Simple Summary
There is limited research on the relationship between comorbidity burden and survival among patients with stage I/II non-small-cell lung cancer (NSCLC). Thus, the purpose of this study was to compare survival by comorbidity burden among stage I/II NSCLC patients who have received thoracoscopic surgery as their primary treatment. We found that increasing comorbidity burden was associated with a higher risk of all-cause mortality and that the impact of comorbidity on survival was stronger in female patients with NSCLC than in male patients. These findings highlight the importance of considering comorbidities to optimize the selection of candidates for thoracoscopic resection.
Abstract
We sought to compare overall survival (OS) by comorbidity burden among patients with stage I/II non-small cell lung cancer (NSCLC) who received thoracoscopic resection. Utilizing data from the National Cancer Database, we conducted a survival analysis among patients aged 50+ with stage I/II NSCLC who received thoracoscopic resection between 2010 and 2017. The comorbidity burden was measured by the Charlson comorbidity index (CCI, 0, 1, 2+). Multivariable Cox proportional hazard models were used to compare overall survival relative to the CCI (CCI of 0 as the referent). Subgroup analyses were conducted considering sex, age groups, days from diagnosis to surgery, facility type, laterality, and type of surgery. For this study, 61,760 patients were included, with a mean age of 69.1 years (SD: 8.5). Notably, 51.2% had a CCI of 0, 31.8% had a CCI of 1, and 17.0% had a CCI of 2+. Most participants were non-Hispanic White (87.5%), and 56.9% were female. We found that an increase in the CCI was associated with a higher risk of all-cause mortality (CCI 1 vs. 0 aHR: 1.24, 95% CI: 1.20–1.28; CCI 2+ vs. 0 aHR: 1.51, 95% CI: 1.45–1.57; p-trend < 0.01). Our subgroup analysis according to sex suggested that the association between CCI and risk of death was stronger in women.
1. Introduction
Lung cancer accounts for the most cancer-related deaths in the US [1]. In 2022, an estimated 236,740 people in the US were diagnosed with lung cancer, and lung cancer deaths accounted for 21% of all cancer-related deaths [1]. With the dissemination of lung cancer screening, the proportion of patients diagnosed with stage I/II lung cancer has continuously increased in the US. Between 2013 and 2017, the average annual percent change in early-stage lung cancer cases was 6.88%, while the average annual percent change in lung cancer cases diagnosed at an advanced stage was −2.74% [2]. Compared with stage III/IV lung cancer patients, patients with stage I/II disease have a more favorable prognosis after treatment.
Non-small-cell lung cancer (NSCLC) accounts for ~85% of incident lung cancer cases in the US. In clinical practice, surgical resection is usually the first choice when treating early-stage NSCLC [3]. In recent years, thoracoscopic resection has become increasingly favored over open surgical resection, as thoracoscopy is associated with fewer postprocedural complications, shorter length of hospital stay, and faster recovery [3,4,5,6,7]. Some clinical studies suggest that patients who undergo thoracoscopic resections have more favorable survival outcomes than those undergoing open surgeries [8,9,10,11,12,13].
Lung cancer is an age-related disease, with a median age at diagnosis of 71 years in the US according to data from the Surveillance, Epidemiology, and End Results (SEER) Program [14]. Therefore, patients with lung cancer may have a higher risk of co-existing illnesses than those with other types of cancer, who tend to be diagnosed at younger ages. Clinical evidence suggests that comorbidity burden is an independent prognostic factor among stage I/II NSCLC patients undergoing open surgery [15]. However, limited studies have explored how the risk of death among stage I/II NSCLC patients treated with thoracoscopic resection varies by comorbidity burden. Understanding the impact of comorbidity burden on the survival of these patients receiving thoracoscopic resection can be fundamental for decision making in clinical practice for stage I/II NSCLC.
Therefore, we leveraged the National Cancer Database (NCDB) to compare the differences in overall survival (OS) by comorbidity burden among adults with early-stage NSCLC who underwent thoracoscopic resection.
2. Methods
2.1. Data Source and Patient Selection
The NCDB is a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons. The NCDB is a hospital-based cancer registry that captures 72% of all newly diagnosed cancers in the US [16]. The data are extracted from electronic health records (EHRs) by certified tumor registrars. Healthcare facilities that participate in the NCDB are also required to capture information regarding their patients’ cancer care that occurred outside of their facilities, including care from facilities that are not accredited by the Commission on Cancer. All data are validated before being released [17], and the variables collected include patient demographic information, tumor characteristics, first-course cancer treatment, and survival [18]. We obtained NCDB data for patients diagnosed with NSCLC between 2010 and 2017 and selected our analytic sample using the following criteria: (1) patients with primary stage I/II NSCLC at the time of diagnosis; (2) patients aged 50 or greater at the time of diagnosis, as there are relatively few younger patients with a high comorbidity burden; (3) patients who received thoracoscopic resection; and (4) those with no missing data on the Charlson comorbidity index (CCI) score, mortality, or our selected covariates. This yielded a total of 61,760 participants for analysis. A flowchart of participant selection is presented in Figure 1. This study was exempt from IRB review as the data used in this study were obtained from a de-identified NCDB file.
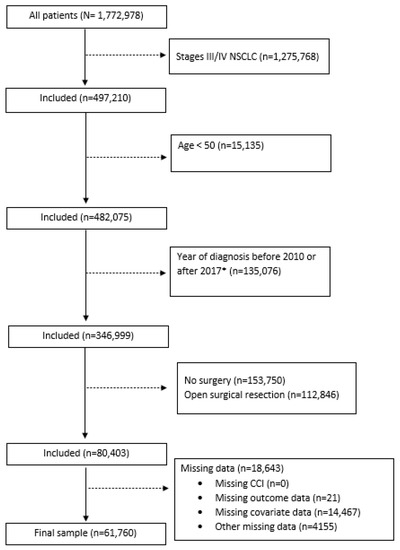
Figure 1.
Flowchart for study participants selection. Abbreviations: NSCLC: non-small-cell lung cancer; CCI: Charlson comorbidity index. *Diagnosis years 2010–2017 selected due to inconsistencies in surgical resection classification for cases diagnosed prior to 2010 and lack of survival data for cases diagnosed in 2018.
2.2. Exposure, Outcome, and Covariates
The exposure of interest in this analysis was comorbidity burden, measured using the Charlson–Deyo version of the CCI and categorized as an ordinal variable [19]. Because only a small fraction of stage I/II NSCLC cases had a CCI score higher than 2, the CCI was truncated to 0, 1, and 2+ in our study, with a score of 0 indicating no comorbid conditions. The outcome of interest in this analysis was all-cause mortality, with the follow-up time measured as time from diagnosis to death or last contact, whichever occurred first. Facilities participating in the NCDB are expected to report patient follow-up annually and have a follow-up rate of at least 90% for all patients diagnosed within the prior 5 years [17]. Patient-level characteristics included age at diagnosis, sex (male or female), and race/ethnicity (Non-Hispanic (NH) White, NH Black, American Indian, Asian/Pacific Islander, Hispanic, or other). Area-level covariates, which included education and income, were inferred by linking patients’ zip codes to the US Census data. Specifically, education was defined as the percentage of adults 25 years or older that did not graduate high school, and we used the median household income to reflect area-level income; in our study, these variables were derived using data from the American Community Survey and were categorized into equally proportioned quartiles based on all US zip codes (education: ≥17.6%, 10.9–17.5%, 6.3–10.8%, <6.3%; income: ≥USD 63,333; USD 50,354–USD 63,332; USD 40,227–USD 50,353; <USD 40,227). Provider-related covariates included insurance status (Medicare, Medicaid, private, uninsured, or other) and facility type (non-academic or academic). Histological subtype, stage, and laterality were the cancer-specific characteristics included in our study. Tumor histological subtype was determined by using the International Classification of Diseases for Oncology (ICD-O-3) morphology codes (adenocarcinoma, squamous cell carcinoma, large cell/neuroendocrine carcinoma, or other). Cancer stage was defined by the 6th or 7th edition of the American Joint Committee of Cancer TNM staging system, as they were the editions in use during the study period. Laterality was included as a cancer-related covariate, as it has been suggested that laterality may affect postsurgical survival in lung cancer [20]. Treatment-related factors included receipt of adjuvant chemotherapy, surgical margin status (no residual tumor, residual tumor not specified, microscopic residuals, macroscopic residuals, or indeterminate), days from diagnosis to surgery, and type of surgery (sublobar resection, lobectomy/bilobectomy, pneumonectomy, or other). The type of surgery was categorized based on prior literature [21]. Covariates were selected based on a priori knowledge regarding their associations with our exposure and outcome of interest as well as based on prior NCDB analyses [22,23].
2.3. Statistical Analysis
We first descriptively summarized study characteristics in the overall study population and according to CCI scores (0, 1, and 2+) by reporting the number of observations and percentages. Kaplan–Meier curves were used to visualize the probability of survival relative to CCI categories, and the log-rank test was used to examine if the risk of mortality differed relative to the CCI. A Kaplan-Meier curve including patients with missing values for the relevant covariates was also used to evaluate if data missingness affected the survival probabilities. Number of deaths and mortality rates were summarized for the overall study population and according to CCI categories. Age-adjusted and multivariable Cox proportional hazard models were used to examine the association between comorbidity burden (reference: CCI = 0) and risk of all-cause mortality. Two multivariable Cox models were conducted; therefore, hazard ratios (HRs) and 95% confidence intervals (95% CIs) were the measures of association in our study; the first model was adjusted for age, race/ethnicity, education, income, insurance, and facility type. The second model was additionally adjusted for the stage at diagnosis, histological type, days from diagnosis to surgery, and receipt of adjuvant chemotherapy. We assessed the proportional hazards assumption by checking Schoenfeld residuals [24]. In both multivariable Cox models, age and sex violated the proportional hazards assumptions; thus, we stratified the multivariable models by sex and included an interaction term between age and follow-up, and no violation was observed afterward. A trend test was performed by treating the CCI as a continuous variable in the model. Subgroup analyses were conducted considering sex (female vs. male), age groups (50–64, 65–74, 75+), days from diagnosis to surgery (<30 days vs. ≥30 days), facility type (academic vs. non-academic), laterality (left vs. right), and type of surgery (sublobar resection, lobectomy/bilobectomy, pneumonectomy, and other). In each subgroup analysis, we created an interaction term between the CCI and the variable used for stratification (i.e., sex, age group, days from diagnosis to surgery, etc.); the Wald test was used to assess if the interaction was significant, with a p-value < 0.05 suggesting statistical heterogeneity between subgroups. Four sets of sensitivity analyses were conducted. The first sensitivity analysis examined if the association obtained in the primary Cox model changed substantially when only analyzing participants who did not receive adjuvant chemotherapy. Then, to assess the stability of the HR relative to the CCI, we created an indicator variable of exclusion due to missing data in our selected covariates and included this indicator in the multivariable models with different sample sizes; in addition to the indicator of exclusion, all these models were adjusted for demographic variables (age, sex, and race/ethnicity) plus (1) socioeconomic variables (education and income), (2) provider-related factors (insurance and facility type), or (3) cancer-related characteristics (histological type, stage, use of chemotherapy, and days from diagnosis to surgery). For our third sensitivity analysis, we evaluated the proportions of deaths within 90 days after surgery relative to the CCI. In our final sensitivity analysis, we explored the relationship between the type of sublobar resection (wedge resection, segmental resection, or other), CCI, and mortality. All analyses were performed in SAS 9.4 (Cary, NC, USA) and R Studio V 4.1.1. All statistical tests were two-sided, and a p-value < 0.05 indicated statistical significance.
3. Results
A total of 61,760 patients who received thoracoscopy for treatment of stage I/II NSCLC between 2010 and 2017 were included in the present analysis. Of those, 51.2% had a CCI of 0, 31.8% had a CCI of 1, and 17.0% had a CCI of 2 or greater. The mean age at diagnosis was 69.1 years (SD: 8.5 years), approximately 57% were female, and a majority (87.5%) of the study population self-identified as NH White. Most patients had stage I NSCLC (88.5%), and the most common histological subtype was adenocarcinoma (53.9%). The majority of the patients had complete resection with no residual margins (95.6%). Among the 4.4% who did not receive complete resection, over 60% were treated in non-academic facilities, which may have a lower volume of surgical resections. The detailed distributions of the study characteristics are presented in Table 1.

Table 1.
Summary of study characteristics.
In our study, the median follow-up time of all patients was 39.2 months (IQR: 24.2–60.8). During the follow-up, 18,833 (30.5%) patients died. The Kaplan–Meier survival curve (Figure 2) relative to the CCI indicated that patients with higher CCI scores had a higher risk of all-cause mortality (log-rank p < 0.01). The Kaplan-Meier survival curve including patients with missing values for relevant covariates (Supplementary Figure S1) produced similar results (log-rank p < 0.01). The all-cause mortality rate was 83.6 (95% CI: 82.5–84.8) per 1000 person-years. Among patients with a CCI of 0, the all-cause mortality rate was 68.7 (95% CI: 67.3–70.2) per 1000 person-years, and it increased with the CCI score (CCI = 1: 91.2 (95% CI: 89.1–93.3); CCI = 2+: 118.2 (95% CI: 114.8–121.7) per 1000 person-years). Similarly, the results of the multivariable Cox proportional hazard models (Table 2) showed that, compared with patients with CCI scores of 0, patients with CCI scores of 1 and 2+ had a 24% and 51% relative increase in the risk of all-cause mortality, respectively (full model aHR (CCI 1 vs. 0): 1.24, 95% CI: 1.20–1.28; aHR (CCI 2+ vs. 0): 1.51; 95% CI: 1.45–1.57; p-trend < 0.01).
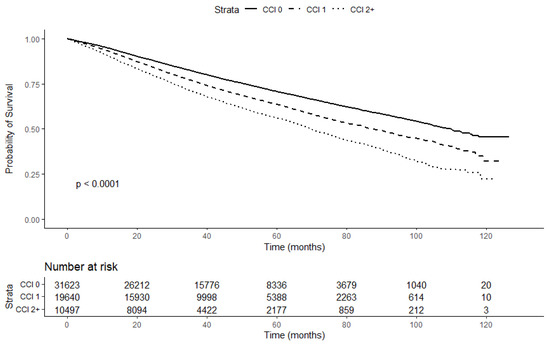
Figure 2.
Kaplan–Meier survival curve stratified by CCI.

Table 2.
Association of all-cause mortality with CCI in stage I-II NSCLC patients undergoing thoracoscopic resection (N = 61,760).
Our subgroup analysis (Table 3) considering sex found that, although the associations between the CCI and all-cause mortality were positive and significant in both males and females, the magnitude of association was stronger for females when comparing CCI = 1 versus CCI = 0 (female aHR for CCI 1: 1.31, 95% CI: 1.25–1.37; male aHR for CCI 1: 1.17, 95% CI: 1.12–1.22; p-interaction < 0.01). This pattern was also observed among patients with a CCI of 2 or greater (female aHR: 1.65, 95% CI: 1.56–1.75; male aHR: 1.40, 95% CI: 1.33–1.47). We also found that the association between the CCI and all-cause mortality varied by days from diagnosis to surgery (p-interaction < 0.01), facility type (p-interaction < 0.01), and the type of surgery (p-interaction < 0.01); however, because of the overlap in 95% CIs of HR in these subgroups, their magnitude of heterogeneity was not as strong as heterogeneity by sex. Our subgroup analyses considering age and tumor laterality did not reveal significant interaction (Table 3). In our sensitivity analysis that only included patients who did not receive adjuvant chemotherapy, the effect measures for the CCI were not substantially different from those obtained in the main analysis (Supplementary Table S1). In models adjusting for the indicators of exclusion, the effect measures of CCI scores did not substantially change (Supplementary Table S2). Our sensitivity analysis for 90-day mortality revealed a similar trend to that of long-term mortality: Participants with a CCI of 0 had the lowest proportion of 90-day mortality (1.8%, 95% CI: 1.7–2.0%), and participants with a CCI of 2 or greater had the highest proportion of 90-day mortality (3.2%, 95% CI: 2.9–3.6%) (Supplementary Table S3). Finally, in our sensitivity analysis among patients who underwent sublobar resection only, effect measures were similar in terms of the type of sublobar resection and did not differ substantially from those obtained in the main analysis (Supplementary Table S4).

Table 3.
Subgroup analysis for the association between CCI and risk of all-cause mortality by selected covariates.
4. Discussion
In the present analysis, we found that a high comorbidity burden was associated with lower overall survival among stage I/II NSCLC patients undergoing thoracoscopic resection. Our data indicated that the impact of comorbidity burden was stronger in females than in males, and we have a hypothesis to explain this phenomenon. Prior studies suggest that female cancer patients tend to have higher levels of frailty than male cancer patients [25,26,27]. For example, our prior study of data from the National Health and Nutrition Examination Survey showed that women with a prior cancer diagnosis were 15% more likely to have frailty than their male counterparts [27]. Therefore, it is possible that pre-existing frailty combined with the comorbidity burden could contribute to poorer survival outcomes for female patients [28].
To our knowledge, this is the first analysis leveraging national cancer registry data to explore the relationship between comorbidity burden and survival among patients undergoing thoracoscopic resection for treatment of early-stage NSCLC. Prior studies have shown that lung cancer patients with comorbidities have poor short-term outcomes following minimally invasive surgery (MIS); specifically, preoperative comorbidity is associated with an increased risk of complications, longer hospital stays, and increased risk of in-hospital mortality [29]. While there is limited research exploring comorbid conditions in relation to long-term outcomes after MIS such as thoracoscopic resection, our findings are similar to what has been reported among patients receiving open surgical resection for the treatment of NSCLC. For instance, an analysis of surgically resected NSCLC patients (N = 3152) within the Danish Lung Cancer Registry (DLCR) found that increased CCI was associated with decreased 5-year survival in early-stage NSCLC patients (for pT1 NSCLC, high comorbidity: 38%, low comorbidity: 69%) [30].
Several underlying mechanisms may explain our findings from this study. Cellular senescence, a hallmark of aging, is the condition in which a cell undergoes permanent growth arrest and is no longer able to proliferate, [31] and it plays an important role in the development of age-related diseases (e.g., cardiovascular disease, dementia, diabetes, etc.) [31,32,33]. Therefore, patients with a high burden of comorbidities are more likely to have cellular senescence, which can adversely affect survival following cancer treatment. In addition, senescence in immune cells can induce immunosenescence, compromising the immune surveillance of NSCLC patients and increasing the risk of cancer-related adverse events such as cancer recurrence or cancer-specific death [34,35,36]. In addition, chronic inflammation, which is tightly connected to the pathogenesis of many chronic diseases [37,38,39,40,41], can negatively impact postsurgical outcomes and cancer prognosis; this can also partially explain why NSCLC patients with a higher burden of comorbidities have shorter survival. Further, NSCLC patients—particularly those with pre-existing chronic conditions—are often at risk of cardiac and/or respiratory complications, including but not limited to pneumonia, atrial fibrillation, and venous thromboembolism [42,43]. Such complications may occur shortly after surgical resection and often cause permanent damage, serving as competing risks for mortality. NSCLC patients with postoperative complications have been found to have reduced 5-year overall survival compared with their counterparts who did not develop postoperative complications [42]. Other factors that can influence prognosis following the surgical resection of NSCLC among patients with an increased comorbidity burden include smoking history [44]—which can also influence the development of postsurgical complications—and histological type at diagnosis [45]. Finally, it is worth noting that the factors related to healthcare delivery may also play a role in the increased risk of mortality seen in patients with higher CCI. Cancer patients with a high comorbidity burden face significantly higher healthcare costs, they are more likely to receive conservative adjuvant therapy, and coordination of healthcare delivery is more complex for patients with multimorbidity, all of which can increase the risk of mortality [46,47,48].
Several limitations should be noted when interpreting our results. First, the NCDB does not provide information on the cause of death, leaving us unable to analyze deaths due to cancer versus deaths due to other causes. Second, residual confounding may exist in our analysis. For example, smoking is an important factor that should be considered in the survival analysis of lung cancer patients; however, the NCDB does not collect this information. Third, differences in staging classification over the years included in the present analysis may also lead to potential stage shifting. The use of both immunotherapies and targeted therapies in adjuvant and neoadjuvant settings may also influence the obtained mortality estimates. Finally, a majority of the patients included in our analysis were non-Hispanic White, indicating that the generalizability of our findings may be compromised in minority populations. Despite these limitations, our analysis has notable strengths. The NCDB is one of the largest cancer registries in the world [17], and it collects standardized, high-quality data that undergo strict quality control methods, which enables the validity of measurement and robust power in analysis. Furthermore, our sample included over 60,000 NSCLC cases; this large sample size strengthens the precision of our estimates and is significantly larger than many studies of NSCLC patients. Finally, our subgroup analyses enabled us to explore the potential effect modification induced by covariates.
5. Conclusions
Our study found that a high comorbidity burden was associated with increased mortality among stage I/II NSCLC patients undergoing thoracoscopic resection, even after controlling for clinically important patient characteristics. This finding indicates that comorbidity burden is an independent predictor of NSCLC patients’ survival following thoracoscopic resection, highlighting the need for clinicians to consider comorbidity burden when evaluating whether a patient is a suitable candidate for thoracoscopy, and to consider comorbidity burden when discussing the benefits and harms of thoracoscopy with their patients. Further research, particularly with prospective study design, is necessary to confirm that comorbidity burden has a more substantial impact on the survival of female stage I/II NSCLC patients undergoing thoracoscopy and to explore the potential biological mechanisms that influence survival differences relative to sex among NSCLC patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers15072075/s1, Table S1: Sensitivity analysis for participants not receiving chemotherapy. Table S2: Sensitivity analysis incorporating different sets of covariates and indicators of exclusion due to missing data. Table S3: Sensitivity analysis for 90-day mortality relative to CCI. Table S4: Sensitivity analysis for participants who received sublobar resection. Figure S1: Kaplan–Meier survival curve relative to CCI, including patients with missing values for relevant covariates.
Author Contributions
Conceptualization, D.Z., D.B. and S.D.K.; methodology, D.Z., D.B., S.D.K., M.K.G. and M.W.; data analysis, M.W. and D.Z.; writing—original draft preparation, M.W., S.D.K., H.J.M., D.Y., L.A.-P., C.W., Y.-R.H., M.K.G., D.B. and D.Z.; writing—review and editing, M.W., S.D.K., H.J.M., D.Y., L.A.-P., C.W., Y.-R.H., M.K.G., D.B. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the National Institutes of Health/National Cancer Institute (Grant Number R01-CA249506).
Institutional Review Board Statement
This study was exempt from IRB review as all data were obtained from a de-identified NCDB file.
Informed Consent Statement
Patient consent was not required as all data included in the study was coded and de-identified.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Dongyu Zhang is an employee at Johnson & Johnson but, at the time of this study, was affiliated with the University of Florida; he has no conflicts of interest to declare. The remaining authors report no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Stage at Diagnosis. 2022. Available online: https://progressreport.cancer.gov/diagnosis/stage (accessed on 22 April 2022).
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Bendixen, M.; Jorgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.P.; Chang, E.; Senagore, A.J.; Broder, M. Clinical outcomes and resource utilization associated with laparoscopic and open colectomy using a large national database. Ann. Surg. 2008, 247, 819–824. [Google Scholar] [CrossRef]
- Boffa, D.J.; Dhamija, A.; Kosinski, A.S.; Kim, A.W.; Detterbeck, F.C.; Mitchell, J.D.; Onaitis, M.W.; Paul, S. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J. Thorac. Cardiovasc. Surg. 2014, 148, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, M.; Tan, L.; Wang, Q. Comparison of the short-term quality of life in patients with esophageal cancer after subtotal esophagectomy via video-assisted thoracoscopic or open surgery. Dis. Esophagus 2010, 23, 408–414. [Google Scholar] [CrossRef]
- Dziedzic, R.; Marjanski, T.; Binczyk, F.; Polanska, J.; Sawicka, W.; Rzyman, W. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: A propensity score-matched analysis. Eur. J. Cardiothorac. Surg. 2018, 54, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Video-assisted thoracoscopic surgery for non-small-cell lung cancer is beneficial to elderly patients. Int. J. Clin. Exp. Med. 2015, 8, 13604–13609. [Google Scholar]
- Laursen, L.O.; Petersen, R.H.; Hansen, H.J.; Jensen, T.K.; Ravn, J.; Konge, L. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur. J. Cardiothorac. Surg. 2016, 49, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Akhtar-Danseh, G.G.; Akhtar-Danesh, N.; Finley, C. Uptake and survival effects of minimally invasive surgery for lung cancer: A population-based study. Eur. J. Surg. Oncol. 2021, 47, 1791–1796. [Google Scholar] [CrossRef]
- Yamashita, S.I.; Tokuishi, K.; Moroga, T.; Nagata, A.; Imamura, N.; Miyahara, S.; Yoshida, Y.; Waseda, R.; Sato, T.; Shiraishi, T.; et al. Long-term survival of thoracoscopic surgery compared with open surgery for clinical N0 adenocarcinoma. J. Thorac. Dis. 2020, 12, 6523–6532. [Google Scholar] [CrossRef]
- Taioli, E.; Lee, D.S.; Lesser, M.; Flores, R. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: A meta-analysis. Eur. J. Cardiothorac. Surg. 2013, 44, 591–597. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 6 May 2022).
- Battafarano, R.J.; Piccirillo, J.F.; Meyers, B.F.; Hsu, H.S.; Guthrie, T.J.; Cooper, J.D.; Patterson, G.A. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2002, 123, 280–287. [Google Scholar] [CrossRef]
- Mallin, K.; Browner, A.; Palis, B.; Gay, G.; McCabe, R.; Nogueira, L.; Yabroff, R.; Shulman, L.; Facktor, M.; Winchester, D.P.; et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012-2014. Ann. Surg. Oncol. 2019, 26, 1604–1612. [Google Scholar] [CrossRef]
- Boffa, D.J.; Rosen, J.E.; Mallin, K.; Loomis, A.; Gay, G.; Palis, B.; Thoburn, K.; Gress, D.; McKellar, D.P.; Shulman, L.N.; et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017, 3, 1722–1728. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann. Surg. Oncol. 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, F.G.; Force, S.D.; Pickens, A.; Kilgo, P.D.; Luu, T.; Miller, D.L. Impact of laterality on early and late survival after pneumonectomy. Ann. Thorac. Surg. 2011, 92, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Blom, E.F.; Ten Haaf, K.; Arenberg, D.A.; de Koning, H.J. Uptake of minimally invasive surgery and stereotactic body radiation therapy for early stage non-small cell lung cancer in the USA: An ecological study of secular trends using the National Cancer Database. BMJ Open Respir. Res. 2020, 7, e000603. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, K.; Cochuyt, J.; Hodge, D.; Qin, H.; Manochakian, R.; Zhao, Y.; Ailawadhi, S.; Adjei, A.A.; Lou, Y. Survival of Black and White Patients With Stage IV Small Cell Lung Cancer. Front. Oncol. 2021, 11, 773958. [Google Scholar] [CrossRef]
- Shi, R.; Diaz, R.; Shi, Z.; Duvall, E.; Mills, G. The Effect of Payer Status on Survival of Patients with Stage I/II Non-small Cell Lung Cancer: NCDB 1998-2011. Anticancer Res. 2016, 36, 319–326. [Google Scholar] [PubMed]
- SCHOENFELD, D. Partial residuals for the proportional hazards regression model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- Gordon, E.H.; Peel, N.M.; Samanta, M.; Theou, O.; Howlett, S.E.; Hubbard, R.E. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 2017, 89, 30–40. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S.; de Rooij, B.H.; Mols, F.; Oerlemans, S.; Husson, O.; Schoormans, D.; Haanen, J.B.; van de Poll-Franse, L.V. Sex-differences in symptoms and functioning in >5000 cancer survivors: Results from the PROFILES registry. Eur. J. Cancer 2021, 156, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mobley, E.M.; Manini, T.M.; Leeuwenburgh, C.; Anton, S.D.; Washington, C.J.; Zhou, D.; Parker, A.S.; Okunieff, P.G.; Bian, J.; et al. Frailty and risk of mortality in older cancer survivors and adults without a cancer history: Evidence from the National Health and Nutrition Examination Survey, 1999–2014. Cancer 2022, 128, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Jawitz, O.K.; Wang, Z.; Boffa, D.J.; Detterbeck, F.C.; Blasberg, J.D.; Kim, A.W. The differential impact of preoperative comorbidity on perioperative outcomes following thoracoscopic and open lobectomies. Eur. J. Cardiothorac. Surg. 2017, 51, 169–174. [Google Scholar] [CrossRef]
- Luchtenborg, M.; Jakobsen, E.; Krasnik, M.; Linklater, K.M.; Mellemgaard, A.; Moller, H. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur. J. Cancer 2012, 48, 3386–3395. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Chkhotua, A.B.; Gabusi, E.; Altimari, A.; D’Errico, A.; Yakubovich, M.; Vienken, J.; Stefoni, S.; Chieco, P.; Yussim, A.; Grigioni, W.F. Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am. J. Kidney Dis. 2003, 41, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Lei, Q.; Zhang, Y. Tumor-intrinsic signaling pathways: Key roles in the regulation of the immunosuppressive tumor microenvironment. J. Hematol. Oncol. 2019, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Webster, M.R.; Marchbank, K.; Behera, R.; Ndoye, A.; Kugel, C.H., 3rd; Dang, V.M.; Appleton, J.; O’Connell, M.P.; Cheng, P.; et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 2016, 532, 250–254. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Kim, Y.; Bayona, P.W.; Kim, M.; Chang, J.; Hong, S.; Park, Y.; Budiman, A.; Kim, Y.J.; Choi, C.Y.; Kim, W.S.; et al. Macrophage Lamin A/C Regulates Inflammation and the Development of Obesity-Induced Insulin Resistance. Front. Immunol. 2018, 9, 696. [Google Scholar] [CrossRef]
- Agere, S.A.; Akhtar, N.; Watson, J.M.; Ahmed, S. RANTES/CCL5 Induces Collagen Degradation by Activating MMP-1 and MMP-13 Expression in Human Rheumatoid Arthritis Synovial Fibroblasts. Front. Immunol. 2017, 8, 1341. [Google Scholar] [CrossRef] [PubMed]
- Espigol-Frigole, G.; Planas-Rigol, E.; Lozano, E.; Corbera-Bellalta, M.; Terrades-Garcia, N.; Prieto-Gonzalez, S.; Garcia-Martinez, A.; Hernandez-Rodriguez, J.; Grau, J.M.; Cid, M.C. Expression and Function of IL12/23 Related Cytokine Subunits (p35, p40, and p19) in Giant-Cell Arteritis Lesions: Contribution of p40 to Th1- and Th17-Mediated Inflammatory Pathways. Front. Immunol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Negera, E.; Tilahun, M.; Bobosha, K.; Lambert, S.M.; Walker, S.L.; Spencer, J.S.; Aseffa, A.; Dockrell, H.M.; Lockwood, D.N. The effects of prednisolone treatment on serological responses and lipid profiles in Ethiopian leprosy patients with Erythema Nodosum Leprosum reactions. PLoS Negl. Trop. Dis. 2018, 12, e0007035. [Google Scholar] [CrossRef]
- Nold-Petry, C.A.; Nold, M.F.; Levy, O.; Kliger, Y.; Oren, A.; Borukhov, I.; Becker, C.; Wirtz, S.; Sandhu, M.K.; Neurath, M.; et al. Gp96 Peptide Antagonist gp96-II Confers Therapeutic Effects in Murine Intestinal Inflammation. Front. Immunol. 2017, 8, 1531. [Google Scholar] [CrossRef]
- Shinohara, S.; Kobayashi, K.; Kasahara, C.; Onitsuka, T.; Matsuo, M.; Nakagawa, M.; Sugaya, M. Long-term impact of complications after lung resections in non-small cell lung cancer. J. Thorac. Dis. 2019, 11, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Sase, K.; Fujisaka, Y.; Shoji, M.; Mukai, M. Cardiovascular Complications Associated with Contemporary Lung Cancer Treatments. Curr. Treat. Options Oncol. 2021, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Heiden, B.T.; Eaton, D.B., Jr.; Chang, S.H.; Yan, Y.; Schoen, M.W.; Chen, L.S.; Smock, N.; Patel, M.R.; Kreisel, D.; Nava, R.G.; et al. The Impact of Persistent Smoking After Surgery on Long-term Outcomes After Stage I Non-small Cell Lung Cancer Resection. Chest 2022, 161, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Grosu, H.B.; Manzanera, A.; Shivakumar, S.; Sun, S.; Noguras Gonzalez, G.; Ost, D.E. Survival disparities following surgery among patients with different histological types of non-small cell lung cancer. Lung Cancer 2020, 140, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Rim, S.H.; Guy, G.P., Jr.; Yabroff, K.R.; McGraw, K.A.; Ekwueme, D.U. The impact of chronic conditions on the economic burden of cancer survivorship: A systematic review. Expert Rev. Pharm. Outcomes Res. 2016, 16, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.D.; Shui, A.M.; Perez, G.K.; Kirchhoff, A.C.; Peppercorn, J.M.; Moy, B.; Kuhlthau, K.; Park, E.R. Patterns in Health Care Access and Affordability Among Cancer Survivors During Implementation of the Affordable Care Act. JAMA Oncol. 2018, 4, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Robinson, L.A.; Jensen, R.E.; Smith, T.G.; Yabroff, K.R. Factors Associated with Health-Related Quality of Life among Cancer Survivors in the United States. JNCI Cancer Spectr. 2021, 5, pkaa123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).