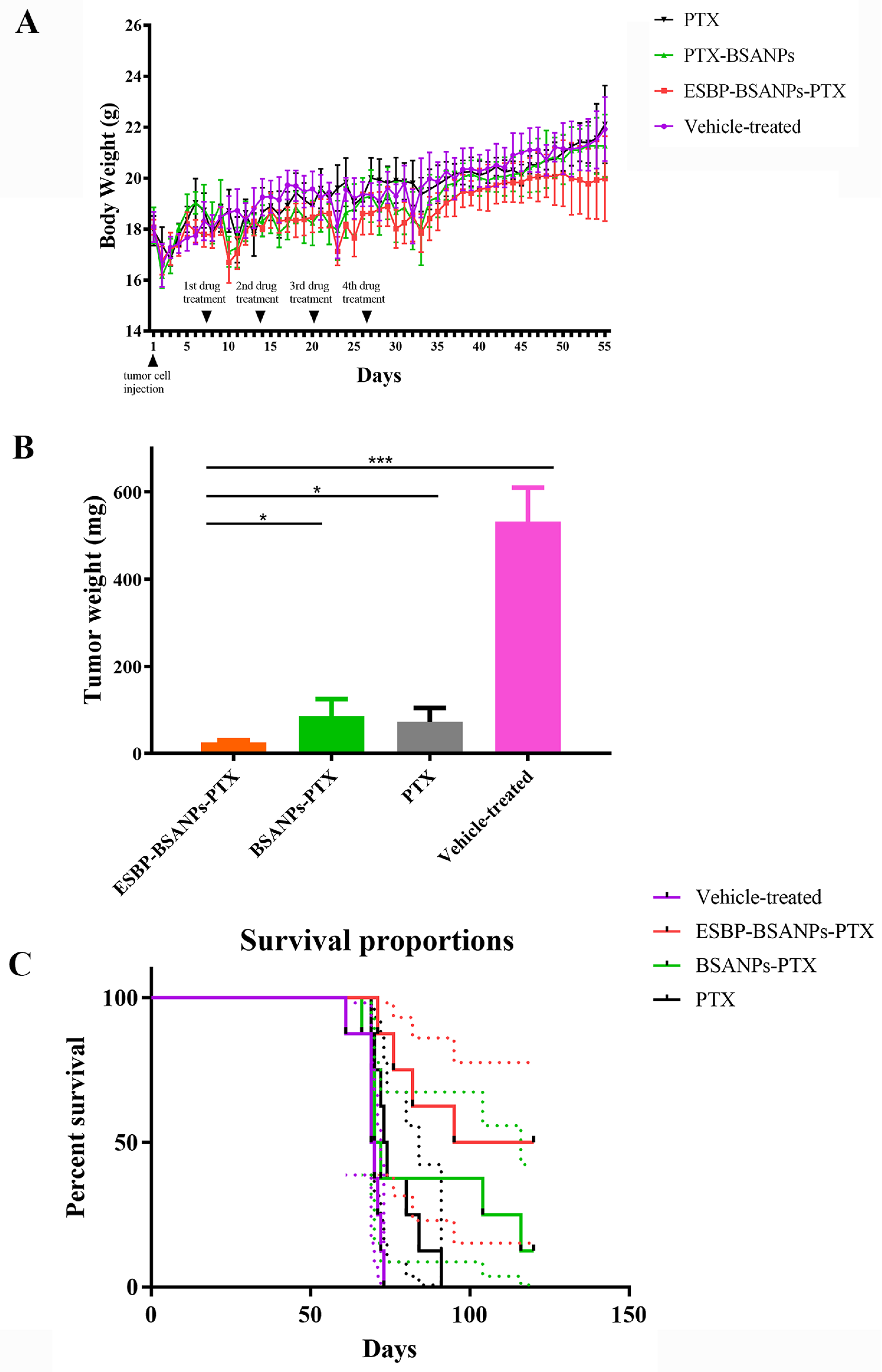
3.6. ESBP-BSANPs-PTX Inhibited Tumor Growth In Vivo and Prolonged Survival Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Arend, R.; Martinez, A.; Szul, T.; Birrer, M.J. Biomarkers in ovarian cancer: To be or not to be. Cancer 2019, 125, 4563–4572. [Google Scholar] [CrossRef]
- Fujiwara, K.; Hasegawa, K.; Nagao, S. Landscape of systemic therapy for ovarian cancer in 2019: Primary therapy. Cancer 2019, 125, 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fidalgo, J.A.; Grau, F.; Farinas, L.; Oaknin, A. Systemic treatment of newly diagnosed advanced epithelial ovarian cancer: From chemotherapy to precision medicine. Crit. Rev. Oncol. Hematol. 2021, 158, 103209. [Google Scholar] [CrossRef]
- Picard, M.; Castells, M.C. Re-visiting hypersensitivity reactions to Taxanes: A comprehensive review. Clin. Rev. Allergy Immunol. 2015, 49, 177–191. [Google Scholar] [CrossRef]
- Caiado, J.; Castells, M.C. Drug desensitizations for chemotherapy: Safety and efficacy in preventing anaphylaxis. Curr. Allergy Asthma Rep. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Lao, D.H.; Chen, Y.; Fan, J.; Zhang, J.Z. Assessing taxane-associated adverse events using the FDA adverse event reporting system database. Chin. Med. J. (Engl.) 2021, 134, 1471–1476. [Google Scholar] [CrossRef]
- Laforgia, M.; Laface, C.; Calabro, C.; Ferraiuolo, S.; Ungaro, V.; Tricarico, D.; Gadaleta, C.D.; Nardulli, P.; Ranieri, G. Peripheral neuropathy under oncologic therapies: A literature review on pathogenetic mechanisms. Int. J. Mol. Sci. 2021, 22, 1980. [Google Scholar] [CrossRef]
- Lee, J.M.; Minasian, L.; Kohn, E.C. New strategies in ovarian cancer treatment. Cancer 2019, 125, 4623–4629. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Jin, K.T.; Wang, S.B.; Ying, X.J.; Lan, H.R.; Lv, J.Q.; Zhang, L.H.; Motallebnezhad, M.; Mou, X.Z. Immune-mediated adverse effects of immune-checkpoint inhibitors and their management in cancer. Immunol. Lett. 2020, 221, 61–71. [Google Scholar] [CrossRef]
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Zhou, J.X.; Feng, L.J.; Zhang, X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2017, 11, 3009–3017. [Google Scholar] [CrossRef]
- Birmingham, K.G.; O’Melia, M.J.; Ban, D.; Mouw, J.; Edwards, E.E.; Marcus, A.I.; McDonald, J.; Thomas, S.N. Analyzing Mechanisms of Metastatic Cancer Cell Adhesive Phenotype Leveraging Preparative Adhesion Chromatography Microfluidic. Adv. Biosyst. 2019, 3, 1800328. [Google Scholar] [CrossRef]
- Barbier, V.; Erbani, J.; Fiveash, C.; Davies, J.M.; Tay, J.; Tallack, M.R.; Lowe, J.; Magnani, J.L.; Pattabiraman, D.R.; Perkins, A.C.; et al. Endothelial E-selectin inhibition improves acute myeloid leukaemia therapy by disrupting vascular niche-mediated chemoresistance. Nat. Commun. 2020, 11, 2042. [Google Scholar] [CrossRef]
- Laird, C.T.; Hassanein, W.; O’Neill, N.A.; French, B.M.; Cheng, X.; Fogler, W.E.; Magnani, J.L.; Parsell, D.; Cimeno, A.; Phelps, C.J.; et al. P- and E-selectin receptor antagonism prevents human leukocyte adhesion to activated porcine endothelial monolayers and attenuates porcine endothelial damage. Xenotransplantation 2018, 25, e12381. [Google Scholar] [CrossRef]
- Federico, C.; Alhallak, K.; Sun, J.; Duncan, K.; Azab, F.; Sudlow, G.P.; de la Puente, P.; Muz, B.; Kapoor, V.; Zhang, L.; et al. Tumor microenvironment-targeted nanoparticles loaded with bortezomib and ROCK inhibitor improve efficacy in multiple myeloma. Nat. Commun. 2020, 11, 6037. [Google Scholar] [CrossRef]
- Winkler, I.G.; Barbier, V.; Nowlan, B.; Jacobsen, R.N.; Forristal, C.E.; Patton, J.T.; Magnani, J.L.; Levesque, J.P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 2012, 18, 1651–1657. [Google Scholar] [CrossRef]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Meng, Q.; Pu, L.; Lu, Q.; Wang, B.; Li, S.; Liu, B.; Li, F. Morin hydrate inhibits atherosclerosis and LPS-induced endothelial cells inflammatory responses by modulating the NFκB signaling-mediated autophagy. Int. Immunopharmacol. 2021, 100, 108096. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K.; Desai, N.; Legha, S.; Soon-Shiong, P.; Theriault, R.L.; Rivera, E.; Esmaeli, B.; Ring, S.E.; Bedikian, A.; Hortobagyi, G.N.; et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin. Cancer Res. 2002, 8, 1038–1044. [Google Scholar] [PubMed]
- Yamada, K.; Yamamoto, N.; Yamada, Y.; Mukohara, T.; Minami, H.; Tamura, T. Phase I and pharmacokinetic study of ABI-007, albumin-bound paclitaxel, administered every 3 weeks in Japanese patients with solid tumors. Jpn. J. Clin. Oncol. 2010, 40, 404–411. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Oh, P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am. J. Physiol. 1992, 263, H1872–H1879. [Google Scholar] [CrossRef]
- Tonissi, F.; Lattanzio, L.; Merlano, M.C.; Infante, L.; Lo Nigro, C.; Garrone, O. The effect of paclitaxel and nab-paclitaxel in combination with anti-angiogenic therapy in breast cancer cell lines. Investig. New Drugs 2015, 33, 801–809. [Google Scholar] [CrossRef]
- Lu, Z.H.; Gu, X.J.; Shi, K.Z.; Li, X.; Chen, D.D.; Chen, L. Association between genetic polymorphisms of inflammatory response genes and the risk of ovarian cancer. J. Formos. Med. Assoc. 2016, 115, 31–37. [Google Scholar] [CrossRef]
- Klaschik, S.; Gehlen, J.; Neumann, C.; Keyver-Paik, M.D.; Soehle, M.; Frede, S.; Velten, M.; Hoeft, A.; Hilbert, T. Network of mediators for vascular inflammation and leakage is dysbalanced during cytoreductive surgery for late-stage ovarian cancer. Mediat. Inflamm. 2019, 2019, 5263717. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Nasr, L.; Kassab, J.; Saba, L.; Ghossein, M.; Yaghi, M.; Dominguez, B.; Chaulagain, C.P. Adhesion molecules in multiple myeloma oncogenesis and targeted therapy. Int. J. Hematol. Oncol. 2022, 11, IJH39. [Google Scholar] [CrossRef]
- Natoni, A.; Farrell, M.L.; Harris, S.; Falank, C.; Kirkham-McCarthy, L.; Macauley, M.S.; Reagan, M.R.; O’Dwyer, M. Sialyltransferase inhibition leads to inhibition of tumor cell interactions with E-selectin, VCAM1, and MADCAM1, and improves survival in a human multiple myeloma mouse model. Haematologica 2020, 105, 457–467. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, N.; Wang, S. MDSCs: Key Criminals of Tumor Pre-metastatic Niche Formation. Front. Immunol. 2019, 10, 172. [Google Scholar] [CrossRef]
- Natoni, A.; Smith, T.A.G.; Keane, N.; McEllistrim, C.; Connolly, C.; Jha, A.; Andrulis, M.; Ellert, E.; Raab, M.S.; Glavey, S.V.; et al. E-selectin ligands recognised by HECA452 induce drug resistance in myeloma, which is overcome by the E-selectin antagonist, GMI-1271. Leukemia 2017, 31, 2642–2651. [Google Scholar] [CrossRef]
- Kang, S.A.; Blache, C.A.; Bajana, S.; Hasan, N.; Kamal, M.; Morita, Y.; Gupta, V.; Tsolmon, B.; Suh, K.S.; Gorenstein, D.G.; et al. The effect of soluble E-selectin on tumor progression and metastasis. BMC Cancer 2016, 16, 331. [Google Scholar] [CrossRef]
- Shirure, V.S.; Liu, T.; Delgadillo, L.F.; Cuckler, C.M.; Tees, D.F.; Benencia, F.; Goetz, D.J.; Burdick, M.M. CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions. Am. J. Physiol. Cell Physiol. 2015, 308, C68–C78. [Google Scholar] [CrossRef]
- Khan, S.U.; Xia, Y.; Goodale, D.; Schoettle, G.; Allan, A.L. Lung-derived selectins enhance metastatic behavior of triple negative breast cancer cells. Biomedicines 2021, 9, 1580. [Google Scholar] [CrossRef]
- Qian, J.; LeSavage, B.L.; Hubka, K.M.; Ma, C.; Natarajan, S.; Eggold, J.T.; Xiao, Y.; Fuh, K.C.; Krishnan, V.; Enejder, A.; et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J. Clin. Investig. 2021, 131, e146186. [Google Scholar] [CrossRef]
- Saha, S.; Parte, S.; Roy, P.; Kakar, S.S. Ovarian Cancer Stem Cells: Characterization and Role in Tumorigenesis. Ovarian Cancer Mol. Diagn. Imaging Treat. Strateg. 2021, 1330, 151–169. [Google Scholar] [CrossRef]
- Martincuks, A.; Li, P.C.; Zhao, Q.; Zhang, C.; Li, Y.J.; Yu, H.; Rodriguez-Rodriguez, L. CD44 in Ovarian Cancer Progression and Therapy Resistance-A Critical Role for STAT3. Front. Oncol. 2020, 10, 589601. [Google Scholar] [CrossRef]
- Li, Y.; Duy Le, T.M.; Nam Bui, Q.; Yang, H.Y.; Lee, D.S. Tumor acidity and CD44 dual targeting hyaluronic acid-coated gold nanorods for combined chemo- and photothermal cancer therapy. Carbohydr. Polym. 2019, 226, 115281. [Google Scholar] [CrossRef]
- Karan Krizanac, D.; Krasic Arapovic, A.; Skocibusic, S.; Pintaric, I.; Trgo, G.; Tomic, S. CD44 immunoexpression is unfavorable predictor in ovarian serous cancer. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.; Kalacheva, M.; Boersma, A.J.; Kedrov, A. The more the merrier: Effects of macromolecular crowding on the structure and dynamics of biological membranes. FEBS J. 2020, 287, 5039–5067. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Yadav, A.; Vashistha, P.; Pandey, V.P.; Dwivedi, U.N. Protein misfolding diseases and therapeutic approaches. Curr. Protein Pept. Sci. 2019, 20, 1226–1245. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Macromolecular crowding: An important but neglected aspect of the intracellular environment. Curr. Opin. Struct. Biol. 2001, 11, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Bascetin, R.; Laurent-Issartel, C.; Blanc-Fournier, C.; Vendrely, C.; Kellouche, S.; Carreiras, F.; Gallet, O.; Leroy-Dudal, J. A biomimetic model of 3D fluid extracellular macromolecular crowding microenvironment fine-tunes ovarian cancer cells dissemination phenotype. Biomaterials 2021, 269, 120610. [Google Scholar] [CrossRef]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Van den Bergh, R.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef]
- Sui, H.; Dongye, S.; Liu, X.; Xu, X.; Wang, L.; Jin, C.Q.; Yao, M.; Gong, Z.; Jiang, D.; Zhang, K.; et al. Immunotherapy of targeting MDSCs in tumor microenvironment. Front. Immunol. 2022, 13, 990463. [Google Scholar] [CrossRef]
- Osipov, A.; Saung, M.T.; Zheng, L.; Murphy, A.G. Small molecule immunomodulation: The tumor microenvironment and overcoming immune escape. J. Immunother. Cancer 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Horikawa, N.; Abiko, K.; Matsumura, N.; Hamanishi, J.; Baba, T.; Yamaguchi, K.; Yoshioka, Y.; Koshiyama, M.; Konishi, I. Expression of vascular rndothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin. Cancer Res. 2017, 23, 587–599. [Google Scholar] [CrossRef]
- Nathan, J.R.; Lakshmanan, G.; Michael, F.M.; Seppan, P.; Ragunathan, M. Expression of adenosine receptors and vegf during angiogenesis and its inhibition by pentoxifylline-A study using zebrafish model. Biomed. Pharmacother. 2016, 84, 1406–1418. [Google Scholar] [CrossRef]
- Du, X.; Khan, A.R.; Fu, M.; Ji, J.; Yu, A.; Zhai, G. Current development in the formulations of non-injection administration of paclitaxel. Int. J. Pharm. 2018, 542, 242–252. [Google Scholar] [CrossRef]
- Saito, Y.; Takekuma, Y.; Kobayashi, M.; Komatsu, Y.; Sugawara, M. Detection of risk factors related to administration suspension and severe neutropenia in gemcitabine and nab-paclitaxel treatment. Support. Care Cancer 2021, 29, 3277–3285. [Google Scholar] [CrossRef]
- Lee, M.; Yee, J.; Kim, J.Y.; Kim, J.Y.; An, S.H.; Lee, K.E.; Gwak, H.S. Risk factors for neutropenia and febrile neutropenia following prophylactic pegfilgrastim. Asia Pac. J. Clin. Oncol. 2019, 15, 231–237. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Nakasya, A.; Matsumoto, T.; Ikoma, T.; Yamamoto, Y.; Kurioka, Y.; Tsuduki, T.; Kajiwara, T.; Nishina, T.; Yamashita, N.; et al. Risk factors and efficacy outcomes of early-onset severe neutropenia due to paclitaxel or nanoparticle albumin-bound paclitaxel combined with ramucirumab in advanced gastric cancer: A multicenter retrospective cohort study. J. Gastrointest. Oncol. 2022, 13, 2769–2778. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Yin, S.; Zhou, Z.; Huang, L.; Xi, M. Inflammation Control and Tumor Growth Inhibition of Ovarian Cancer by Targeting Adhesion Molecules of E-Selectin. Cancers 2023, 15, 2136. https://doi.org/10.3390/cancers15072136
Yang B, Yin S, Zhou Z, Huang L, Xi M. Inflammation Control and Tumor Growth Inhibition of Ovarian Cancer by Targeting Adhesion Molecules of E-Selectin. Cancers. 2023; 15(7):2136. https://doi.org/10.3390/cancers15072136
Chicago/Turabian StyleYang, Bowen, Shanmei Yin, Zishuo Zhou, Luyao Huang, and Mingrong Xi. 2023. "Inflammation Control and Tumor Growth Inhibition of Ovarian Cancer by Targeting Adhesion Molecules of E-Selectin" Cancers 15, no. 7: 2136. https://doi.org/10.3390/cancers15072136
APA StyleYang, B., Yin, S., Zhou, Z., Huang, L., & Xi, M. (2023). Inflammation Control and Tumor Growth Inhibition of Ovarian Cancer by Targeting Adhesion Molecules of E-Selectin. Cancers, 15(7), 2136. https://doi.org/10.3390/cancers15072136





