Baicalein as Promising Anticancer Agent: A Comprehensive Analysis on Molecular Mechanisms and Therapeutic Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
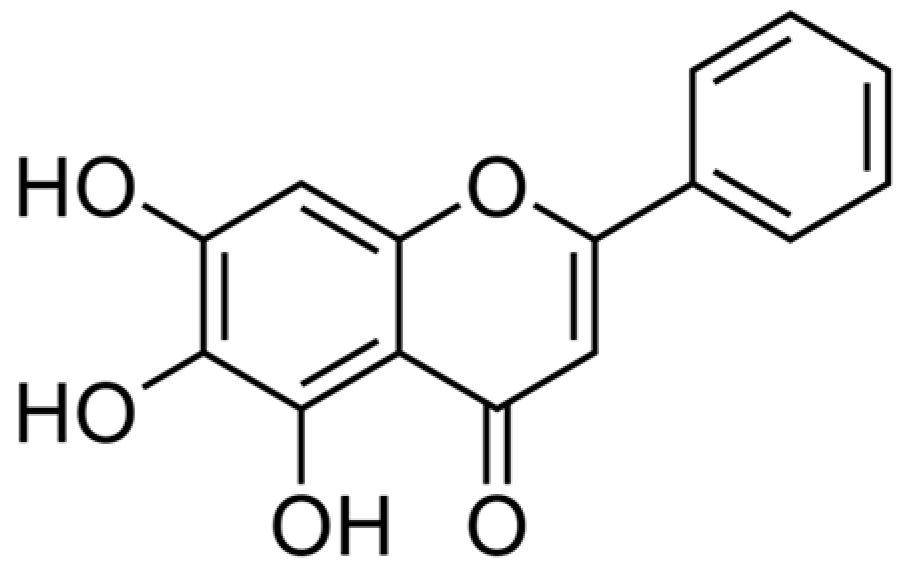
2. Overview of Baicalein
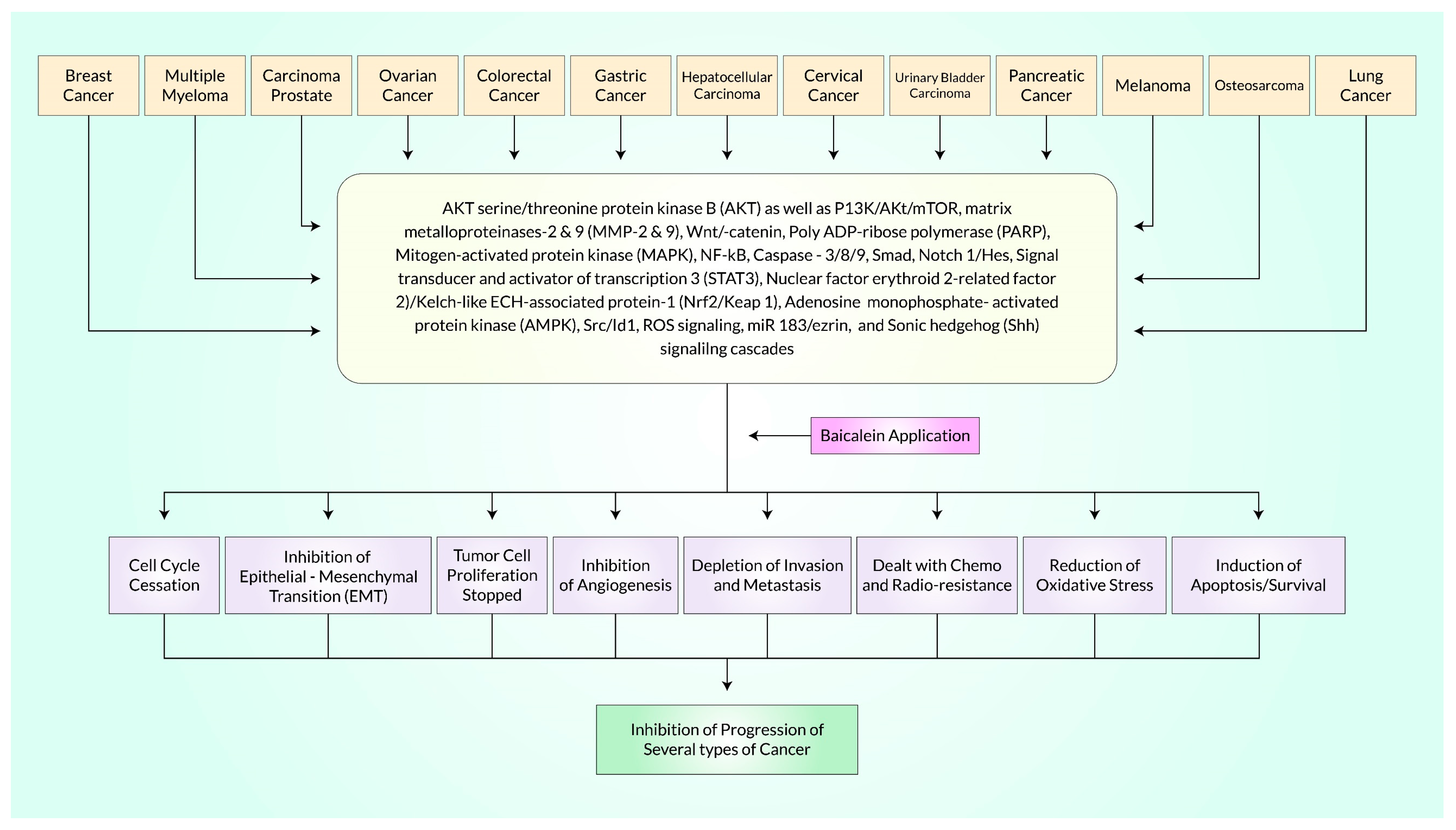
3. Molecular Pathway-Based Anticancer Properties of Baicalein
3.1. Baicalein Induces ROS for Cancer Treatment
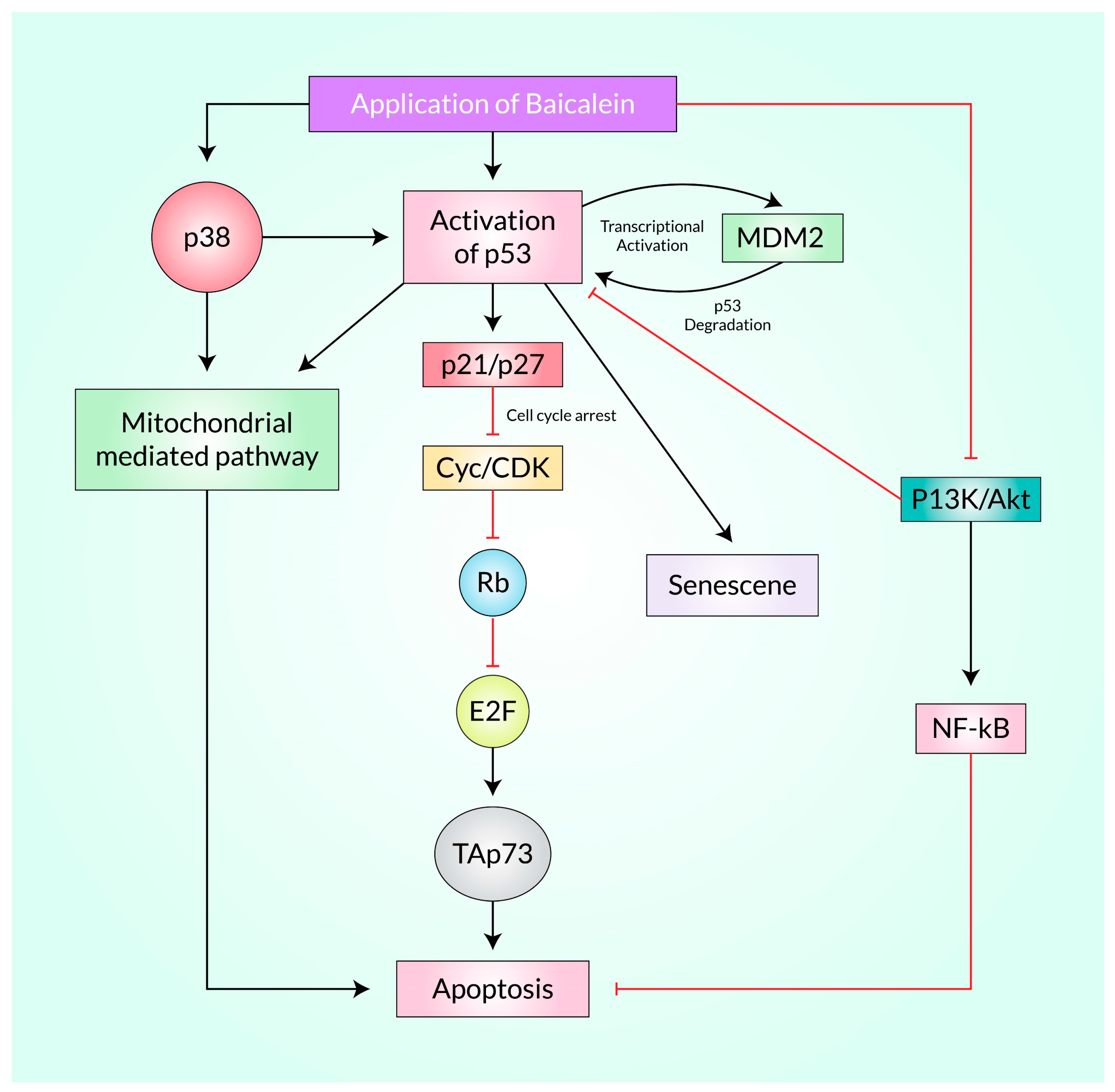
3.2. Baicalein Activates p53 in Cancer
3.3. Baicalein Induces Apoptosis in Cancer
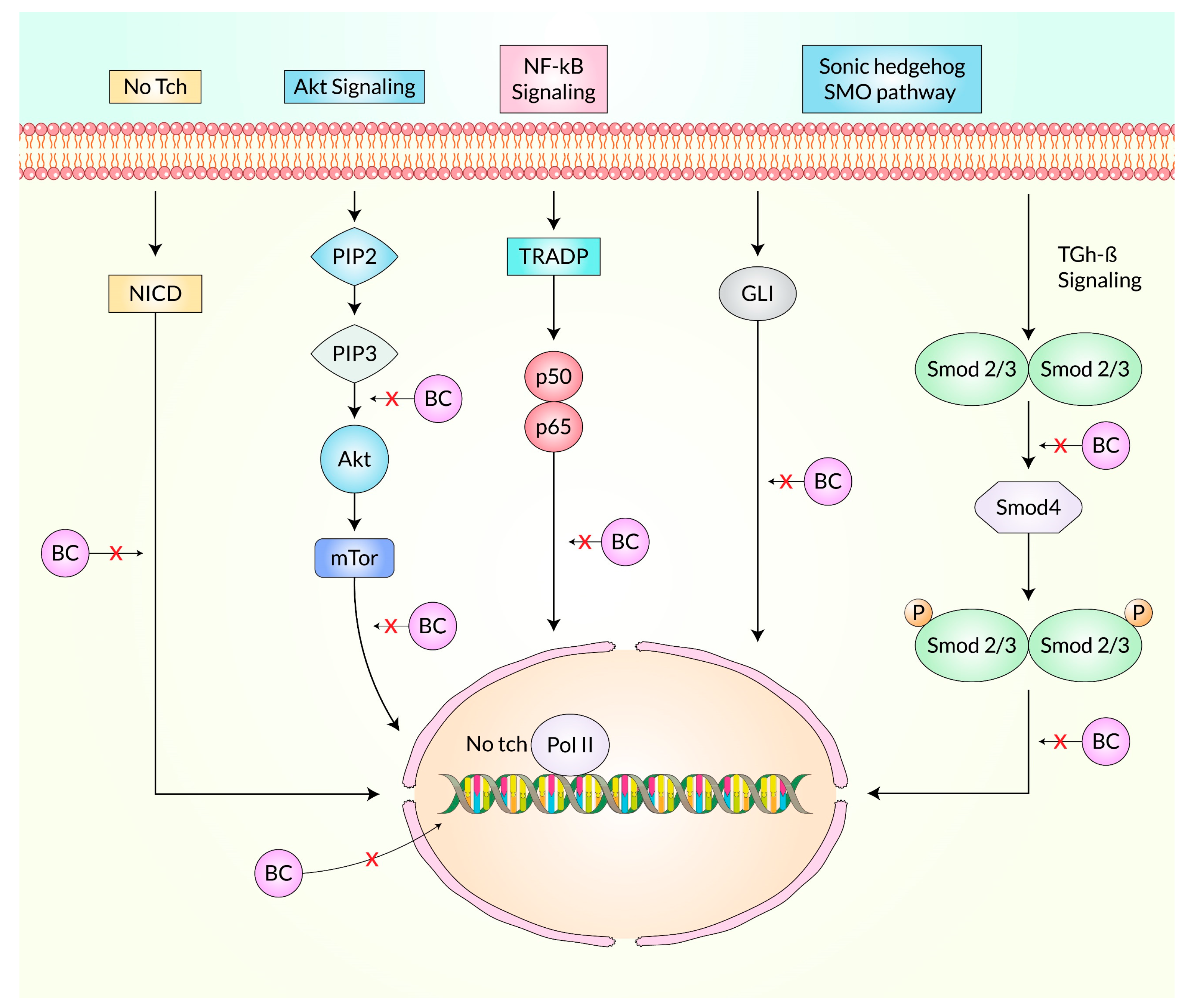
3.4. Suppression of Cancer Stem Cells by Baicalein
3.5. Cell Cycle Arrest Induction by Baicalein in Cancer
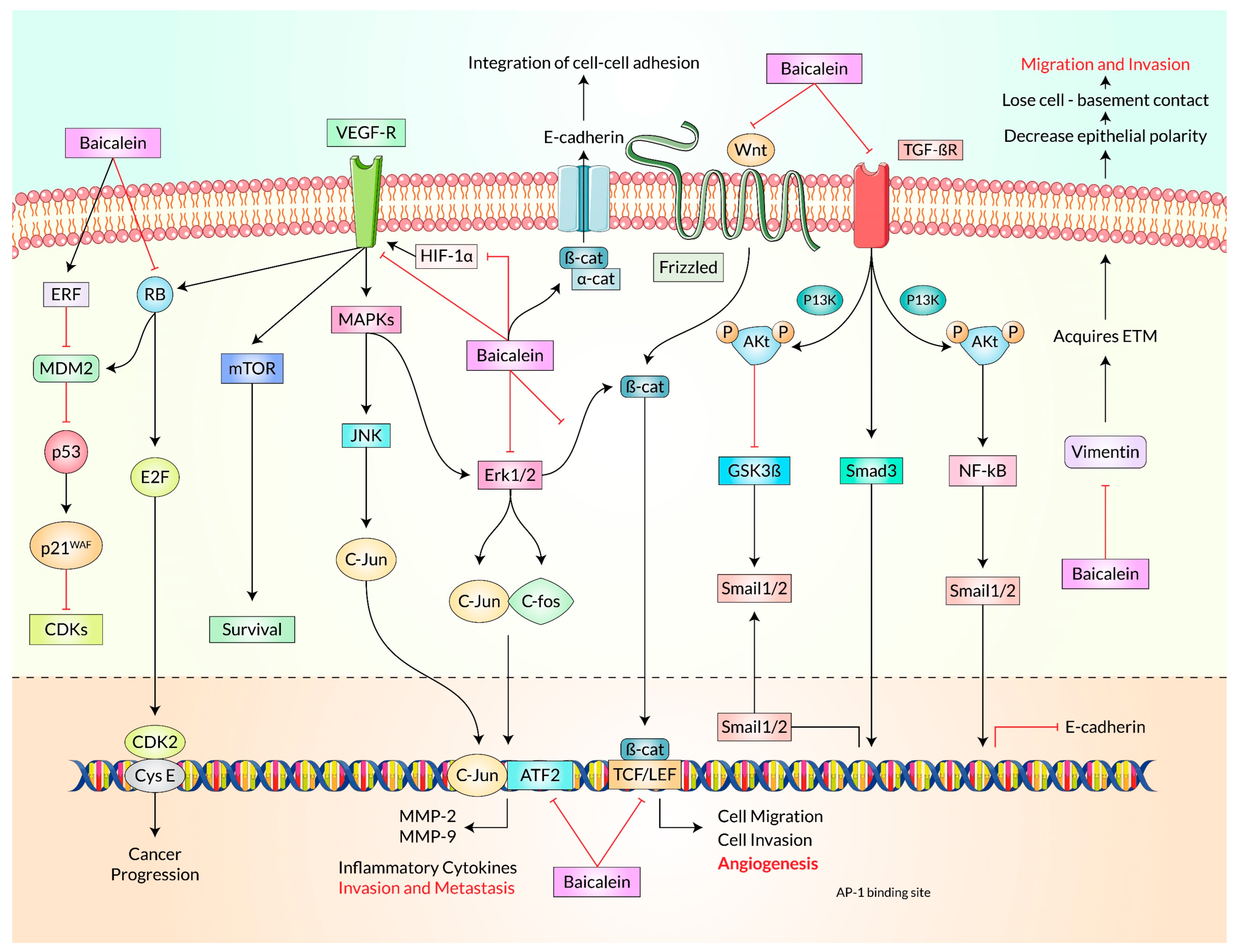
4. Cell-Signaling Molecular Mechanisms of Baicalein for Cancer Treatment
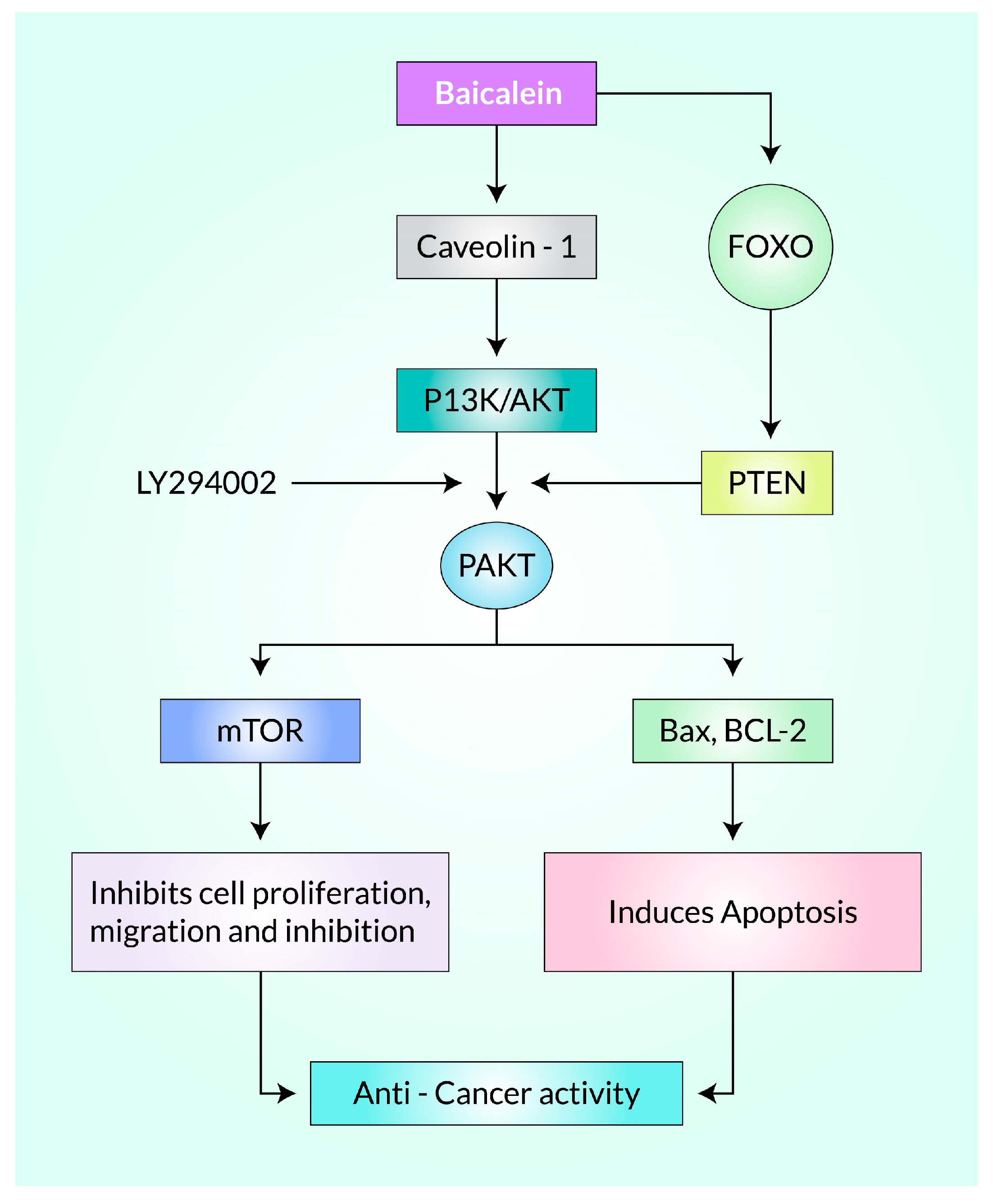
Akt Is the Principal Target of Baicalein Following PI3K/Akt, mTOR, MAPK, PI3K/FoxO, and NF-κB Signaling Pathway in Cancer
5. Baicalein-Based Drug Design for Cancer Treatment: A Focus on Nanomedicine Development
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Tuorkey, M.; Kaur, G.; Savla, R.; Sak, K. Baicalein: A metabolite with promising antineoplastic activity. Life Sci. 2020, 259, 118183. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, X.; Li, H.; Zhang, X.; Liu, X.; Song, Y. Baicalein inhibits RLS3-induced ferroptosis in melanocytes. Biochem. Biophys. Res. Commun. 2021, 561, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Yang, L.; Haase, A.; Yang, X. Health benefits of vanadium and its potential as an anticancer agent. Met. Ions Life Sci 2018, 18, 251–279. [Google Scholar]
- Sajwani, F.H. Frondoside A is a potential anticancer agent from sea cucumbers. J. Cancer Res. Ther. 2019, 15, 953. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Z.; Li, W.; Kong, F.; Yi, P.; Huang, J.; Mao, D.; Peng, W.; Zhang, S. Network pharmacology-based prediction and verification of the active ingredients and potential targets of zuojinwan for treating colorectal cancer. Drug Des. Dev. Ther. 2020, 14, 2725. [Google Scholar] [CrossRef] [PubMed]
- Donald, G.; Hertzer, K.; Eibl, G. Baicalein-an intriguing therapeutic phytochemical in pancreatic cancer. Curr. Drug Targets 2012, 13, 1772–1776. [Google Scholar] [CrossRef]
- Tuan, N.M.; Lee, C.H. Penfluridol as a candidate of drug repurposing for anticancer agent. Molecules 2019, 24, 3659. [Google Scholar] [CrossRef]
- Baby, J.; Devan, A.R.; Kumar, A.R.; Gorantla, J.N.; Nair, B.; Aishwarya, T.S.; Nath, L.R. Cogent role of flavonoids as key orchestrators of chemoprevention of hepatocellular carcinoma: A review. J. Food Biochem. 2021, 45, e13761. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wu, J.; Chen, Y.; Chen, B.; Hu, J.; Zhou, J. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014, 354, 5–11. [Google Scholar] [CrossRef]
- Gupta, S.; Buttar, H.S.; Kaur, G.; Tuli, H.S. Baicalein: Promising therapeutic applications with special reference to published patents. Pharm. Pat. Anal. 2022, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Huang, T.S.; Cheng, W.F.; Lu, F.J. Baicalein and baicalin are potent inhibitors of angiogenesis: Inhibition of endothelial cell proliferation, migration and differentiation. Int. J. Cancer 2003, 106, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Zhang, M.; Song, Y.; Zhang, Y.; Fan, S.; Ren, S.; Fu, L.; Zhang, N.; Hui, H. Baicalein resensitizes tamoxifen-resistant breast cancer cells by reducing aerobic glycolysis and reversing mitochondrial dysfunction via inhibition of hypoxia-inducible factor-1α. Clin. Transl. Med. 2021, 11, e577. [Google Scholar] [CrossRef] [PubMed]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef]
- Gong, W.Y.; Zhao, Z.X.; Liu, B.J.; Lu, L.W.; Dong, J.C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017, 126, 844–852. [Google Scholar] [CrossRef]
- Kuo, Y.-T.; Liu, C.-H.; Wong, S.H.; Pan, Y.-C.; Lin, L.-T. Small molecules baicalein and cinnamaldehyde are potentiators of measles virus-induced breast cancer oncolysis. Phytomedicine 2021, 89, 153611. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Z.; Zhang, H.; Zhen, Z.; Calway, T.; Wang, Y.; Yuan, C.S.; Wang, C.Z. Pretreatment of baicalin and wogonoside with glycoside hydrolase: A promising approach to enhance anticancer potential. Oncol. Rep. 2013, 30, 2411–2418. [Google Scholar] [CrossRef]
- Park, Y.G.; Choi, J.; Jung, H.K.; Kim, B.; Kim, C.; Park, S.Y.; Seol, J.W. Baicalein inhibits tumor progression by inhibiting tumor cell growth and tumor angiogenesis. Oncol. Rep. 2017, 38, 3011–3018. [Google Scholar] [CrossRef]
- Huang, X.; Mao, W.; Zhang, T.; Wang, M.; Wang, X.; Li, Y.; Zhang, L.; Yao, D.; Cai, X.; Wang, L. Baicalin promotes apoptosis and inhibits proliferation and migration of hypoxia-induced pulmonary artery smooth muscle cells by up-regulating A2a receptor via the SDF-1/CXCR4 signaling pathway. BMC Complement. Altern. Med. 2018, 18, 330. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Wang, S.; Zhang, H.; Lu, Y.Y.; Wang, X.F.; Motoo, Y.; Su, S.B. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol. Sin. 2009, 30, 1648–1658. [Google Scholar] [CrossRef]
- Kumagai, T.; Müller, C.I.; Desmond, J.C.; Imai, Y.; Heber, D.; Koeffler, H.P. Scutellaria baicalensis, a herbal medicine: Anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk. Res. 2007, 31, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tsai, S.C.; Tseng, M.T.; Peng, S.F.; Kuo, S.C.; Lin, M.W.; Hsu, Y.M.; Lee, M.R.; Amagaya, S.; Huang, W.W.; et al. AKT serine/threonine protein kinase modulates baicalin-triggered autophagy in human bladder cancer T24 cells. Int. J. Oncol. 2013, 42, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mei, J.; Tan, Y. Baicalin attenuates DDP (cisplatin) resistance in lung cancer by downregulating MARK2 and p-Akt. Int. J. Oncol. 2017, 50, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, N.; Pandi, A. Baicalein: A review on its anti-cancer effects and mechanisms in lung carcinoma. J. Food Biochem. 2022, 46, e14230. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Li, N.; Liu, Y.; Su, H. Combination lung cancer chemotherapy: Design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed. Pharmacother. 2017, 95, 548–555. [Google Scholar] [CrossRef]
- Andouard, D.; Gueye, R.; Hantz, S.; Fagnère, C.; Liagre, B.; Bernardaud, L.; Pouget, C.; Duroux, J.; Alain, S. Impact of new cyclooxygenase 2 inhibitors on human cytomegalovirus replication in vitro. Antivir. Ther. 2021, 26, 117–125. [Google Scholar] [CrossRef]
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef]
- Yu, X.; Tang, W.; Yang, Y.; Tang, L.; Dai, R.; Pu, B.; Feng, C.; Xia, J. Long noncoding RNA NKILA enhances the anti-cancer effects of baicalein in hepatocellular carcinoma via the regulation of NF-κB signaling. Chem. Biol. Interact. 2018, 285, 48–58. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The fascinating effects of baicalein on cancer: A review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef]
- Nik Salleh, N.N.H.; Othman, F.A.; Kamarudin, N.A.; Tan, S.C. The biological activities and therapeutic potentials of baicalein extracted from oroxylum indicum: A systematic review. Molecules 2020, 25, 5677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, J.; Zhong, L.; Li, Y.; Er, A.N.; Li, T.; Yang, L.; Dong, L. Combination of a biopharmaceutic classification system and physiologically based pharmacokinetic models to predict absorption properties of baicalein in vitro and in vivo. J. Tradit. Chin. Med. Sci. 2021, 8, 238–247. [Google Scholar] [CrossRef]
- Li, L.; Gao, H.; Lou, K.; Luo, H.; Hao, S.; Yuan, J.; Liu, Z.; Dong, R. Safety, tolerability, and pharmacokinetics of oral baicalein tablets in healthy Chinese subjects: A single-center, randomized, double-blind, placebo-controlled multiple-ascending-dose study. Clin. Transl. Sci. 2021, 14, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, Y.; Yu, N.; Sun, Y.; Xing, Y.; Yang, F.; Yu, X.; Sun, W.; Sun, J.; Li, X.; et al. Intestinal metabolism of baicalein after oral administration in mice: Pharmacokinetics and mechanisms. J. Funct. Foods 2019, 54, 53–63. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Lin, G.; Krajcsi, P.; Zuo, Z. Hepatic metabolism and disposition of baicalein via the coupling of conjugation enzymes and transporters-in vitro and in vivo evidences. AAPS J. 2011, 13, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Yang, L.; Chen, Y.; Wang, Q.F.; Sun, Q.S.; Che, Y.X.; Che, Q.M. Identification of the metabolites of baicalein in human plasma. J. Asian Nat. Prod. Res. 2011, 13, 861–868. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, G.; Zuo, Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm. Res. 2007, 24, 81–89. [Google Scholar] [CrossRef]
- Zhang, R.; Cui, Y.; Wang, Y.; Tian, X.; Zheng, L.; Cong, H.; Wu, B.; Huo, X.; Wang, C.; Zhang, B.; et al. Catechol-O-Methyltransferase and UDP-Glucuronosyltransferases in the Metabolism of Baicalein in Different Species. Eur. J. Drug Metab. Pharm. 2017, 42, 981–992. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Hosseinzadeh, H. Promising influences of Scutellaria baicalensis and its two active constituents, baicalin, and baicalein, against metabolic syndrome: A review. Phytother. Res. 2021, 35, 3558–3574. [Google Scholar] [CrossRef]
- Liu, H.T.; Lin, Y.N.; Tsai, M.C.; Wu, Y.C.; Lee, M.C. Baicalein Exerts Therapeutic Effects against Endotoxin-Induced Depression-like Behavior in Mice by Decreasing Inflammatory Cytokines and Increasing Brain-Derived Neurotrophic Factor Levels. Antioxidants 2022, 11, 947. [Google Scholar] [CrossRef]
- Pan, L.; Cho, K.S.; Yi, I.; To, C.H.; Chen, D.F.; Do, C.W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxid. Med. Cell Longev. 2021, 2021, 8377362. [Google Scholar] [CrossRef]
- Commoner, B.; Townsend, J.; Pake, G.E. Free radicals in biological materials. Nature 1954, 174, 689–691. [Google Scholar] [CrossRef]
- Dahlgren, C.; Karlsson, A. Respiratory burst in human neutrophils. J. Immunol. Methods 1999, 232, 3–14. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Kang, K.A.; Zhang, R.; Kim, B.J.; Kang, S.S.; Hyun, J.W. Mitochondria protection of baicalein against oxidative damage via induction of manganese superoxide dismutase. Environ. Toxicol. Pharmacol. 2011, 31, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bao, Y.; Ju, X.; Li, K.; Shang, H.; Ha, L.; Qian, Y.; Zou, L.; Sun, X.; Li, J. BA-j as a novel CDK1 inhibitor selectively induces apoptosis in cancer cells by regulating ROS. Sci. Rep. 2015, 5, 13626. [Google Scholar] [CrossRef]
- Lee, J.-H.; Li, Y.-C.; Ip, S.-W.; Hsu, S.-C.; Chang, N.-W.; Tang, N.-Y.; Yu, C.-S.; Chou, S.-T.; Lin, S.-S.; Lin, C.-C. The role of Ca2+ in baicalein-induced apoptosis in human breast MDA-MB-231 cancer cells through mitochondria-and caspase-3-dependent pathway. Anticancer Res. 2008, 28, 1701–1711. [Google Scholar]
- Lin, Y.-T.; Yang, J.-S.; Lin, H.-J.; Tan, T.-W.; Tang, N.-Y.; Chaing, J.-H.; Chang, Y.-H.; Lu, H.-F.; Chung, J.-G. Baicalein induces apoptosis in SCC-4 human tongue cancer cells via a Ca2+-dependent mitochondrial pathway. In Vivo 2007, 21, 1053–1058. [Google Scholar] [PubMed]
- Taniguchi, H.; Yoshida, T.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Konishi, M.; Wakada, M.; Kataoka, K.; Yoshikawa, T.; Sakai, T. Baicalein overcomes tumor necrosis factor–related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008, 68, 8918–8927. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yang, C.X.; Zhang, L.; Yang, C.Y.; Xu, X.Q. Baicalein, as a Prooxidant, Triggers Mitochondrial Apoptosis in MCF-7 Human Breast Cancer Cells Through Mobilization of Intracellular Copper and Reactive Oxygen Species Generation. OncoTargets Ther. 2019, 12, 10749–10761. [Google Scholar] [CrossRef]
- Yang, S.; Liao, Y.; Li, L.; Xu, X.; Cao, L. Zeylenone induces mitochondrial apoptosis and inhibits migration and invasion in gastric cancer. Molecules 2018, 23, 2149. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Liu, W.-J.; Yin, Y.-B.; Sun, J.-Y.; Feng, S.; Ma, J.-K.; Fu, X.-Y.; Hou, Y.-J.; Yang, M.-F.; Sun, B.-L.; Fan, C.-D. Natural borneol is a novel chemosensitizer that enhances temozolomide-induced anticancer efficiency against human glioma by triggering mitochondrial dysfunction and reactive oxide species-mediated oxidative damage. OncoTargets Ther. 2018, 11, 5429. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Tsai, K.-W.; Li, Y.-Z.; Chang, Y.-S.; Lai, Y.-C.; Laio, Y.-H.; Wu, J.-D.; Liu, Y.-W. Anti-bladder-tumor effect of baicalein from Scutellaria baicalensis Georgi and its application in vivo. Evid. Based Complement. Altern. Med. 2013, 2013, 579751. [Google Scholar]
- Guo, Z.; Hu, X.; Xing, Z.; Xing, R.; Lv, R.; Cheng, X.; Su, J.; Zhou, Z.; Xu, Z.; Nilsson, S. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol. Cell. Biochem. 2015, 406, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Guo, C.; Yang, Y.; Li, F.; Zhang, Y.; Jiang, B.; Li, Q. Baicalein induces apoptosis of human cervical cancer HeLa cells in vitro. Mol. Med. Rep. 2015, 11, 2129–2134. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Selvamani, A.; Subramanian, R.; Pandi, A.; Thiruvengadam, D. Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in-vivo. Toxicol. Appl. Pharmacol. 2012, 261, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Attardi, L.D.; de Vries, A.; Jacks, T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene 2004, 23, 973–980. [Google Scholar] [CrossRef]
- Gao, C.-F.; Ren, S.; Wang, J.; Zhang, S.-L.; Jin, F.; Nakajima, T.; Ikeda, M.; Tsuchida, N. P130 and its truncated form mediate p53-induced cell cycle arrest inRb−/− Saos2 cells. Oncogene 2002, 21, 7569–7579. [Google Scholar] [CrossRef]
- Ling, Y.; Chen, Y.; Chen, P.; Hui, H.; Song, X.; Lu, Z.; Li, C.; Lu, N.; Guo, Q. Baicalein potently suppresses angiogenesis induced by vascular endothelial growth factor through the p53/Rb signaling pathway leading to G1/S cell cycle arrest. Exp. Biol. Med. 2011, 236, 851–858. [Google Scholar] [CrossRef]
- Qi, J.; Li, J.; Bie, B.; Shi, M.; Zhu, M.; Tian, J.; Zhu, K.; Sun, J.; Mu, Y.; Li, Z. Baicalein Attenuates Hepatocellular Carcinoma Cell Survival and Induces Apoptosis Through the miR-3178/HDAC10 pathway. ResearchSquare 2021. [Google Scholar] [CrossRef]
- Cai, P.; Lu, Y.; Yin, Z.; Wang, X.; Zhou, X.; Li, Z. Baicalein ameliorates osteoporosis via AKT/FOXO1 signaling. Aging (Albany NY) 2021, 13, 17370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wan, Y.; Chen, R.; Zhang, C.; Li, X.; Meng, F.; Glaser, S.; Wu, N.; Zhou, T.; Li, S. The emerging role of cellular senescence in renal diseases. J. Cell. Mol. Med. 2020, 24, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Zhang, C.-F.; Chen, L.; Anderson, S.; Lu, F.; Yuan, C.-S. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int. J. Oncol. 2015, 47, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 12831. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, J.; Zheng, J.; Li, J.; Wei, T.; Zheng, Z.; Chen, Y. Down-regulation of the PI3K/Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin. J. Exp. Clin. Cancer Res. 2012, 31, 48. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Lu, P.; Guo, Q.-Y.; Zhang, Z.-H.; Meng, X.-Y. Baicalein induces apoptosis in esophageal squamous cell carcinoma cells through modulation of the PI3K/Akt pathway. Oncol. Lett. 2013, 5, 722–728. [Google Scholar] [CrossRef]
- Huang, K.; Huang, Y.; Diao, Y. Wogonin induces apoptosis and down-regulates survivin in human breast cancer MCF-7 cells by modulating PI3K–AKT pathway. Int. Immunopharmacol. 2012, 12, 334–341. [Google Scholar] [CrossRef]
- Seo, B.R.; Min, K.-j.; Cho, I.J.; Kim, S.C.; Kwon, T.K. Curcumin significantly enhances dual PI3K/Akt and mTOR inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma Caki cells through down-regulation of p53-dependent Bcl-2 expression and inhibition of Mcl-1 protein stability. PLoS ONE 2014, 9, e95588. [Google Scholar] [CrossRef]
- Annovazzi, L.; Mellai, M.; Caldera, V.; Valente, G.; Tessitore, L.; Schiffer, D. mTOR, S6 and AKT expression in relation to proliferation and apoptosis/autophagy in glioma. Anticancer Res. 2009, 29, 3087–3094. [Google Scholar] [PubMed]
- Li, H.; Gao, Q.; Guo, L.; Lu, S.H. The PTEN/PI3K/Akt pathway regulates stem-like cells in primary esophageal carcinoma cells. Cancer Biol. Ther. 2011, 11, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qiu, S.; Qin, J. Baicalein induced apoptosis and autophagy of undifferentiated thyroid cancer cells by the ERK/PI3K/Akt pathway. Am. J. Transl. Res. 2019, 11, 3341. [Google Scholar] [PubMed]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Dev. Ther. 2018, 12, 3961. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Hui, Y.; Xiaoping, T.; Wei, T.; Jiyi, X.; Xiaolan, Y. Baicalein suppresses the proliferation of human cervical cancer cells via Notch 1/Hes signaling pathway. J. Cancer Res. Ther. 2019, 15, 1216. [Google Scholar]
- Agarwal, S.; Achari, C.; Praveen, D.; Roy, K.R.; Reddy, G.V.; Reddanna, P. Inhibition of 12-LOX and COX-2 reduces the proliferation of human epidermoid carcinoma cells (A431) by modulating the ERK and PI3K-Akt signalling pathways. Exp. Dermatol. 2009, 18, 939–946. [Google Scholar] [CrossRef]
- Chao, J.-I.; Su, W.-C.; Liu, H.-F. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol. Cancer Ther. 2007, 6, 3039–3048. [Google Scholar] [CrossRef]
- Morgan, D.; Garg, M.; Tergaonkar, V.; Tan, S.Y.; Sethi, G. Pharmacological significance of the non-canonical NF-κB pathway in tumorigenesis. Biochim. Biophys. Acta BBA Rev. Cancer 2020, 1874, 188449. [Google Scholar] [CrossRef]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the involvement of the master transcription factor NF-κB in cancer initiation and progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef]
- He, K.; Yu, X.; Wang, X.; Tang, L.; Cao, Y.; Xia, J.; Cheng, J. Baicalein and Ly294002 induces liver cancer cells apoptosis via regulating phosphatidyl inositol 3-kinase/Akt signaling pathway. J. Cancer Res. Ther. 2018, 14, 519. [Google Scholar]
- Hu, J.; Wang, R.; Liu, Y.; Zhou, J.; Shen, K.; Dai, Y. Baicalein represses cervical cancer cell growth, cell cycle progression and promotes apoptosis via blocking akt/mtor pathway by the regulation of circhiat1/mir-19a-3p axis. OncoTargets Ther. 2021, 14, 905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, L.; Cai, L.; Wei, R.; Hu, H.; Jin, W. Effects of baicalein on apoptosis, cell cycle arrest, migration and invasion of osteosarcoma cells. Food Chem. Toxicol. 2013, 53, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-H.; Chen, C.-H.; Gau, R.-J.; Lin, C.-C.; Tsai, C.-L.; Tsai, K.; Lu, F.-J. Effect of baicalein on apoptosis of the human Hep G2 cell line was induced by mitochondrial dysfunction. Planta Med. 2002, 68, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Porter, A. Jaenicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Zhu, H.; Chen, M.; Zhao, Y. Inhibitory effect of baicalein combined with gemcitabine in human pancreatic cancer cell lines. Oncol. Lett. 2018, 15, 5459–5464. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Ye, F.; Wang, H.; Zhang, L.; Zou, Y.; Han, H.; Huang, J. Baicalein induces human osteosarcoma cell line MG-63 apoptosis via ROS-induced BNIP3 expression. Tumor Biol. 2015, 36, 4731–4740. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Zhu, L.; Guo, S.; Yi, X.; Cui, T.; He, Y.; Chang, Y.; Liu, B.; Li, C. Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway. Free Radic. Biol. Med. 2018, 129, 492–503. [Google Scholar] [CrossRef]
- Sarker, M.T.; Saha, S.; Biswas, P.; Islam, M.T.; Sheikh, M.A.; Hasan, M.N.; Islam, N.; Rabbe, M.M.I.; Rafi, M.O. Identification of blood-based inflammatory biomarkers for the early-stage detection of acute myocardial infarction. Netw. Model. Anal. Health Inform. Bioinform. 2022, 11, 28. [Google Scholar] [CrossRef]
- Xiang, L.; Gao, Y.; Chen, S.; Sun, J.; Wu, J.; Meng, X. Therapeutic potential of Scutellaria baicalensis Georgi in lung cancer therapy. Phytomedicine 2022, 95, 153727. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Tangah, J.; Inoue, T.; Chan, H.T. A synopsis on flavonoids from the roots of Scutellaria baicalensis with some insights on baicalein and its anti-cancer properties. J. Chin. Pharm. Sci. 2019, 28, 217–228. [Google Scholar]
- Yu, X.; Liu, Y.; Wang, Y.; Mao, X.; Zhang, Y.; Xia, J. Baicalein induces cervical cancer apoptosis through the NF-κB signaling pathway. Mol. Med. Rep. 2018, 17, 5088–5094. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cao, Y.; Tang, L.; Yang, Y.; Chen, F.; Xia, J. Baicalein inhibits breast cancer growth via activating a novel isoform of the long noncoding RNA PAX8-AS1-N. J. Cell. Biochem. 2018, 119, 6842–6856. [Google Scholar] [CrossRef]
- Jiang, L.; Song, H.; Guo, H.; Wang, C.; Lu, Z. RETRACTED: Baicalein inhibits proliferation and migration of bladder cancer cell line T24 by down-regulation of microRNA-106. Biomed. Pharmacother. 2018, 107, 1583–1590. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Zhou, Y.B.; Xiang, Y.X.; Wang, L.S.; Hu, W.K.; Wang, W.J. Baicalein inhibits osteosarcoma cell proliferation and invasion through the miR-183/Ezrin pathway. Mol. Med. Rep. 2018, 18, 1104–1112. [Google Scholar] [CrossRef]
- Xia, X.; Xia, J.; Yang, H.; Li, Y.; Liu, S.; Cao, Y.; Tang, L.; Yu, X. Baicalein blocked cervical carcinoma cell proliferation by targeting CCND1 via Wnt/β-catenin signaling pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2729–2736. [Google Scholar] [CrossRef]
- Dai, G.; Zheng, D.; Wang, Q.; Yang, J.; Liu, G.; Song, Q.; Sun, X.; Tao, C.; Hu, Q.; Gao, T. Baicalein inhibits progression of osteosarcoma cells through inactivation of the Wnt/β-catenin signaling pathway. Oncotarget 2017, 8, 86098. [Google Scholar] [CrossRef]
- He, N.; Zhang, Z. Baicalein suppresses the viability of MG-63 osteosarcoma cells through inhibiting c-MYC expression via Wnt signaling pathway. Mol. Cell. Biochem. 2015, 405, 187–196. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Chen, J.; He, L.; Meng, X.; Liu, S. Baicalein suppresses the proliferation of acute T-lymphoblastic leukemia Jurkat cells by inhibiting the Wnt/β-catenin signaling. Ann. Hematol. 2016, 95, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Siegler, E.L.; Siriwon, N.; Wang, P. Therapeutic strategies for targeting cancer stem cells. J. Cancer Metastasis Treat. 2016, 2, 233–242. [Google Scholar] [CrossRef]
- Koh, S.Y.; Moon, J.Y.; Unno, T.; Cho, S.K. Baicalein suppresses stem cell-like characteristics in radio-and chemoresistant MDA-MB-231 human breast cancer cells through up-regulation of IFIT2. Nutrients 2019, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.; Liu, J.; Li, Y.; Chen, D.; Zhou, L.; Lang, T.; Zhou, Q. Baicalin attenuates YAP activity to suppress ovarian cancer stemness. OncoTargets Ther. 2020, 13, 7151. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, H.; Chen, S.; Yang, R.; Li, H.; Zhang, G. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway. J. Cell. Mol. Med. 2018, 22, 2478–2487. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Chen, A.Y.; Ye, X.; Luo, H.; Rankin, G.O.; Chen, Y.C. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int. J. Mol. Sci. 2013, 14, 6012–6025. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Wang, P.; Gao, S.; Qu, C.; Liu, L. Effects of baicalein on pancreatic cancer stem cells via modulation of sonic Hedgehog pathway. Acta Biochim. Biophys. Sin. 2018, 50, 586–596. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Dang, H.; Peng, H.; Dai, Z. Baicalein Inhibits the Proliferation of Cervical Cancer Cells Through the GSK3β-Dependent Pathway. Oncol. Res. 2018, 26, 645. [Google Scholar] [CrossRef]
- Verma, E.; Kumar, A.; Daimary, U.D.; Parama, D.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Potential of baicalein in the prevention and treatment of cancer: A scientometric analyses based review. J. Funct. Foods 2021, 86, 104660. [Google Scholar] [CrossRef]
- Xu, D.; Chen, Q.; Liu, Y.; Wen, X. Baicalein suppresses the androgen receptor (AR)-mediated prostate cancer progression via inhibiting the AR NC dimerization and AR-coactivators interaction. Oncotarget 2017, 8, 105561. [Google Scholar] [CrossRef]
- Marc, J.; Bellé, R.; Morales, J.; Cormier, P.; Mulner-Lorillon, O. Formulated glyphosate activates the DNA-response checkpoint of the cell cycle leading to the prevention of G2/M transition. Toxicol. Sci. 2004, 82, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Uzbekov, R.E. Analysis of the cell cycle and a method employing synchronized cells for study of protein expression at various stages of the cell cycle. Biochem. Biokhimiia 2004, 69, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.; O’Connor, P.M. Methods for synchronizing cells at specific stages of the cell cycle. Curr. Protoc. Cell Biol. 2001, 8, 8-3. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, S. Cell-cycle regulation. In The Online Review of C. elegans Biology [Internet]; WormBook: Pasadena, CA, USA, 2005. [Google Scholar]
- Passegué, E.; Wagers, A.J.; Giuriato, S.; Anderson, W.C.; Weissman, I.L. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J. Exp. Med. 2005, 202, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Z.; Leung, H.W.C.; Lai, M.Y.; Wu, C.H. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res. 2005, 25, 959–964. [Google Scholar]
- Sui, X.; Han, X.; Chen, P.; Wu, Q.; Feng, J.; Duan, T.; Chen, X.; Pan, T.; Yan, L.; Jin, T. Baicalin induces apoptosis and suppresses the cell cycle progression of lung cancer cells through downregulating Akt/mTOR signaling pathway. Front. Mol. Biosci. 2021, 7, 602282. [Google Scholar] [CrossRef]
- Yu, Z.; Zhan, C.; Du, H.; Zhang, L.; Liang, C.; Zhang, L. Baicalin suppresses the cell cycle progression and proliferation of prostate cancer cells through the CDK6/FOXM1 axis. Mol. Cell. Biochem. 2020, 469, 169–178. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef]
- Shah, M.A.; Schwartz, G.K. Cell cycle-mediated drug resistance: An emerging concept in cancer therapy. Clin. Cancer Res. 2001, 7, 2168–2181. [Google Scholar]
- Elledge, S.J.; Winston, J.; Harper, J.W. A question of balance: The role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol. 1996, 6, 388–392. [Google Scholar] [CrossRef]
- McDonald, E.R.; El-Deiry, W.S. Checkpoint genes in cancer. Ann. Med. 2001, 33, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Diederich, M. Power from the garden: Plant compounds as inhibitors of the hallmarks of cancer. Curr. Med. Chem. 2012, 19, 2061–2087. [Google Scholar] [CrossRef]
- Pidgeon, G.P.; Kandouz, M.; Meram, A.; Honn, K.V. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002, 62, 2721–2727. [Google Scholar] [PubMed]
- Gioti, K.; Tenta, R. Bioactive natural products against prostate cancer: Mechanism of action and autophagic/apoptotic molecular pathways. Planta Med. 2015, 81, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Huang, T.-S.; Wong, C.-H.; Hong, C.-L.; Tsai, Y.-H.; Liang, C.-C.; Lu, F.-J.; Chang, W.-H. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem. Toxicol. 2009, 47, 638–644. [Google Scholar] [CrossRef]
- Shi, M.-D.; Lin, H.-H.; Lee, Y.-C.; Chao, J.-K.; Lin, R.-A.; Chen, J.-H. Inhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem. Biol. Interact. 2008, 174, 201–210. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chen, Y.-F.; Chen, Y.-T.; Chiu, W.-T.; Shen, M.-R. The STIM1-Orai1 pathway of store-operated Ca2+ entry controls the checkpoint in cell cycle G1/S transition. Sci. Rep. 2016, 6, 22142. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.-H. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine 2008, 15, 683–690. [Google Scholar] [CrossRef]
- Roy, M.K.; Kobori, M.; Takenaka, M.; Nakahara, K.; Shinmoto, H.; Tsushida, T. Inhibition of colon cancer (HT-29) cell proliferation by a triterpenoid isolated from Azadirachta indica is accompanied by cell cycle arrest and up-regulation of p21. Planta Med. 2006, 72, 917–923. [Google Scholar] [CrossRef]
- Havermann, S.; Chovolou, Y.; Humpf, H.-U.; Wätjen, W. Modulation of the Nrf2 signalling pathway in Hct116 colon carcinoma cells by baicalein and its methylated derivative negletein. Pharm. Biol. 2016, 54, 1491–1502. [Google Scholar] [CrossRef]
- Yu, M.; Qi, B.; Xiaoxiang, W.; Xu, J.; Liu, X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2017, 90, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Li, Y.; Xing, J.; Sun, P.; Wang, Y.; Zhang, Y.; Chen, L.; Ren, X.; Lin, Z.; Jin, J. Baicalein inhibits PI3K/AKT signaling pathway and induces autophagy of MGC-803 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2019, 35, 613–618. [Google Scholar]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci. 2022, 25, 14. [Google Scholar] [PubMed]
- Deng, X.; Liu, J.; Liu, L.; Sun, X.; Huang, J.; Dong, J. Drp1-mediated mitochondrial fission contributes to baicalein-induced apoptosis and autophagy in lung cancer via activation of AMPK signaling pathway. Int. J. Biol. Sci. 2020, 16, 1403. [Google Scholar] [CrossRef]
- Naveenkumar, C.; Raghunandhakumar, S.; Asokkumar, S.; Devaki, T. Baicalein abrogates reactive oxygen species (ROS)-mediated mitochondrial dysfunction during experimental pulmonary carcinogenesis in vivo. Basic Clin. Pharmacol. Toxicol. 2013, 112, 270–281. [Google Scholar] [CrossRef]
- D’Yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Chapter 2—Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 21–86. [Google Scholar]
- Das, B.B.; Sen, N.; Roy, A.; Dasgupta, S.B.; Ganguly, A.; Mohanta, B.C.; Dinda, B.; Majumder, H.K. Differential induction of Leishmania donovani bi-subunit topoisomerase I-DNA cleavage complex by selected flavones and camptothecin: Activity of flavones against camptothecin-resistant topoisomerase I. Nucleic Acids Res. 2006, 34, 1121–1132. [Google Scholar] [CrossRef]
- Lacombe, O.K.; Zuma, A.A.; da Silva, C.C.; de Souza, W.; Motta, M.C. Effects of camptothecin derivatives and topoisomerase dual inhibitors on Trypanosoma cruzi growth and ultrastructure. J. Negat. Results Biomed. 2014, 13, 11. [Google Scholar] [CrossRef]
- Tian, Y.; Zhen, L.; Bai, J.a.; Mei, Y.; Li, Z.; Lin, A.; Li, X. Anticancer effects of Baicalein in pancreatic Neuroendocrine tumors in vitro and in vivo. Pancreas 2017, 46, 1076. [Google Scholar] [CrossRef]
- Gu, L.; Xu, F.; Zhang, X.; Gu, Z. Baicalein Inhibits the SMYD2/RPS7 Signaling Pathway to Inhibit the Occurrence and Metastasis of Lung Cancer. J. Oncol. 2022, 2022, 3796218. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, Y.; Zhou, H.; Lv, J. Baicalein inhibits the growth of oral squamous cell carcinoma cells by downregulating the expression of transcription factor Sp1. Int. J. Oncol. 2020, 56, 273–282. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Y.; Li, Y.; Cao, Y.; Tang, L.; Chen, F.; Xia, J. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3 K/Akt pathway. Int. J. Biochem. Cell Biol. 2018, 94, 107–118. [Google Scholar] [CrossRef]
- Zhang, F.-W.; Peng, L.-Y.; Shi, C.-J.; Li, J.-C.; Pang, F.-X.; Fu, W.-M.; Pan, X.-H.; Zhang, J.-F. Baicalein mediates the anti-tumor activity in Osteosarcoma through lncRNA-NEF driven Wnt/β-catenin signaling regulatory axis. J. Orthop. Transl. 2022, 33, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, K.; Tan, J.; Meng, M.; Liu, C.M.; Chen, B.; Huang, C.; Wong, H.L.X.; Bian, Z.; Su, T. Baicalein is a novel TLR4-targeting therapeutics agent that inhibits TLR4/HIF-1α/VEGF signaling pathway in colorectal cancer. Clin. Transl. Med. 2021, 11, e564. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Wang, Z.; Ma, L.; Peng, B.; Mao, K.; Li, C.; Su, M.; Zhou, C.; Peng, G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget 2018, 9, 20089. [Google Scholar] [CrossRef]
- Guo, J.; You, H.; Li, D. Baicalein exerts anticancer effect in nasopharyngeal carcinoma in vitro and in vivo. Oncol. Res. 2019, 27, 601. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.S.; Pal, D.; Checker, R.; Sharma, D.; Sandur, S.K. Baicalein induces cell death in murine T cell lymphoma via inhibition of thioredoxin system. Int. J. Biochem. Cell Biol. 2017, 91, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Chen, M.C.; Pham, H.; Angst, E.; King, J.C.; Park, J.; Brovman, E.Y.; Ishiguro, H.; Harris, D.M.; Reber, H.A. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011, 1813, 1465–1474. [Google Scholar] [CrossRef]
- Liu, B.; Ding, L.; Zhang, L.; Wang, S.; Wang, Y.; Wang, B.; Li, L. Baicalein induces autophagy and apoptosis through AMPK pathway in human glioma cells. Am. J. Chin. Med. 2019, 47, 1405–1418. [Google Scholar] [CrossRef]
- Han, S.E.; Park, C.H.; Nam-Goong, I.S.; Kim, Y.I.; Kim, E.S. Anticancer effects of baicalein in FRO thyroid cancer cells through the up-regulation of ERK/p38 MAPK and akt pathway. In Vivo 2019, 33, 375–382. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Xu, Y.-L.; Tang, Z.-H.; Li, T.; Zhang, L.-L.; Chen, X.; Lu, J.-H.; Leung, C.-H.; Ma, D.-L.; Qiang, W.-A. Baicalein induces beclin 1-and extracellular signal-regulated kinase-dependent autophagy in ovarian cancer cells. Am. J. Chin. Med. 2017, 45, 123–136. [Google Scholar] [CrossRef]
- Ye, C.; Yu, X.; Zeng, J.; Dai, M.; Zhang, B. Effects of baicalein on proliferation, apoptosis, migration and invasion of Ewing’s sarcoma cells. Int. J. Oncol. 2017, 51, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-P.; He, L.; Zhang, Q.-P.; Zeng, X.-T.; Liu, S.-Q. Baicalein inhibits proliferation of myeloma U266 cells by downregulating IKZF1 and IKZF3. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 2809. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, J.; Zhang, C.; Li, Y. Baicalein restrains proliferation, migration, and invasion of human malignant melanoma cells by down-regulating colon cancer associated transcript-1. Braz. J. Med. Biol. Res. 2019, 52, e8934. [Google Scholar] [CrossRef] [PubMed]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Madhunapantula, S.V.; Mosca, P.J.; Robertson, G.P. The Akt signaling pathway: An emerging therapeutic target in malignant melanoma. Cancer Biol. Ther. 2011, 12, 1032–1049. [Google Scholar] [CrossRef] [PubMed]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, Q.; Li, Y.; Li, R.; Feng, J.; Chen, W.; Ahmed, W.; Soufiany, I.; Huang, S.; Long, J.; et al. The PI3K/AKT Pathway-The Potential Key Mechanisms of Traditional Chinese Medicine for Stroke. Front. Med. 2022, 9, 900809. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Yang, Y.; Wang, R.; Wang, Y.; Wu, C.; Du, G. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 117, 109102. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, J.; Liu, S.; Wang, P.; Ma, D.; Zhang, G.; Cao, Y.; Hu, L.; Wang, Z.; Wu, J. CFDP1 promotes hepatocellular carcinoma progression through activating NEDD4/PTEN/PI3K/AKT signaling pathway. Cancer Med. 2022, 12, 425–444. [Google Scholar] [CrossRef]
- Li, P.; Hu, J.; Shi, B.; Tie, J. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem. Biophys. Res. Commun. 2020, 531, 320–327. [Google Scholar] [CrossRef]
- Park, C.H.; Han, S.E.; Nam-Goong, I.S.; Kim, Y.I.; Kim, E.S. Combined effects of baicalein and docetaxel on apoptosis in 8505c anaplastic thyroid cancer cells via downregulation of the ERK and Akt/mTOR pathways. Endocrinol. Metab. 2018, 33, 121–132. [Google Scholar] [CrossRef]
- Yi, S.; Liu, G.; Wu, Y.; Liang, Q.; Li, L. Baicalein suppresses the growth of the human thyroid cancer cells by inducing mitotic catastrophe, apoptosis and autophagy via NF-kB signalling pathway. J. BUON 2020, 25, 389–394. [Google Scholar]
- Wang, Y.F.; Li, T.; Tang, Z.H.; Chang, L.L.; Zhu, H.; Chen, X.P.; Wang, Y.T.; Lu, J.J. Baicalein triggers autophagy and inhibits the protein kinase B/mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Phytother. Res. 2015, 29, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Choi, H.S.; Seo, E.-K.; Kang, D.-H.; Oh, E.-S. Baicalin and baicalein inhibit transforming growth factor-β1-mediated epithelial-mesenchymal transition in human breast epithelial cells. Biochem. Biophys. Res. Commun. 2015, 458, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ahn, K.S.; Wang, L.Z.; Kim, C.; Deivasigamni, A.; Arfuso, F.; Um, J.-Y.; Kumar, A.P.; Chang, Y.-C.; Kumar, D. Ascochlorin Enhances the Sensitivity of Doxorubicin Leading to the Reversal of Epithelial-to-Mesenchymal Transition in Hepatocellular CarcinomaAscochlorin Enhances Anticancer Effects of Doxorubicin. Mol. Cancer Ther. 2016, 15, 2966–2976. [Google Scholar] [CrossRef] [PubMed]
- Katoch, A.; Nayak, D.; Faheem, M.M.; Kumar, A.; Sahu, P.K.; Gupta, A.P.; Kumar, L.D.; Goswami, A. Natural podophyllotoxin analog 4DPG attenuates EMT and colorectal cancer progression via activation of checkpoint kinase 2. Cell Death Discov. 2021, 7, 25. [Google Scholar] [CrossRef]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, M.; Zhong, C.; Peng, J.; Wang, X.; Li, J.; Chen, Z.; Huang, Y. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol. Rep. 2015, 33, 457–463. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, M.; Peng, J.; Wang, X.; Huang, T.; Li, S.; Lin, M.; Lin, H.; Xu, Y.; Li, J. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-β signaling pathway. Mol. Med. Rep. 2014, 10, 1999–2003. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Li, L.-A.; Lin, P.; Cheng, L.-C.; Hung, C.-H.; Chang, N.W.; Lin, C. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol. Appl. Pharmacol. 2012, 263, 360–367. [Google Scholar] [CrossRef]
- Lin, H.; Hao, Y.; Wan, X.; He, J.; Tong, Y. Baicalein inhibits cell development, metastasis and EMT and induces apoptosis by regulating ERK signaling pathway in osteosarcoma. J. Recept. Signal Transduct. 2020, 40, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Chen, H.; Sun, X. Baicalein suppresses non small cell lung cancer cell proliferation, invasion and Notch signaling pathway. Cancer Biomark. 2018, 22, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.H.; Yin, L.H.; Grahn, T.H.M.; Ye, A.F.; Zhao, Y.R.; Zhang, Q.Y. Anticancer effects of baicalein on hepatocellular carcinoma cells. Phytother. Res. 2014, 28, 1342–1348. [Google Scholar] [CrossRef]
- Li, B.; Lu, M.; Jiang, X.-X.; Pan, M.-X.; Mao, J.-W.; Chen, M. Inhibiting reactive oxygen species-dependent autophagy enhanced baicalein-induced apoptosis in oral squamous cell carcinoma. J. Nat. Med. 2017, 71, 433–441. [Google Scholar] [CrossRef]
- Aryal, P.; Kim, K.; Park, P.H.; Ham, S.; Cho, J.; Song, K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of m TORC 1 complex components in human cancer cells. FEBS J. 2014, 281, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, H.-J.; Kim, H.-R.; Lee, S.-H.; Cho, S.-D.; Choi, C.-S.; Nam, J.-S.; Jung, J.-Y. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol. Med. Rep. 2012, 6, 1443–1449. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, K.; Yang, L.; Zhang, G. Bladder cancer cell viability inhibition and apoptosis induction by baicalein through targeting the expression of anti-apoptotic genes. Saudi J. Biol. Sci. 2018, 25, 1478–1482. [Google Scholar] [CrossRef]
- Yan, H.; Xin, S.; Wang, H.; Ma, J.; Zhang, H.; Wei, H. Baicalein inhibits MMP-2 expression in human ovarian cancer cells by suppressing the p38 MAPK-dependent NF-κB signaling pathway. Anticancer Drugs 2015, 26, 649–656. [Google Scholar] [CrossRef]
- Moslehi, M.; Meshkini, A.; Yazdanparast, R. Flavonoid baicalein modulates H2O2-induced mitogen-activated protein kinases activation and cell death in SK-N-MC cells. Cell. Mol. Neurobiol. 2012, 32, 549–560. [Google Scholar] [CrossRef]
- Yaylagül, E.Ö.; Ülger, C. The effect of baicalein on Wnt/ß-catenin pathway and miR-25 expression in Saos-2 osteosarcoma cell line. Turk. J. Med. Sci. 2020, 50, 1168–1179. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; Dai, Z.; Gao, X.; Ma, Y.; Xu, Q.; Jiang, J.; Zhang, S. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Des. Dev. Ther. 2016, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ling, Y.; Chen, Y.; Li, C.-L.; Feng, F.; You, Q.-D.; Lu, N.; Guo, Q.-L. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010, 297, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Silva, M.; Li, S.; Yan, F.; Fang, J.; Peng, T.; Hu, J.; Tsao, M.S.; Little, P.; Zheng, W. The role of FOXOs and autophagy in cancer and metastasis—Implications in therapeutic development. Med. Res. Rev. 2020, 40, 2089–2113. [Google Scholar] [CrossRef] [PubMed]
- Jiramongkol, Y.; Lam, E.W.-F. FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 2020, 39, 681–709. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Gao, L.; Zhai, Y.; Shi, M.; Li, J.; Xiu, C.; Cao, J.; Cheng, S.; Jiang, L. Preparation and characterization of baicalein-loaded nanoliposomes for antitumor therapy. J. Nanomater. 2016, 2016, 2861915. [Google Scholar] [CrossRef]
- You, G.; Feng, T.; Zhang, G.; Chen, M.; Liu, F.; Sun, L.; Wang, M.; Ren, X. Preparation, optimization, characterization and in vitro release of baicalein-solubilizing glycyrrhizic acid nano-micelles. Int. J. Pharm. 2021, 601, 120546. [Google Scholar] [CrossRef]
- Liang, J.; Wu, W.; Liu, Q.; Chen, S. Long-circulating nanoliposomes (LCNs) sustained delivery of baicalein (BAI) with desired oral bioavailability in vivo. Drug Deliv. 2013, 20, 319–323. [Google Scholar] [CrossRef]
- I El-Gogary, R.; Gaber, S.A.A.; Nasr, M. Polymeric nanocapsular baicalin: Chemometric optimization, physicochemical characterization and mechanistic anticancer approaches on breast cancer cell lines. Sci. Rep. 2019, 9, 11064. [Google Scholar] [CrossRef]
- Babu, V.N.; Kannan, S. Enhanced delivery of baicalein using cinnamaldehyde cross-linked chitosan nanoparticle inducing apoptosis. Int. J. Biol. Macromol. 2012, 51, 1103–1108. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Pu, G.; Zhang, F.; Liu, H.; Zhang, Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv. 2016, 23, 1364–1368. [Google Scholar] [CrossRef]
- Meng, L.; Xia, X.; Yang, Y.; Ye, J.; Dong, W.; Ma, P.; Jin, Y.; Liu, Y. Co-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibition. Int. J. Pharm. 2016, 513, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xiang, C.; Wang, P.; Yin, Y.; Hou, Y. Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability. Int. J. Nanomed. 2017, 12, 2923. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Wang, S.; Li, W.; Kebebe, D.; Zhang, Y.; Zhang, B.; Qi, D.; Guo, P.; Li, N.; Liu, Z. A nano-cocrystal strategy to improve the dissolution rate and oral bioavailability of baicalein. Asian J. Pharm. Sci. 2019, 14, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Singh, S.; Saraf, S.A.; Chourasia, M.K.; Mathew, J.; Pandey, A.C. Encapsulation of baicalein in cinnamon essential oil nanoemulsion for enhanced anticancer efficacy against MDA-MB-231 cells. BioNanoScience 2021, 11, 1049–1060. [Google Scholar] [CrossRef]

| In Vivo | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Experimental Model | Dose | Treatment Duration | Standard/ Control | Targeted Pathways | Mechanism of Actions | Outcomes | References |
| Baicalein | Mice pulmonary carcinogenesis model | 12 mg/kg body weight | 16 weeks | Mice that received corn oil during the research period. | ROS-induced mitochondrial DNA damage by free radical scavenging potential | ↓Mitochondrial ROS Production ↓Mitochondrial swelling, ↑VDAC expression, ↑Activity of Krebs Cycle enzymes, ↑Activity of METC enzymes | Inhibited lung carcinogenesis in mice | [136] |
| Baicalein | Human breast carcinoma MCF-7 and MDA-MB-231 cells xenograft in female BALB/c nude mice | 100 mg/kg body weight | 21 days | Untreated nude mice that received in-vitro-cultured MCF-7 and MDA-MB-231 cells into the second left breast pad via subcutaneous injection. | PI3K/AKT/mTOR Pathway | ↓Manifestation of p-AKT, p-mTOR, NF-κB, and p-IκB, ↑Manifestation of IκB at the protein level, ↓Proportion of p-AKT/AKT and p-mTOR/mTOR, ↓Bax/BCL-2 ratio | Induced apoptotic cell demise and autophagy by blocking cell multiplication in breast cancer cells. Significantly reduced cell progression and metastasis. | [74] |
| Baicalein | BON1 (Pancreatic neuroendocrine tumor cell line) cells xenograft in female nude mice | 10 mg/kg body weight | 7 weeks | Untreated female nude mice that received BON1 cells into the head of the pancreas. | Mitochondrial pathway, cleavage of caspase 3 | ↓Expression of Survivin in BON1 Cells, ↓Bcl-2, ↑Bax, ↓MMP-2, ↑MMP-9 | Induced apoptosis, inhibited cell invasion and migration, and reduced tumor volume. | [140] |
| Baicalein | Human lung carcinoma A549 and NCI-H1299 cells xenograft in 24 SPF female nude mice | __ | __ | Mice were inoculated subcutaneously with A549 cells in the right axillary and treated with saline. | SMYD2/RPS7 signaling pathway | ↓Expression of SMYD2 | The carcinogenic effect of SMYD2 was blocked; thus, the growth, migration, and infiltration of human non-small cell lung cancer cells were stopped. | [141] |
| Baicalein | Human oral Cancer SCC25 cells heterograft in BALB/c nude lab rat | 30 mg/kg body weight | 21 days | Control group mice were treated with PBS (0.01% DMSO). | Sp1/NF-κB-dependent pathway | ↓expression of transcription factor Sp1, ↓NF-κB, ↓p65 and p50, cessation of cellular maturation process at the G0/G1 level | Suppressed the development of OSCC (human mouth squamous cell carcinoma) cells, induced apoptosis. | [142] |
| Baicalein | Human cervical cancer HeLa, SiHa, ME-180, and Caski cell lines xenograft in female athymic BALB/c nude mice | 10 mg/kg/day | 28 days | Tumor-inoculated mice were treated with 0.1 mL DMSO (0.25%) | Stimulated PIK3CA manifestation and PI3K/Akt axis | ↓long noncoding RNA (BDLNR) expression, ↑Akt phosphorylation | Diminished cell multiplication, enhanced malignant cell death, blocked migration, and in vivo tumor progression reduction of malignancy of the cervix. | [143] |
| Baicalein | Human osteosarcoma 143 B, MG63 and U2OS cell lines xenograft in female BALB/c nude mice | 40 mg/kg body weight | 14 days | Tumor-inoculated mice were treated with buffer solution (10% DMSO + 40% PEG300 + 5% Tween-80 + 45% saline). | lncRNA-NEF oriented Wnt/β-catenin signaling axis | ↓lncRNA-NEF, ↓Wnt/β-catenin | Inhibited tumor growth, invasion, and metastasis. | [144] |
| Baicalein | Colorectal cancer cell xenograft mouse model | 10 & 20 mg/kg body weight | 21 days | Untreated tumor-inoculated mice. | TLR4/HIF-1α/VEGF signaling trail | ↓HIF-1α and VEGF expressions, ↓NFκB phosphorylation, ↓VEGF, ↓CD31, ↓MMP-2 | Inhibited colorectal cancer growth and angiogenesis. Reduced the metastatic potential. | [145] |
| Baicalein and Baicalin | Human colon cancer cell line HCT116 in humanized NOD-scid IL2Rγnull (NSG) mouse xenograft model | 50 mg/kg body weight | 29 days | Tumor-inoculated mice were treated with intraperitoneally injected solvent. | MAPK/p38/ERK1/2 signaling axis | The tumor cell cycle seized in the S phase and decreased in the G0/G1 phase, ↑phosphorylation of ERK and p38, ↓ hTERT expression | Induced cell cycle cessation of the malignant cells, apoptosis, and senescence. Blocked cell cycle progression. It also inhibited colony formation and migration. | [146] |
| Baicalein | Human nasopharyngeal cancer CNE1 and CNE2 cell lines heterograft in BALB/C nude mice | 1.0 mg/kg, 2.0 mg/kg, and 3.0 mg/kg | 12 days | Tumor-inoculated mice were treated with intraperitoneally injected cisplatin DDP and saline. | Extrinsic and intrinsic apoptotic axis | ↑caspase-3, ↑caspase-8, ↑p62, ↑Bcl-2, ↓p-ERK/ERK, ↓p-Akt/Akt, ↓Bcl-2/Bax, ↑Atg12, ↑Atg5 | Inhibited the development and multiplication of malignant nasopharyngeal cells, modified the cell cycle, and induced apoptosis | [147] |
| Baicalein | Murine model T cell lymphoma (EL4) cells heterograft in C57BL/6 male mice | 10 mg/kg body weight | 3 days | Tumor-inoculated mice were left untreated. | ASK1/Cytochrome-C/Caspase-3 cascade | ↓Gli-2, ↓Sox-2, ↓SHH, ↓SMO, ↓Oct-4 | Diminished the recurrence of cancer stem cells, Induced apoptosis | [148] |
| In Vitro | ||||||||
| Human prostatic carcinoma PC-3 and DU145 cell lines | 20 and 40 μM | 24, 48, and 72 h | Untreated PC-3 and DU145 cells | Caveolin-1/PI3K/AKT/mTOR pathway | Inhibition of Cav-1/PI3K/AKT/mTOR pathway, ↑Bax expression, ↓Bcl-2 expression | Triggered apoptosis in androgen-free malignancy of prostatic cells by impeding their development. Also showed anti-metastatic properties. | [55] | |
| Baicalin, Baicalein, and Wogonin | Human pancreatic carcinoma BxPC-3, HPAF-II, Capan-2, AsPc-1, MIA PaCa-2, and Panc-1 cell lines | 0, 5, 15, and 50 μM | 24 and 48 h | Untreated BxPC-3, HPAF-II, Capan-2, AsPc-1, MIA PaCa-2, and Panc-1 cells | Cleavage of caspase-3, -7, and Poly-ADP ribose polymerase (PARP) | ↓Bcl-2, ↓Mcl-1, ↓Bcl-xL | Diminished proliferation and triggered apoptosis of malignant pancreatic cells. | [149] |
| Baicalein | Human bladder transitional cell carcinoma (BTCC) T24 cells | 0, 50, 100, 150, and 200 μM | 24 h | Untreated T24 cells | c-JNK and MEK/ERK trails, regulation of miR-106 | ↓miR-106, ↑p16, ↑p21, ↓cyclinD1, ↓Bcl-2, ↑Bax, ↓MMP-2, ↑MMP-9 | Repressed proliferation and spread and caused apoptosis. | [96] |
| Baicalein | Human osteogenic sarcoma MG-63 and Saos-2 cell populace | 0, 25, 50, 75, and 100 µM | 24, 48, or 72 h | Untreated MG-63 and Saos-2 cells | miR-183/Ezrin pathway | ↑miR-183, ↓Ezrin | Diminished cell proliferation, migration, and infiltration of osteosarcoma and induction of apoptosis in mentioned cancer | [97] |
| Baicalein | Human glioma cell line U251MG | 80 μM | 12, 24, 36, and 48 h | Untreated U251MG cells | AMPK Pathway | ↑LC3II, ↓p-AMPK, ↓Caspase-3 | Induced autophagy and apoptosis. | [150] |
| Baicalein | Human anaplastic thyroid carcinoma cells (FRO) | 10 μM, 20 μM, 40 μM, and 80 μM | 12, 24, 36, and 48 h | Untreated FRO cells | ERK/PI3K and Akt trail | ↓Bcl-2/Bax, ↓p-ERK/ERK, ↑caspase-3, ↑caspase-8, ↑Bcl-2, ↑Atg5, ↑p62, ↑Atg12, ↓p-Akt/Akt | Induced apoptosis and autophagy, reduced cell colony formation, and arrested tumor cell cycles. | [73] |
| Baicalein | Human anaplastic thyroid carcinoma cells (FRO) | 0, 10, 20, 50, and 100 μM | 24, 48, and 72 h | Untreated FRO cells | ERK/p38, MAPK and Akt trail | ↓Bax, ↓cytochrome-C, ↓PARP, ↓cleaved caspase-3, ↓Cox-2, ↑Bcl-2, ↑p-ERK, ↑Akt, ↓pJNK, ↑p38-MAPK | Induced apoptosis. | [151] |
| Baicalein | Human gastric cancer MGC-803 cell line | 0, 5, 15, 25, 50 μmol/L | 24, 48, 72 h | Untreated MGC-803 cells | PI3K/AKT signaling pathway | ↑Lysosomal acid, ↑LC3, ↓p-AKT, ↑LC3-II/LC3-I, ↓p-PI3K, ↑p62 | Induced autophagy. | [133] |
| Baicalein | Ovarian cancer HEY and A2780 cells | 12.5, 25, and 50 μM | 24 h | Untreated HEY and A2780 cells | Beclin 1 and ERK signaling pathway | ↓Beclin 1, ↑LC3-II, ↑PARP, ↑p-ERK, ↑p-AKT | Induced autophagy, decreased cell viability | [152] |
| Baicalein | Human Ewing Sarcoma SK-ES-1 and RD-ES cell populace | 5, 10, 20, 40, 80, and 160 μM | 24, 36, and 48 h | Untreated SK-ES-1 and RD-ES cells | Mitochondrial apoptotic and death receptor pathway | ↑Bax, ↓Bcl-2, ↑Bax/Bcl-2, ↑Cytochrome-C, ↑Caspase-3, ↑Caspase-9, ↑Caspase-8, ↑MMP-2, ↑MMP-9, ↑PARP | Inhibited Ewing’s Sarcoma cell viability and induced apoptosis. | [153] |
| Baicalein | Human Multiple Myeloma U266 cells | 0, 20, 40, 80, and 160 μmol/L | 0, 6, 12,24, and 48 h | Untreated U266 cells | Proteasomal degradation of IKZF1 and IKZF3 | ↓ IKZF3, ↑CRBN, ↓cIKZF1 | Suppressed growth and promoted apoptosis of myeloma cells. | [154] |
| Baicalein | Malignant melanoma A375 and SK-MEL-28 cell lines | 100, 50, 20, and 10 μM | 24 h | Untreated A375 and SK-MEL-28 cells | Wnt/β-catenin or MEK/ERK signaling axis by regulating CCAT1 (Colon cancer-associated transcript-1) | ↓Caspase-3, ↓MMP-2, ↓vimentin, ↓Wnt/β-catenin, ↓MEK/ERK, ↓PARP | Inhibited proliferation, spread, and infiltration of melanoma cells. | [155] |
| Baicalein | Human gastric carcinoma HGC-27, SGC-7901, MGC-803,and BGC-823 cell lines | 0, 5, 15,25, and 50 µmol/L | 24, 48, and 72 h | Untreated HGC-27, SGC-7901, MGC-803,and BGC-823 cells | miR-7/FAK/AKT signaling axis | ↑miR-7, ↓FAK expression, ↓p-PI3K, ↓p-mTOR, ↓p-FAK, ↓p-AKT | Repressed gastric cancer progression, metastasis, and angiogenesis. | [156] |
| Baicalein | Human colon carcinoma Hct116 cell lines | 0, 10, 20, and 40 μM | 1–4 h | Untreated HGC-27, SGC-7901, MGC-803,and BGC-823 cells | Nrf2 (Nuclear factor erythroid 2-related factor 2) signaling axis | ↓Ser40 phosphorylation, ↓NFκB | Anti-inflammatory response, induced apoptosis. | [132] |
| Baicalein | Human lung adenocarcinoma PC9, H1299, H1650, H358, A549, and H1975 cell lines | 0, 25, 50, 75, 100, and 125 μmol/L | 24, 48, and 72 h | Untreated PC9, H1299, H1650, H358, A549, and H1975 cells | AMPKα/MEK/ERK1&2/FoxO signaling axis | ↑FOXO3a and RUNX3 | Induced apoptosis, inhibited cell growth. | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morshed, A.K.M.H.; Paul, S.; Hossain, A.; Basak, T.; Hossain, M.S.; Hasan, M.M.; Hasibuzzaman, M.A.; Rahaman, T.I.; Mia, M.A.R.; Shing, P.; et al. Baicalein as Promising Anticancer Agent: A Comprehensive Analysis on Molecular Mechanisms and Therapeutic Perspectives. Cancers 2023, 15, 2128. https://doi.org/10.3390/cancers15072128
Morshed AKMH, Paul S, Hossain A, Basak T, Hossain MS, Hasan MM, Hasibuzzaman MA, Rahaman TI, Mia MAR, Shing P, et al. Baicalein as Promising Anticancer Agent: A Comprehensive Analysis on Molecular Mechanisms and Therapeutic Perspectives. Cancers. 2023; 15(7):2128. https://doi.org/10.3390/cancers15072128
Chicago/Turabian StyleMorshed, A K M Helal, Supti Paul, Arafat Hossain, Tuli Basak, Md. Sanower Hossain, Md. Mehedi Hasan, Md. Al Hasibuzzaman, Tanjim Ishraq Rahaman, Md. Abdur Rashid Mia, Pollob Shing, and et al. 2023. "Baicalein as Promising Anticancer Agent: A Comprehensive Analysis on Molecular Mechanisms and Therapeutic Perspectives" Cancers 15, no. 7: 2128. https://doi.org/10.3390/cancers15072128
APA StyleMorshed, A. K. M. H., Paul, S., Hossain, A., Basak, T., Hossain, M. S., Hasan, M. M., Hasibuzzaman, M. A., Rahaman, T. I., Mia, M. A. R., Shing, P., Sohel, M., Bibi, S., Dey, D., Biswas, P., Hasan, M. N., Ming, L. C., & Tan, C. S. (2023). Baicalein as Promising Anticancer Agent: A Comprehensive Analysis on Molecular Mechanisms and Therapeutic Perspectives. Cancers, 15(7), 2128. https://doi.org/10.3390/cancers15072128











