Consensus for Flow Cytometry Clinical Report on Multiple Myeloma: A Multicenter Harmonization Process Merging Laboratory Experience and Clinical Needs
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
Participants and Screening Policy
3. Results
3.1. Patient Personal Information, Clinical Data and Biological Material
3.2. Cytometry Analysis
3.3. Quality Control of the Sample: Cytometric Myelogram
3.4. Flow Cytometry Analysis of PC and Lymphoid Populations
3.5. Final Comment/Conclusion of Report
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Nasillo, V.; Ottomano, A.M.; Bergonzini, G.; Paolini, A.; Forghieri, F.; Lusenti, B.; Barozzi, P.; Lagreca, I.; Fiorcari, S.; et al. Multiparametric Flow Cytometry for MRD Monitoring in Hematologic Malignancies: Clinical Applications and New Challenges. Cancers 2021, 13, 4582. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, P.; Mejstrikova, E.; Sedek, L.; van der Sluijs-Gelling, A.J.; Gaipa, G.; Bartels, M.; da Costa, E.S.; Kotrová, M.; Novakova, M.; Sonneveld, E.; et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017, 129, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.-C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- Del Giudice, I.; Raponi, S.; Della Starza, I.; De Propris, M.S.; Cavalli, M.; De Novi, L.A.; Cappelli, L.V.; Ilari, C.; Cafforio, L.; Guarini, A.; et al. Minimal Residual Disease in Chronic Lymphocytic Leukemia: A New Goal? Front. Oncol. 2019, 9, 689. [Google Scholar] [CrossRef]
- Legarda, M.A.; Cejalvo, M.J.; de la Rubia, J. Recent Advances in the Treatment of Patients with Multiple Myeloma. Cancers 2020, 12, 3576. [Google Scholar] [CrossRef]
- Landgren, O.; Devlin, S.; Boulad, M.; Mailankody, S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: A meta-analysis. Bone Marrow Transplant. 2016, 51, 1565–1568. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.C.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of Minimal Residual Disease with Superior Survival Outcomes in Patients with Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Gozzetti, A.; Raspadori, D.; Bacchiarri, F.; Sicuranza, A.; Pacelli, P.; Ferrigno, I.; Tocci, D.; Bocchia, M. Minimal Residual Disease in Multiple Myeloma: State of the Art and Applications in Clinical Practice. J. Pers. Med. 2020, 10, 120. [Google Scholar] [CrossRef]
- Diamond, B.T.; Rustad, E.; Maclachlan, K.; Thoren, K.; Ho, C.; Roshal, M.; Ulaner, G.A.; Landgren, C.O. Defining the undetectable: The current landscape of minimal residual disease assessment in multiple myeloma and goals for future clarity. Blood Rev. 2021, 46, 100732. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Stetler-Stevenson, M.; Paiva, B.; Stoolman, L.; Lin, P.; Jorgensen, J.L.; Orfao, A.; Van Dongen, J.; Rawstron, A.C. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytom. Part B Clin. Cytom. 2016, 90, 26–30. [Google Scholar] [CrossRef]
- Arroz, M.; Came, N.; Lin, P.; Chen, W.; Yuan, C.; Lagoo, A.; Monreal, M.; de Tute, R.; Vergilio, J.A.; Rawstron, A.C.; et al. Consensus guidelines on plasma cell myeloma minimal residual disease analysis and reporting. Cytom. Part B Clin. Cytom. 2016, 90, 31–39. [Google Scholar] [CrossRef]
- Caers, J.; Garderet, L.; Kortüm, K.M.; O’Dwyer, M.E.; van de Donk, N.; Binder, M.; Dold, S.M.; Gay, F.; Corre, J.; Beguin, Y.; et al. European Myeloma Network recommendations on tools for the diagnosis and monitoring of multiple myeloma: What to use and when. Haematologica 2018, 103, 1772–1784. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Costa, L.J.; Derman, B.A.; Bal, S.; Sidana, S.; Chhabra, S.; Silbermann, R.; Ye, J.C.; Cook, G.; Cornell, R.F.; Holstein, S.A.; et al. International harmonization in performing and reporting minimal residual disease assessment in multiple myeloma trials. Leukemia 2021, 35, 18–30. [Google Scholar] [CrossRef]
- Olteanu, H. Role of Flow Cytometry in the Diagnosis and Prognosis of Plasma Cell Myeloma. Surg. Pathol. Clin. 2016, 9, 101–116. [Google Scholar] [CrossRef]
- Maia, C.; Puig, N.; Cedena, M.T.; Goicoechea, I.; Valdes-Mas, R.; Vazquez, I.; Chillon, M.C.; Aguirre, P.; Sarvide, S.; Gracia-Aznárez, F.J.; et al. Biological and clinical significance of dysplastic hematopoiesis in patients with newly diagnosed multiple myeloma. Blood 2020, 135, 2375–2387. [Google Scholar] [CrossRef]
- Kalina, T.; Flores-Montero, J.; van der Velden, V.H.; Martin-Ayuso, M.; Böttcher, S.; Ritgen, M.; Almeida, J.; Lhermitte, L.; Asnafi, V.; Mendonça, A.; et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012, 26, 1986–2010. [Google Scholar] [CrossRef]
- Scott, S.D.; Fletcher, M.; Whitehouse, H.; Whitby, L.; Yuan, C.; Mazzucchelli, S.; Lin, P.; de Tute, R.; Dorwal, P.; Wallace, P.K.; et al. Assessment of plasma cell myeloma minimal residual disease testing by flow cytometry in an international inter-laboratory study: Is it ready for primetime use? Cytom. Part B Clin. Cytom. 2019, 96, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Puig, N.; Flores-Montero, J.; Burgos, L.; Cedena, M.T.; Cordón, L.; Pérez, J.J.; Sanoja-Flores, L.; Manrique, I.; Rodríguez-Otero, P.; Rosiñol, L.; et al. Reference Values to Assess Hemodilution and Warn of Potential False-Negative Minimal Residual Disease Results in Myeloma. Cancers 2021, 13, 4924. [Google Scholar] [CrossRef] [PubMed]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.J.; Vidriales, M.B.; García-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Rawstron, A.C.; Child, J.A.; de Tute, R.M.; Davies, F.E.; Gregory, W.M.; Bell, S.E.; Szubert, A.J.; Navarro-Coy, N.; Drayson, M.T.; Feyler, S.; et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: Impact on outcome in the Medical Research Council Myeloma IX Study. J. Clin. Oncol. 2013, 31, 2540–2547. [Google Scholar] [CrossRef]
- Chong, L.L.; Soon, Y.Y.; Soekojo, C.Y.; Ooi, M.; Chng, W.J.; de Mel, S. Daratumumab-based induction therapy for multiple myeloma: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 159, 103211. [Google Scholar] [CrossRef]
- Frampton, J.E. Isatuximab: A Review of Its Use in Multiple Myeloma. Target. Oncol. 2021, 16, 675–686. [Google Scholar] [CrossRef]
- Oberle, A.; Brandt, A.; Alawi, M.; Langebrake, C.; Janjetovic, S.; Wolschke, C.; Schütze, K.; Bannas, P.; Kröger, N.; Koch-Nolte, F.; et al. Long-term CD38 saturation by daratumumab interferes with diagnostic myeloma cell detection. Haematologica 2017, 102, e368–e370. [Google Scholar] [CrossRef]
- Krejcik, J.; Frerichs, K.A.; Nijhof, I.S.; van Kessel, B.; van Velzen, J.F.; Bloem, A.C.; Broekmans, M.E.C.; Zweegman, S.; van Meerloo, J.; Musters, R.J.P.; et al. Monocytes and Granulocytes Reduce CD38 Expression Levels on Myeloma Cells in Patients Treated with Daratumumab. Clin. Cancer Res. 2017, 23, 7498–7511. [Google Scholar] [CrossRef]
- Broijl, A.; de Jong, A.C.M.; van Duin, M.; Sonneveld, P.; Kühnau, J.; van der Velden, V.H.J. VS38c and CD38-Multiepitope Antibodies Provide Highly Comparable Minimal Residual Disease Data in Patients with Multiple Myeloma. Am. J. Clin. Pathol. 2022, 157, 494–497. [Google Scholar] [CrossRef]
- Soh, K.T.; Tario, J.D., Jr.; Hahn, T.; Hillengass, J.; McCarthy, P.L.; Wallace, P.K. CD319 (SLAMF7) an alternative marker for detecting plasma cells in the presence of daratumumab or elotuzumab. Cytom. Part B Clin. Cytom. 2021, 100, 497–508. [Google Scholar] [CrossRef]
- Hofste op Bruinink, D.; Oliva, S.; Rihova, L.; Schmitz, A.; Gilestro, M.; Te Marvelde, J.; Kralova, R.; Høholt, H.; Broijl, A.; Johnsen, H.E.; et al. Standardization of flow cytometric minimal residual disease assessment in international clinical trials—A feasibility study from the European Myeloma Network. Haematologica 2020, 106, 1496–1499. [Google Scholar] [CrossRef]
- Yang, J.; Terebelo, H.R.; Zonder, J.A. Secondary primary malignancies in multiple myeloma: An old NEMESIS revisited. Adv. Hematol. 2012, 2012, 801495. [Google Scholar] [CrossRef]
- Mailankody, S.; Pfeiffer, R.M.; Kristinsson, S.Y.; Korde, N.; Bjorkholm, M.; Goldin, L.R.; Turesson, I.; Landgren, O. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood 2011, 118, 4086–4092. [Google Scholar] [CrossRef]
- Bolli, N.; Genuardi, E.; Ziccheddu, B.; Martello, M.; Oliva, S.; Terragna, C. Next-Generation Sequencing for Clinical Management of Multiple Myeloma: Ready for Prime Time? Front. Oncol. 2020, 10, 189. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Dong, N.; Zhang, X.; Hussaini, M.; Jain, A.; Moscinski, L.; Shain, K.; Baz, R.; Alsina, M.; et al. The Application of NextGen Sequencing in the Diagnosis of Myeloid Neoplasms in Myeloma Patients with Cytopenia. Clin. Lymphoma Myeloma Leuk. 2022, 22, e414–e426. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Leone, G.; Pagano, L.; Ben-Yehuda, D.; Voso, M.T. Therapy-related leukemia and myelodysplasia: Susceptibility and incidence. Haematologica 2007, 92, 1389–1398. [Google Scholar] [CrossRef]
- Gertz, M.A.; Terpos, E.; Dispenzieri, A.; Kumar, S.; Shah, R.A.; Orlowski, R.; Kastritis, E.; Dimopoulos, M.A.; Shah, J. Therapy-related myelodysplastic syndrome/acute leukemia after multiple myeloma in the era of novel agents. Leuk. Lymphoma 2015, 56, 1723–1726. [Google Scholar] [CrossRef]
- Poh, C.; Keegan, T.; Rosenberg, A.S. Second primary malignancies in multiple myeloma: A review. Blood Rev. 2021, 46, 100757. [Google Scholar] [CrossRef]
- Jonsdottir, G.; Björkholm, M.; Turesson, I.; Hultcrantz, M.; Diamond, B.; Porwit, A.; Landgren, O.; Kristinsson, S.Y. Cumulative exposure to melphalan chemotherapy and subsequent risk of developing acute myeloid leukemia and myelodysplastic syndromes in patients with multiple myeloma. Eur. J. Haematol. 2021, 107, 275–282. [Google Scholar] [CrossRef]
- Liu, D.; Lin, P.; Hu, Y.; Zhou, Y.; Tang, G.; Powers, L.; Medeiros, L.J.; Jorgensen, J.L.; Wang, S.A. Immunophenotypic heterogeneity of normal plasma cells: Comparison with minimal residual plasma cell myeloma. J. Clin. Pathol. 2012, 65, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Boquoi, A.; Banahan, S.M.; Mohring, A.; Savickaite, I.; Strapatsas, J.; Hildebrandt, B.; Kobbe, G.; Gattermann, N.; Haas, R.; Schroeder, T.; et al. Therapy-related myeloid neoplasms following treatment for multiple myeloma-a single center analysis. Ann. Hematol. 2022, 101, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Alhan, C.; Westers, T.M.; Cremers, E.M.; Cali, C.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. Application of flow cytometry for myelodysplastic syndromes: Pitfalls and technical considerations. Cytom. Part B Clin. Cytom. 2016, 90, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E.; Zhang, D.; Cui, W. Flow Cytometric Analysis of Monocytes and Granulocytes May Be Useful in the Distinction of Myeloid Neoplasms from Reactive Conditions: A Single Institution Experience and Literature Review. Ann. Clin. Lab. Sci. 2020, 50, 327–332. [Google Scholar] [PubMed]
- Campana, S.; De Pasquale, C.; Sidoti Migliore, G.; Pezzino, G.; Cavaliere, R.; Venanzi Rullo, E.; Nunnari, G.; Caramori, G.; David, A.; Bonaccorsi, I.; et al. Cutting Edge: Hyperinflammatory Monocytes Expressing CD56 Abound in Severe COVID-19 Patients. J. Immunol. 2022, 209, 655–659. [Google Scholar] [CrossRef]
- Dutt, T.S.; LaVergne, S.M.; Webb, T.L.; Baxter, B.A.; Stromberg, S.; McFann, K.; Berry, K.; Tipton, M.; Alnachoukati, O.; Zier, L.; et al. Comprehensive Immune Profiling Reveals CD56(+) Monocytes and CD31(+) Endothelial Cells Are Increased in Severe COVID-19 Disease. J. Immunol. 2022, 208, 685–696. [Google Scholar] [CrossRef]
- Friedrich, K.; Sommer, M.; Strobel, S.; Thrum, S.; Blüher, M.; Wagner, U.; Rossol, M. Perturbation of the Monocyte Compartment in Human Obesity. Front. Immunol. 2019, 10, 1874. [Google Scholar] [CrossRef]
- Landgren, O.; Iskander, K. Modern multiple myeloma therapy: Deep, sustained treatment response and good clinical outcomes. J. Intern. Med. 2017, 281, 365–382. [Google Scholar] [CrossRef]
- Mina, R.; Oliva, S.; Boccadoro, M. Minimal Residual Disease in Multiple Myeloma: State of the Art and Future Perspectives. J. Clin. Med. 2020, 9, 2142. [Google Scholar] [CrossRef]
- Oliva, S.; Bruinink, D.H.O.; Rihova, L.; D’Agostino, M.; Pantani, L.; Capra, A.; van der Holt, B.; Troia, R.; Petrucci, M.T.; Villanova, T.; et al. Minimal residual disease assessment by multiparameter flow cytometry in transplant-eligible myeloma in the EMN02/HOVON 95 MM trial. Blood Cancer J. 2021, 11, 106. [Google Scholar] [CrossRef]
- San-Miguel, J.; Avet-Loiseau, H.; Paiva, B.; Kumar, S.; Dimopoulos, M.A.; Facon, T.; Mateos, M.V.; Touzeau, C.; Jakubowiak, A.; Usmani, S.Z.; et al. Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE. Blood 2022, 139, 492–501. [Google Scholar] [CrossRef]
- Paiva, B.; Cedena, M.T.; Puig, N.; Arana, P.; Vidriales, M.B.; Cordon, L.; Flores-Montero, J.; Gutierrez, N.C.; Martín-Ramos, M.L.; Martinez-Lopez, J.; et al. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood 2016, 127, 3165–3174. [Google Scholar] [CrossRef]
- Soh, K.T.; Wallace, P.K. Evaluation of measurable residual disease in multiple myeloma by multiparametric flow cytometry: Current paradigm, guidelines, and future applications. Int. J. Lab. Hematol. 2021, 43 (Suppl. S1), 43–53. [Google Scholar] [CrossRef]
- Flores-Montero, J.; de Tute, R.; Paiva, B.; Perez, J.J.; Böttcher, S.; Wind, H.; Sanoja, L.; Puig, N.; Lecrevisse, Q.; Vidriales, M.B.; et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytom. Part B Clin. Cytom. 2016, 90, 61–72. [Google Scholar] [CrossRef]
- Cordone, I.; Marchesi, F.; Masi, S.; Summa, V.; Pisani, F.; Merola, R.; Cigliana, G.; Orlandi, G.; Gumenyuk, S.; Palombi, F.; et al. Flow cytometry remission by Ig light chains ratio is a powerful marker of outcome in multiple myeloma after tandem autologous transplant: A real-life study. J. Exp. Clin. Cancer Res. 2016, 35, 49. [Google Scholar] [CrossRef]
- Paiva, B.; Montes, M.C.; García-Sanz, R.; Ocio, E.M.; Alonso, J.; de Las Heras, N.; Escalante, F.; Cuello, R.; de Coca, A.G.; Galende, J.; et al. Multiparameter flow cytometry for the identification of the Waldenström’s clone in IgM-MGUS and Waldenström’s Macroglobulinemia: New criteria for differential diagnosis and risk stratification. Leukemia 2014, 28, 166–173. [Google Scholar] [CrossRef]
- Wood, B.; Jevremovic, D.; Béné, M.C.; Yan, M.; Jacobs, P.; Litwin, V. Validation of cell-based fluorescence assays: Practice guidelines from the ICSH and ICCS—Part V—Assay performance criteria. Cytom. Part B Clin. Cytom. 2013, 84, 315–323. [Google Scholar] [CrossRef]
- Soh, K.T.; Tario, J.D., Jr.; Hahn, T.E.; Hillengass, J.; McCarthy, P.L.; Wallace, P.K. Methodological considerations for the high sensitivity detection of multiple myeloma measurable residual disease. Cytom. Part B Clin. Cytom. 2020, 98, 161–173. [Google Scholar] [CrossRef]
- Roshal, M.; Flores-Montero, J.A.; Gao, Q.; Koeber, M.; Wardrope, J.; Durie, B.G.M.; Dogan, A.; Orfao, A.; Landgren, O. MRD detection in multiple myeloma: Comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood Adv. 2017, 1, 728–732. [Google Scholar] [CrossRef]

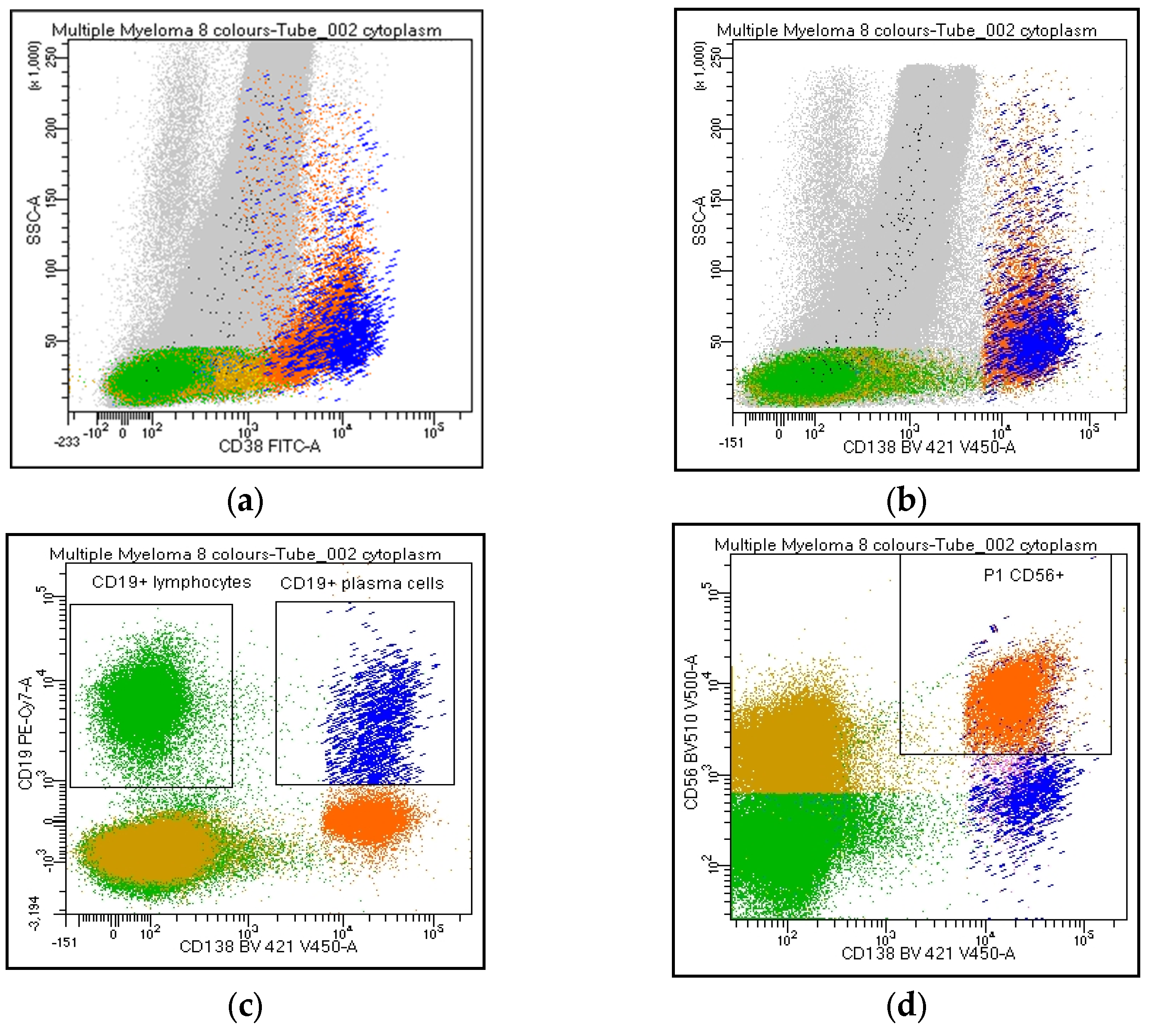
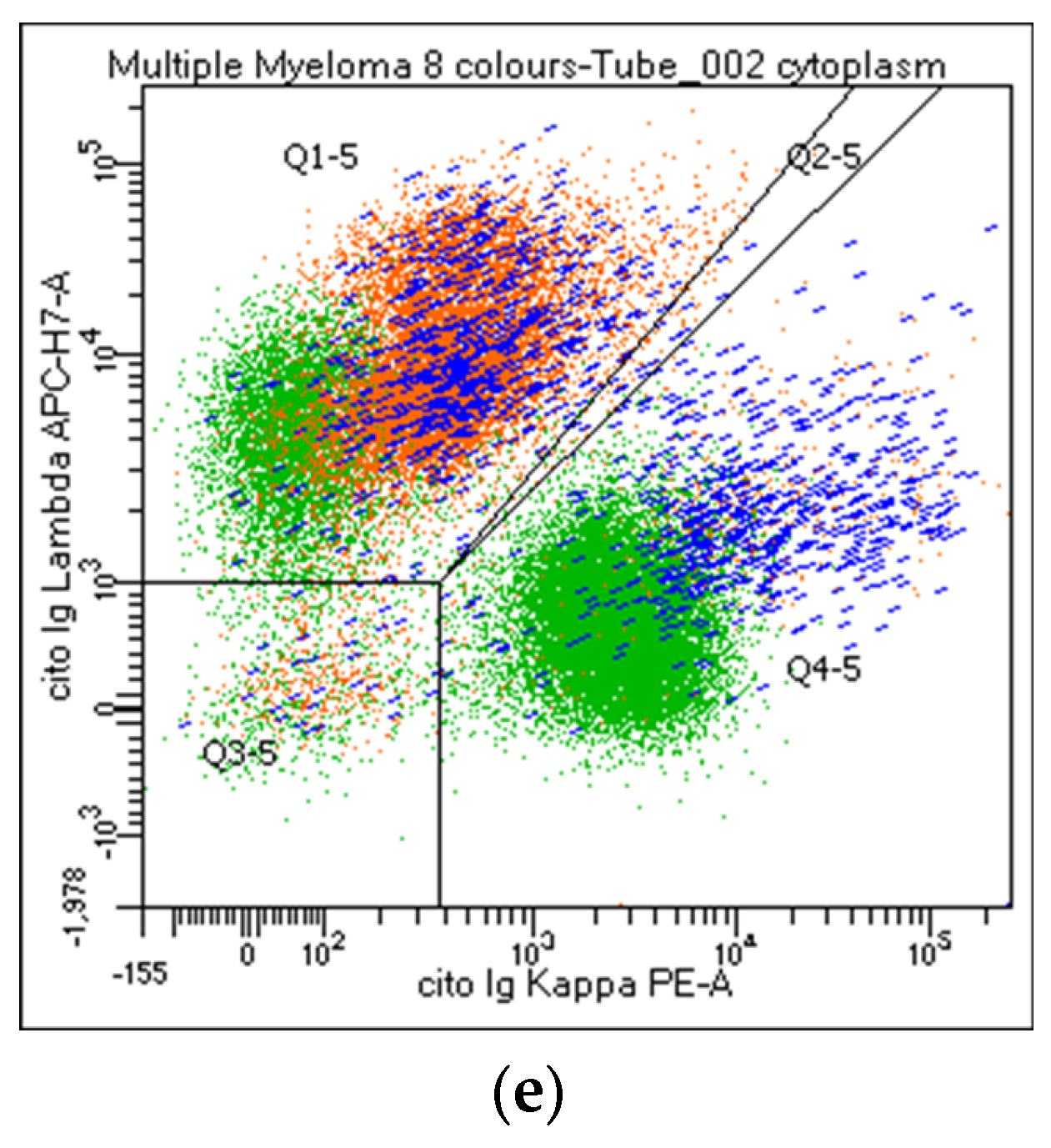
| # | Y | N | NR | |
| 1 | Name | 8/8 | ||
| 2 | Sex | 7/8 | 1/8 | |
| 3 | Date of birth | 8/8 | ||
| 4 | Code of sample identification | 8/8 | ||
| 5 | Medical center of origin | 8/8 | ||
| 6 | Requesting physician | 8/8 | ||
| 7 | Diagnostic query | 8/8 | ||
| 8 | Timepoint of assessment/stage | 8/8 | ||
| 9 | Type of biological material (bone marrow, peripheral blood | 8/8 | ||
| 10 | Type of anticoagulant | 3/8 | 5/8 | |
| 11 | Amount of biological material (ml) | 6/8 | 2/8 | |
| 12 | First pool (for MRD studies) | 7/8 | 1/8 | |
| 13 | Cellularity/mm3 (from automatic cell counter) | 6/8 | 2/8 | |
| 14 | Date of sampling | 8/8 | ||
| 15 | Date of processing | 8/8 | ||
| 16 | Date of report | 8/8 | ||
| 17 | Treatment (to specify MoAb treatment such as anti-CD38) | 8/8 |
| # | Y | N | NR | |
| 18 | Bulk lysis (yes/no) | 4/8 | 1/8 | 3/8 |
| 19 | Cell fixation and permeabilizing reagents for intra-cytoplasmic staining | 1/8 | 7/8 | |
| 20 | Gating strategy | 7/8 | 1/8 | |
| 21 | Number of total events acquired/analyzed | 8/8 | ||
| 22 | Number of plasma cells acquired/analyzed | 8/8 |
| # | Cell Population % | Markers | Warning/Potential Pitfalls | Y | N | NR |
| 23 | NRBC | CD45neg/CD38 and or CD138neg | If reduced, possible risk of hemodilution | 7/8 | 1/8 | |
| 24 | CD45neg/SSClow | NRBC overestimation if PCs CD45neg/small size | 5/8 | 3/8 | ||
| 25 | NRBC value from the WBC count | 1/8 | 1/8 | 6/8 | ||
| 26 | Lymphocytes | FSC/SSC | 6/8 | 2/8 | ||
| 27 | SSClow/CD45++ | 6/8 | 2/8 | |||
| 28 | Hematogones | CD19/CD38++ or CD81++ lymphocytes | If reduced, possible risk of hemodilution | 6/8 | 2/8 | |
| 29 | Myelocytes | SSChi/CD45+ | 6/8 | 2/8 | ||
| 30 | Myeloid precursors | CD117/CD45 | CD45 CD117+ PCs included | 7/8 | 1/8 | |
| 31 | CD117+/CD138neg | 7/8 | 1/8 | |||
| 32 | CD117/CD45 > 5% | Marker of putative myelodysplasia | 7/8 | 1/8 | ||
| 33 | Mast cells | CD117hi/SSChi | If reduced, possible risk of hemodilution | 6/8 | 2/8 | |
| 34 | Monocytes | FSC/SSC | PCs can be included | 5/8 | 3/8 | |
| 35 | CD45/SSCint | CD45+ PCs included | 6/8 | 2/8 | ||
| 36 | CD38+/SSCint | 5/8 | 3/8 | |||
| 37 | CD56 on CD38/SSCint | If >50% marker of putative myelodysplasia | 4/8 | 4/8 | ||
| 38 | Plasma cells | CD38++/SSC | Exclude CD38neg MM | 7/8 | 1/8 | |
| 39 | PCs negative after anti-CD38 therapy | 8/8 | ||||
| 40 | CD138++/SSC | Rare CD138dim/neg MM | 8/8 | |||
| 41 | CD38/CD138++ | CD38 or CD138neg PCs excluded | 8/8 | |||
| 42 | CD38 multi-epitope or VS38c/other markers | 8/8 |
| # | Y | N | NR | ||
| 43 | List of the antibodies tested | 6/8 | 1/8 | 1/8 | |
| 44 | Percentage/number of plasma cells on total cellularity | 8/8 | |||
| 45 | Percentage/number of neoplastic cells on total cellularity | 5/5 | 3/8 | ||
| 46 | Percentage of normal plasma cells on total cellularity | 5/8 | 1/8 | 2/8 | |
| 47 | Percentage/number of neoplastic cells within the plasma cell population | 7/8 | 1/8 | ||
| 48 | Percentage of normal plasma cells within the plasma cell population | 7/8 | 1/8 | ||
| 49 | Ratio clonal PCs/total PCs | 5/8 | 3/8 | ||
| Markers to identify the PC population | |||||
| 50 | CD38+ bright | 8/8 | |||
| 51 | CD138+ bright | 8/8 | |||
| 52 | CD38/CD138+ bright | 8/8 | |||
| 53 | CD38 multi-epitope, VS38c, CD319 or other markers | 8/8 | |||
| Analysis on plasma cell (CD38 and/or CD138bright) population (%) | |||||
| 54 | CD19 | 8/8 | |||
| 55 | CD20 | 8/8 | |||
| 56 | CD27 | 7/8 | 1/8 | ||
| 57 | CD28 | 7/8 | 1/8 | ||
| 58 | CD45 | 8/8 | |||
| 59 | CD56 | 8/8 | |||
| 60 | CD81 | 6/8 | 2/8 | ||
| 61 | CD117 | 8/8 | |||
| 62 | Cytoplasmic Ig Kappa light chains | 8/8 | |||
| 63 | Cytoplasmic Ig Lambda light chains | 8/8 | |||
| 64 | CD19+/CD45+ | Putative polyclonal PCs on CD19neg MM | 5/8 | 3/8 | |
| 65 | CD19+/SAM+ | Putative monoclonal PCs on CD19+ MM | 6/8 | 2/8 | |
| 66 | CD19+/SAMneg | Putative polyclonal PCs on CD19neg MM | 6/8 | 2/8 | |
| 67 | CD19neg/SAM+ | Putative monoclonal PCs | 6/8 | 2/8 | |
| 68 | Ig Kappa/Lambda ratio on | Total PCs | 6/8 | 2/8 | |
| 69 | CD19+ PCs | 6/8 | 2/8 | ||
| 70 | SAM+ PCs | 6/8 | 2/8 | ||
| 71 | CD19neg/SAM+ PCs | 6/8 | 2/8 | ||
| 72 | CD19+/SAMneg PCs | 6/8 | 2/8 | ||
| 73 | CD19+/CD45+ PCs | 6/8 | 2/8 | ||
| Analysis of lymphoid (SSC low/CD45++) population (%) | |||||
| 74 | CD19 | 8/8 | |||
| 75 | CD19+/CD38+ | 7/8 | 1/8 | ||
| 76 | CD19+/CD38neg | 7/8 | 1/8 | ||
| 77 | CD20 | 8/8 | |||
| 78 | CD27 | 3/8 | 2/8 | 3/8 | |
| 79 | CD28 | 3/8 | 2/8 | 3/8 | |
| 80 | CD56 | 4/8 | 2/8 | 2/8 | |
| 81 | CD81 | 3/8 | 2/8 | 3/8 | |
| 82 | Other | 4/8 | 2/8 | 2/8 | |
| 83 | Ig Kappa/Lambda ratio on | CD19+ | 6/8 | 1/8 | 1/8 |
| 84 | CD19+/CD38+ | 5/8 | 1/8 | 2/8 | |
| 85 | CD19+/CD38neg | 4/8 | 2/8 | 2/8 | |
| # | Y | N | NR | |
| 86 | State if clonal plasma cells are present or absent | 8/8 | ||
| 87 | List of the aberrant markers expressed in the clonal plasma cell population | 8/8 | ||
| 88 | List of the markers expressed in the normal plasma cell population | 5/8 | 1/8 | 2/8 |
| 89 | Quality of the sample: adequate, inadequate | 8/8 | ||
| 90 | Maximum sensitivity of the method: 10−5/<0.001% (or the sensitivity reached) | 8/8 | ||
| 91 | MRD status: MRD-positive; MRD-negative | 7/8 | 1/8 | |
| 92 | MRD threshold: 0.01%/10−4 or 0.001%/10−5 | 7/8 | 1/8 | |
| 93 | MRD negative <20 clonal plasma cells MRD positive not quantifiable ≥ 20 <50 clonal plasma cells MRD positive ≥50 clonal plasma cells | 6/8 | 1/8 | 1/8 |
| 94 | Up-to-date reference for MRD | 3/8 | 2/8 | 3/8 |
| 95 | LOD (20 events) LLOQ (50 events) | 8/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordone, I.; Amodeo, R.; Bellesi, S.; Bottan, F.; Buccisano, F.; De Propris, M.S.; Masi, S.; Panichi, V.; Scerpa, M.C.; Annibali, O.; et al. Consensus for Flow Cytometry Clinical Report on Multiple Myeloma: A Multicenter Harmonization Process Merging Laboratory Experience and Clinical Needs. Cancers 2023, 15, 2060. https://doi.org/10.3390/cancers15072060
Cordone I, Amodeo R, Bellesi S, Bottan F, Buccisano F, De Propris MS, Masi S, Panichi V, Scerpa MC, Annibali O, et al. Consensus for Flow Cytometry Clinical Report on Multiple Myeloma: A Multicenter Harmonization Process Merging Laboratory Experience and Clinical Needs. Cancers. 2023; 15(7):2060. https://doi.org/10.3390/cancers15072060
Chicago/Turabian StyleCordone, Iole, Rachele Amodeo, Silvia Bellesi, Fiorella Bottan, Francesco Buccisano, Maria Stefania De Propris, Serena Masi, Valentina Panichi, Maria Cristina Scerpa, Ombretta Annibali, and et al. 2023. "Consensus for Flow Cytometry Clinical Report on Multiple Myeloma: A Multicenter Harmonization Process Merging Laboratory Experience and Clinical Needs" Cancers 15, no. 7: 2060. https://doi.org/10.3390/cancers15072060
APA StyleCordone, I., Amodeo, R., Bellesi, S., Bottan, F., Buccisano, F., De Propris, M. S., Masi, S., Panichi, V., Scerpa, M. C., Annibali, O., Bongarzoni, V., Caravita di Toritto, T., Coppetelli, U., Cupelli, L., de Fabritiis, P., Franceschini, L., Garzia, M., Fiorini, A., Laverde, G., ... Petrucci, M. T. (2023). Consensus for Flow Cytometry Clinical Report on Multiple Myeloma: A Multicenter Harmonization Process Merging Laboratory Experience and Clinical Needs. Cancers, 15(7), 2060. https://doi.org/10.3390/cancers15072060








