The Risk of Recurrence in Endometrial Cancer Patients with Low-Volume Metastasis in the Sentinel Lymph Nodes: A Retrospective Multi-Institutional Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sentinel Lymph Node Mapping Protocol
2.2. Statistical Analysis
3. Results
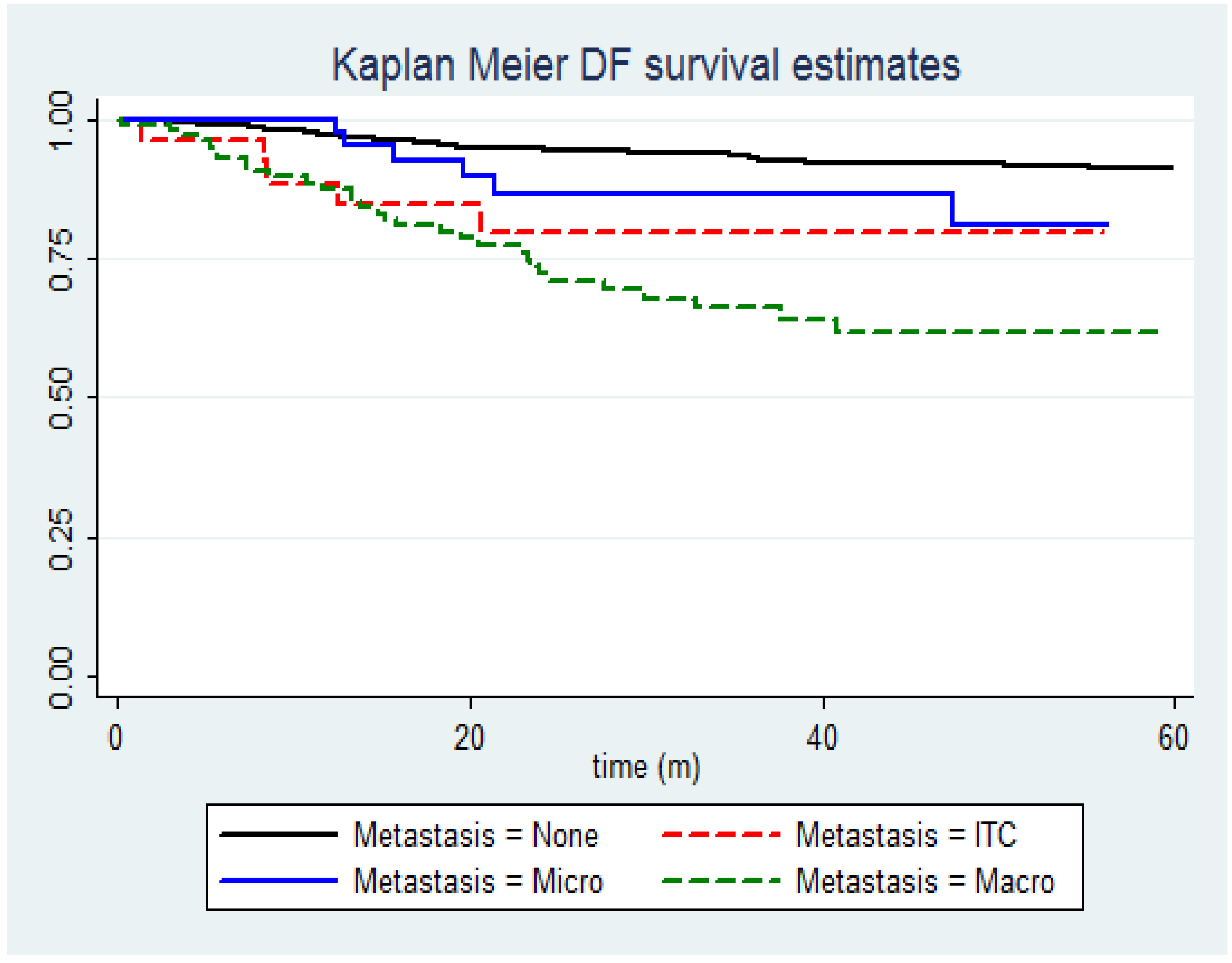
Disease-Free Survival Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.M.; Kapp, D.S.; Cheung, M.K.; Shin, J.Y.; Osann, K.; Usain, A.; Teng, N.N.; Berek, J.S.; Chan, J.K. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am. J. Obstet. Gynecol. 2008, 198, 218.e1–218.e6. [Google Scholar] [CrossRef] [PubMed]
- Benedetti Panici, P.; Basile, S.; Salerno, M.G.; Di Donato, V.; Marchetti, C.; Perniola, G.; Palagiano, A.; Perutelli, A.; Maneschi, F.; Lissoni, A.A.; et al. Secondary analyses from a randomized clinical trial: Age as the key prognostics factor in endometrial carcinoma. Am. J. Obstet. Gynecol. 2014, 210, 363.e1–363.e10. [Google Scholar] [CrossRef]
- Doll, K.M.; Tseng, J.; Denslow, S.A.; Fader, A.N.; Gehrig, P.A. High-grade endometrial cancer: Revisiting the impact of tumor size and location on outcomes. Gynecol. Oncol. 2014, 132, 44–49. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 149: Endometrial cancer. Obstet. Gynecol. 2015, 125, 1006–1026. [Google Scholar] [CrossRef] [PubMed]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 3, 1707–1716. [Google Scholar] [CrossRef]
- ASTEC study group; Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R. Sentinel lymph node mapping for endometrial cancer: A modern approach to surgical staging. J. Natl. Compr. Cancer Netw. 2014, 12, 288–297. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Daraï, E.; Dubernard, G.; Bats, A.S.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Sentinel node biopsy for the management of early stage endometrial cancer: Long-term results of the SENTI-ENDO study. Gynecol. Oncol. 2015, 136, 54–59. [Google Scholar] [CrossRef]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Barlin, J.N.; Khoury-Collado, F.; Kim, C.H.; Leitao, M.M., Jr.; Chi, D.S.; Sonoda, Y.; Alektiar, K.; DeLair, D.F.; Barakat, R.R.; Abu-Rustum, N.R. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol. Oncol. 2012, 125, 531–535. [Google Scholar] [CrossRef]
- Bogani, G.; Mariani, A.; Paolini, B.; Ditto, A.; Raspagliesi, F. Low-volume disease in endometrial cancer: The role of micrometastasis and isolated tumor cells. Gynecol. Oncol. 2019, 153, 670–675. [Google Scholar] [CrossRef]
- Ghoniem, K.; Larish, A.M.; Dinoi, G.; Zhou, X.C.; Alhilli, M.; Wallace, S.; Wohlmuth, C.; Baiocchi, G.; Tokgozoglu, N.; Raspagliesi, F.; et al. Oncologic outcomes of endometrial cancer in patients with low-volume metastasis in the sentinel lymph nodes: An international multi-institutional study. Gynecol. Oncol. 2021, 162, 590–598. [Google Scholar] [CrossRef]
- Gómez-Hidalgo, N.R.; Ramirez, P.T.; Ngo, B.; Pérez-Hoyos, S.; Coreas, N.; Sanchez-Iglesias, J.L.; Cabrera, S.; Franco, S.; Benavente, A.P.; Gil-Moreno, A. Oncologic impact of micrometastases or isolated tumor cells in sentinel lymph nodes of patients with endometrial cancer: A meta-analysis. Clin. Transl. Oncol. 2020, 22, 1272–1279. [Google Scholar] [CrossRef]
- Pineda, V.; Hernández Gutiérrez, A.; Gracia Segovia, M.; Siegrist Ridruejo, J.; Diestro Tejeda, M.D.; Zapardiel, I. Low-Volume Nodal Metastasis in Endometrial Cancer: Risk Factors and Prognostic Significance. J. Clin. Med. 2020, 9, 1999. [Google Scholar] [CrossRef]
- St Clair, C.M.; Eriksson, A.G.; Ducie, J.A.; Jewell, E.L.; Alektiar, K.M.; Hensley, M.L.; Soslow, R.A.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Low-Volume Lymph Node Metastasis Discovered During Sentinel Lymph Node Mapping for Endometrial Carcinoma. Ann. Surg. Oncol. 2016, 23, 1653–1659. [Google Scholar] [CrossRef]
- Plante, M.; Stanleigh, J.; Renaud, M.C.; Sebastianelli, A.; Grondin, K.; Grégoire, J. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: Does adjuvant treatment matter? Gynecol. Oncol. 2017, 146, 240–246. [Google Scholar] [CrossRef]
- Backes, F.J.; Felix, A.S.; Plante, M.; Grégoire, J.; Sullivan, S.A.; Rossi, E.C.; Tanner, E.J., 3rd; Stewart, K.I.; Soliman, P.T.; Holloway, R.W.; et al. Sentinel lymph node (SLN) isolated tumor cells (ITCs) in otherwise stage I/II endometrioid endometrial cancer: To treat or not to treat? Gynecol. Oncol. 2021, 161, 347–352. [Google Scholar] [CrossRef] [PubMed]
- How, A.J.; Frumovitz, M.; Stewart, K.I.; Soliman, P.T. Lymphatic mapping and sentinel node biopsy in high-grade uterine cancers. Curr. Oncol. Rep. 2022, 24, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Lebius, C.; Ignatov, T.; Ivros, S.; Knueppel, R.; Papathemelis, T.; Ortmann, O.; Eggemann, H. Lymph node micrometastases and outcome of endometrial cancer. Gynecol. Oncol. 2019, 154, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Di Donato, V.; Papadia, A.; Buda, A.; Casarin, J.; Multinu, F.; Plotti, F.; Gasparri, M.L.; Pinelli, C.; Perrone, A.M.; et al. Hysterectomy alone vs. hysterectomy plus sentinel node mapping in endometrial cancer: Perioperative and long-term results from a propensity-score based study. Eur. J. Surg. Oncol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.L. Pathology evaluation of sentinel lymph nodes in breast cancer: Protocol recommendations and rationale. Mod. Pathol. 2010, 23 (Suppl. S2), S26–S32. [Google Scholar] [CrossRef]
- Euscher, E.; Sui, D.; Soliman, P.; Westin, S.; Ramalingam, P.; Bassett, R.; Malpica, A. Ultrastaging of Sentinel Lymph Nodes in Endometrial Carcinoma According to Use of 2 Different Methods. Int. J. Gynecol. Pathol. 2018, 37, 242–251. [Google Scholar] [CrossRef]
- Malpica, A.; Euscher, E.D.; Hecht, J.L.; Ali-Fehmi, R.; Quick, C.M.; Singh, N.; Horn, L.C.; Alvarado-Cabrero, I.; Matias-Guiu, X.; Hirschowitz, L.; et al. Endometrial Carcinoma, Grossing and Processing Issues: Recommendations of the International Society of Gynecologic Pathologists. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S9–S24. [Google Scholar] [CrossRef]
- Grassi, T.; Mariani, A.; Cibula, D.; Soliman, P.T.; Suman, V.J.; Weaver, A.L.; Pedra Nobre, S.; Weigelt, B.; Glaser, G.E.; Cappuccio, S.; et al. A prospective multicenter international single-arm observational study on the oncological safety of the sentinel lymph node algorithm in stage I intermediate-risk endometrial cancer (SELECT, SEntinel Lymph node Endometrial Cancer Trial). Int. J. Gynecol. Cancer 2020, 30, 1627–1632. [Google Scholar] [CrossRef]
- Obermair, A.; Nicklin, J.; Gebski, V.; Hayes, S.C.; Graves, N.; Mileshkin, L.; Lin, M.Y.; Beale, P.; Baxter, E.; Robledo, K.; et al. A phase III randomized clinical trial comparing sentinel node biopsy with no retroperitoneal node dissection in apparent early-stage endometrial cancer—ENDO-3: ANZGOG trial 1911/2020. Int. J. Gynecol. Cancer 2021, 31, 1595–1601. [Google Scholar] [CrossRef]
- Sullivan, S.A.; Hawkins, G.; Zhao, X.; Jo, H.; Hayes, N.; Deng, X.; Bandyopadhyay, D.; Bae-Jump, V.L.; Rossi, E.C. Genomic profiling of endometrial cancer and relationship with volume of endometrial cancer disease spread. Gynecol. Oncol. Rep. 2021, 36, 100720. [Google Scholar] [CrossRef]
| Variables | No. Patients (%) |
|---|---|
| Age (at surgery), median (IQR) | 63 (55–71) |
| BMI, median (IQR) Unknown | 28 (24–33) 100 |
| Histology Endometrioid Other histologies | 1223 (85.6%) 205 (14.4%) |
| Grade Grade 1 Grade 2 Grade 3 Unknown | 558 (39.1%) 541 (37.9%) 310 (21.7%) 19 (1.3%) |
| Myometrial infiltration <50% >50% Unknown | 969 (67.9%) 456 (31.9%) 3 (0.2%) |
| Cervical stromal invasion NO YES Unknown | 1268 (88.8%) 91 (6.4%) 69 (4.8%) |
| LVSI NO YES Unknown | 1043 (73.0%) 310 (21.7%) 75 (5.3%) |
| FIGO stage final pathology IA IB II IIIA IIIB IIIC1 IIIC2 IVA IVB | 877 (61.4%) 282 (19.8%) 60 (4.2%) 38 (2.7%) 6 (0.4%) 113 (7.9%) 46 (3.2%) 2 (0.1%) 4 (0.3%) |
| Surgical approach Minimally invasive surgery (MIS + Robotic) Open surgery MIS + open | 1277 (89.4%) 144 (10.1%) 7 (0.5%) |
| Dye used ICG TC99 + blue Blue alone ICG + TC99 Other | 1088 (76.2%) 72 (5.0%) 186 (13.0%) 79 (5.5%) 3 (0.2%) |
| Mapping Bilateral mapping Unilateral mapping No migration | 1126 (78.8%) 261 (18.3%) 41 (2.9%) |
| Nr. SLN removed, median (IQR) | 2 (2–4) |
| Pelvic LND NO YES | 665 (46.6%) 763 (53.4%) |
| Aortic LND NO YES | 1109 (77.7%) 319 (2.3%) |
| Tot patients SLN+ N° patients with only SLN+ N° patients with only Non-SLN+ N° patients with both SLN and non-SLN+ | 186 (13.0%) 107 (7.5 %) 19 (1.3%) 60 (4.2%) |
| Type of nodal metastasis SLN+ and non SLN+ ITC Micro-Mets Macro-Mets | 26 (14.0%) 50 (26.9%) 110 (59.1%) |
| Adjuvant therapy NO YES Unknown | 814 (57.0%) 603 (42.2%) 11 (0.8%) |
| Recurrence NO YES | 1314 (92.0%) 114 (8.0%) |
| ITC (N = 26) | Micro-Mets (N = 50) | Macro-Mets (N = 110) | p-Value | |
|---|---|---|---|---|
| Histology Endometrioid Other histologies | 19 (73.1%) 7 (26.9%) | 41 (82.0%) 9 (18.0%) | 78 (70.9%) 32 (29.1%) | 0.353 |
| Grade Grade 1 Grade 2 Grade 3 Unknown | 8 (32.0%) 9 (36.0%) 8 (32.0%) 1 | 10 (20.4%) 31 (63.3%) 8 (16.3%) - | 12 (10.9%) 37 (33.6%) 61 (55.5%) - | <0.0001 |
| Myometrial infiltration <50% >50% Unknown | 16 (61.5%) 10 (38.5%) - | 16 (32.7%) 33 (67.4%) 1 | 25 (22.7%) 85 (77.3%) - | 0.001 |
| Cervical stromal invasion NO YES Unknown | 24 (92.3%) 2 (7.7%) - | 40 (83.3%) 8 (16.7%) 2 | 90 (82.6%) 19 (17.4%) 1 | 0.528 |
| LVSI NO YES Unknown | 8 (30.8%) 18 (69.2%) - | 10 (20.8%) 38 (79.2%) 2 | 24 (21.8%) 86 (78.2%) - | 0.593 |
| Adjuvant therapy none EBRT +/− BRT RCT CT Other Unknown | 7 (26.9%) 5 (19.2%) 12 (46.2%) 2 (7.7%) 0 (0.0%) - | 5 (10.0%) 5 (10.0%) 25 (50.0%) 7 (14.0%) 8 (16.0%) - | 5 (4.6%) 7 (6.4%) 78 (71.6%) 11 (10.1%) 8 (7.3%) 1 | 0.002 |
| Recurrences NO YES | 21 (80.8%) 5 (19.2%) | 44 (88.0%) 6 (12.0%) | 79 (71.8%) 31 (28.2%) | 0.066 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% IC) | p-Value | OR (95% IC) | p-Value | |
| Age at surgery | 1.04 (1.02–1.06) | <0.0001 | 1.03 (1.00–1.08) | 0.085 |
| Grade | 2.72 (2.08–357) | <0.0001 | 1.21 (0.64–2.28) | 0.560 |
| LVSI | 5.50 (3.69–8.19) | <0.0001 | 7.93 (1.74–36.07) | 0.007 |
| Histology | 3.43 (2.25–5.24) | <0.0001 | 2.16 (0.90–5.21) | 0.086 |
| Nodal status (ITC vs. Micro-Mets vs. Macro-Mets) | 1.58 (0.93–2.68) | 0.094 | 1.56 (0.86–2.83) | 0.142 |
| Adjuvant therapy | 5.70 (3.58–9.06) | <0.0001 | 4.20 (0.46–38.19) | 0.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buda, A.; Paniga, C.; Taskin, S.; Mueller, M.; Zapardiel, I.; Fanfani, F.; Puppo, A.; Casarin, J.; Papadia, A.; De Ponti, E.; et al. The Risk of Recurrence in Endometrial Cancer Patients with Low-Volume Metastasis in the Sentinel Lymph Nodes: A Retrospective Multi-Institutional Study. Cancers 2023, 15, 2052. https://doi.org/10.3390/cancers15072052
Buda A, Paniga C, Taskin S, Mueller M, Zapardiel I, Fanfani F, Puppo A, Casarin J, Papadia A, De Ponti E, et al. The Risk of Recurrence in Endometrial Cancer Patients with Low-Volume Metastasis in the Sentinel Lymph Nodes: A Retrospective Multi-Institutional Study. Cancers. 2023; 15(7):2052. https://doi.org/10.3390/cancers15072052
Chicago/Turabian StyleBuda, Alessandro, Cristiana Paniga, Salih Taskin, Michael Mueller, Ignacio Zapardiel, Francesco Fanfani, Andrea Puppo, Jvan Casarin, Andrea Papadia, Elena De Ponti, and et al. 2023. "The Risk of Recurrence in Endometrial Cancer Patients with Low-Volume Metastasis in the Sentinel Lymph Nodes: A Retrospective Multi-Institutional Study" Cancers 15, no. 7: 2052. https://doi.org/10.3390/cancers15072052
APA StyleBuda, A., Paniga, C., Taskin, S., Mueller, M., Zapardiel, I., Fanfani, F., Puppo, A., Casarin, J., Papadia, A., De Ponti, E., Grassi, T., Mauro, J., Turan, H., Vatansever, D., Gungor, M., Ortag, F., Imboden, S., Garcia-Pineda, V., Mohr, S., ... Fruscio, R. (2023). The Risk of Recurrence in Endometrial Cancer Patients with Low-Volume Metastasis in the Sentinel Lymph Nodes: A Retrospective Multi-Institutional Study. Cancers, 15(7), 2052. https://doi.org/10.3390/cancers15072052









