Independent Reproduction of the FLASH Effect on the Gastrointestinal Tract: A Multi-Institutional Comparative Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. MD Anderson
2.1.1. Animals
2.1.2. Irradiation Setup and Parameters
2.1.3. Endpoints
2.1.4. Statistical Analysis
2.2. CHUV
2.2.1. Animals
2.2.2. Irradiation Setup and Parameters
2.2.3. Endpoints
2.2.4. Statistical Analysis
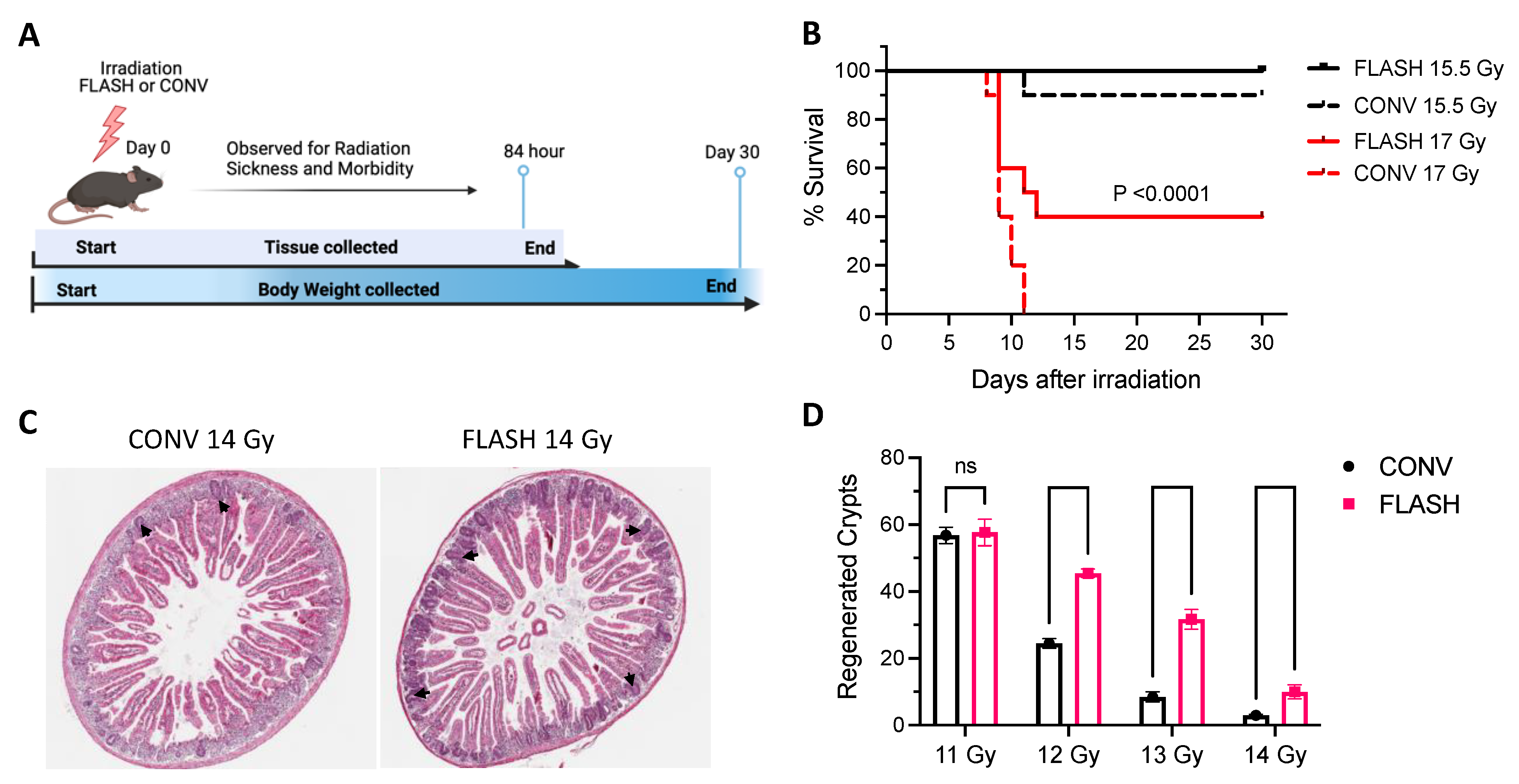
3. Results
3.1. MD Anderson Experiments
3.1.1. Body Weight
3.1.2. Survival
3.1.3. Histologic Evaluation and Crypt Assays
3.2. CHUV Experiments
3.2.1. Body Weight
3.2.2. Survival
3.2.3. Histologic Evaluation and Crypt Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra293. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J. Ultrahigh Dose-rate Radiotherapy: Next Steps for FLASH-RT. Clin. Cancer Res. 2019, 25, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Schüler, E.; Acharya, M.; Montay-Gruel, P.; Loo, B.W., Jr.; Vozenin, M.C.; Maxim, P.G. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med. Phys. 2022, 49, 2082–2095. [Google Scholar] [CrossRef] [PubMed]
- Okoro, C.M.; Schüler, E.; Taniguchi, C.M. The Therapeutic Potential of FLASH-RT for Pancreatic Cancer. Cancers 2022, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Z.; Perentesis, J.P.; Khuntia, D.; Pfister, S.X.; Sharma, R.A. Can Rational Combination of Ultra-high Dose Rate FLASH Radiotherapy with Immunotherapy Provide a Novel Approach to Cancer Treatment? Clin. Oncol. R Coll. Radiol. 2021, 33, 713–722. [Google Scholar] [CrossRef]
- Lin, B.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. FLASH Radiotherapy: History and Future. Front. Oncol. 2021, 11, 644400. [Google Scholar] [CrossRef]
- Levy, K.; Natarajan, S.; Wang, J.; Chow, S.; Eggold, J.T.; Loo, P.E.; Manjappa, R.; Melemenidis, S.; Lartey, F.M.; Schuler, E.; et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci. Rep. 2020, 10, 21600. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Gaide, O.; Herrera, F.; Sozzi, W.J.; Jorge, P.G.; Kinj, R.; Bailat, C.; Duclos, F.; Bochud, F.; Germond, J.-F.; Gondré, M. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother. Oncol. 2022, 174, 87–91. [Google Scholar] [CrossRef]
- Daugherty, E.; Mascia, A.; Sertorio, M.; Zhang, Y.; Lee, E.; Xiao, Z.; Speth, J.; Woo, J.; McCann, C.; Russell, K. FAST-01: Results of the First-in-Human Study of Proton FLASH Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, S4. [Google Scholar] [CrossRef]
- Mascia, A.E.; Daugherty, E.C.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; Backus, L.R.; McDonald, J.M.; McCann, C. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial. JAMA Oncol. 2022, 9, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Yue, J. Radiotherapy and the gut microbiome: Facts and fiction. Radiat. Oncol. 2021, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, B.A.; Cassatt, D.R.; DiCarlo, A.L.; Rios, C.I.; Satyamitra, M.M.; Winters, T.A.; Taliaferro, L.P. Acute Radiation Syndrome and the Microbiome: Impact and Review. Front. Pharmacol. 2021, 12, 643283. [Google Scholar] [CrossRef]
- Valayer, S.; Kim, D.; Fogtman, A.; Straube, U.; Winnard, A.; Caplan, N.; Green, D.A.; van Leeuwen, F.H.P.; Weber, T. The Potential of Fasting and Caloric Restriction to Mitigate Radiation Damage-A Systematic Review. Front. Nutr. 2020, 7, 584543. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, T.T.; Germond, J.F.; Bourhis, J.; Bailat, C.; Bochud, F.; Moeckli, R. The minimal FLASH sparing effect needed to compensate the increase of radiobiological damage due to hypofractionation for late-reacting tissues. Med. Phys. 2022, 49, 7672–7682. [Google Scholar] [CrossRef] [PubMed]
- Moeckli, R.; Goncalves Jorge, P.; Grilj, V.; Oesterle, R.; Cherbuin, N.; Bourhis, J.; Vozenin, M.C.; Germond, J.F.; Bochud, F.; Bailat, C. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT preclinical animal experiments and future clinical human protocols. Med. Phys. 2021, 48, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Palmiero, A.; Chopra, N.; Velasquez, B.; Li, Z.; Beddar, S.; Schüler, E. Dual beam-current transformer design for monitoring and reporting of electron ultra-high dose rate (FLASH) beam parameters. J. Appl. Clin. Med. Phys. 2023, 24, e13891. [Google Scholar] [CrossRef] [PubMed]
- Schüler, E.; Trovati, S.; King, G.; Lartey, F.; Rafat, M.; Villegas, M.; Praxel, A.J.; Loo, B.W., Jr.; Maxim, P.G. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 195–203. [Google Scholar] [CrossRef]
- Loo, B.W.; Schüler, E.; Lartey, F.M.; Rafat, M.; King, G.J.; Trovati, S.; Koong, A.C.; Maxim, P.G. Delivery of ultra-rapid flash radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, E16. [Google Scholar] [CrossRef]
- Schüler, E.; Trovati, S.; King, G.; Lartey, F.; Rafat, M.; Loo, B.; Maxim, P. FLASH irradiation improves the therapeutic index following GI tract irradiation. Med. Phys. 2016, 43, 3783. [Google Scholar] [CrossRef]
- Withers, H.R.; Elkind, M.M. Microcolony Survival Assay for Cells of Mouse Intestinal Mucosa Exposed to Radiation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1970, 17, 261–267. [Google Scholar] [CrossRef]
- Jorge, P.G.; Jaccard, M.; Petersson, K.; Gondré, M.; Durán, M.T.; Desorgher, L.; Germond, J.-F.; Liger, P.; Vozenin, M.-C.; Bourhis, J.; et al. Dosimetric and preparation procedures for irradiating biological models with pulsed electron beam at ultra-high dose-rate. Radiother. Oncol. 2019, 139, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bernier-Latmani, J.; Petrova, T.V. High-resolution 3D analysis of mouse small-intestinal stroma. Nat. Protoc. 2016, 11, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010, 327, 542–545. [Google Scholar] [CrossRef]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; de Sauvage, F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [CrossRef]
- Roughton, K.; Bostrom, M.; Kalm, M.; Blomgren, K. Irradiation to the young mouse brain impaired white matter growth more in females than in males. Cell Death Dis. 2013, 4, e897. [Google Scholar] [CrossRef] [PubMed]
- Roughton, K.; Kalm, M.; Blomgren, K. Sex-dependent differences in behavior and hippocampal neurogenesis after irradiation to the young mouse brain. Eur. J. Neurosci. 2012, 36, 2763–2772. [Google Scholar] [CrossRef]
- Cheng, H.; Leblond, C. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine I. Columnar cell. Am. J. Anat. 1974, 141, 461–479. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Goncalves Jorge, P.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondre, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Böhlen, T.T.; Germond, J.-F.; Bourhis, J.; Vozenin, M.-C.; Ozsahin, E.M.; Bochud, F.; Bailat, C.; Moeckli, R. Normal Tissue Sparing by FLASH as a Function of Single-Fraction Dose: A Quantitative Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 1032–1044. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Values at MD Anderson | Values at CHUV |
|---|---|---|
| Nominal electron beam energy | 9 MeV | 9 MeV |
| Total absorbed dose * | 10.5–17 Gy | 15.8–16.3 Gy |
| Number of pulses | 7–12 | 8 |
| Fractionation schedule | Single fraction | Single fraction |
| Mean dose rate * | 185–225 Gy/s | 199 Gy/s |
| Instantaneous dose rate * | 1.24 × 106 ± 5.74 × 104 Gy/s | 0.97 × 106 Gy/s |
| Pulse frequency | 120 Hz | 90 Hz |
| Dose per pulse * | 1.5 ± 0.07 Gy | 1.94 Gy |
| Pulse width | 1.2 µs | 2 µs |
| Duration of exposure | 42–75 ms | 78 ms |
| Beam field size ** | 40 × 40 mm | 40 mm diameter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés Zayas, A.; Kumari, N.; Liu, K.; Neill, D.; Delahoussaye, A.; Gonçalves Jorge, P.; Geyer, R.; Lin, S.H.; Bailat, C.; Bochud, F.; et al. Independent Reproduction of the FLASH Effect on the Gastrointestinal Tract: A Multi-Institutional Comparative Study. Cancers 2023, 15, 2121. https://doi.org/10.3390/cancers15072121
Valdés Zayas A, Kumari N, Liu K, Neill D, Delahoussaye A, Gonçalves Jorge P, Geyer R, Lin SH, Bailat C, Bochud F, et al. Independent Reproduction of the FLASH Effect on the Gastrointestinal Tract: A Multi-Institutional Comparative Study. Cancers. 2023; 15(7):2121. https://doi.org/10.3390/cancers15072121
Chicago/Turabian StyleValdés Zayas, Anet, Neeraj Kumari, Kevin Liu, Denae Neill, Abagail Delahoussaye, Patrik Gonçalves Jorge, Reiner Geyer, Steven H. Lin, Claude Bailat, François Bochud, and et al. 2023. "Independent Reproduction of the FLASH Effect on the Gastrointestinal Tract: A Multi-Institutional Comparative Study" Cancers 15, no. 7: 2121. https://doi.org/10.3390/cancers15072121
APA StyleValdés Zayas, A., Kumari, N., Liu, K., Neill, D., Delahoussaye, A., Gonçalves Jorge, P., Geyer, R., Lin, S. H., Bailat, C., Bochud, F., Moeckli, R., Koong, A. C., Bourhis, J., Taniguchi, C. M., Herrera, F. G., & Schüler, E. (2023). Independent Reproduction of the FLASH Effect on the Gastrointestinal Tract: A Multi-Institutional Comparative Study. Cancers, 15(7), 2121. https://doi.org/10.3390/cancers15072121








