Rotating Gantries Provide Individualized Beam Arrangements for Charged Particle Therapy
Abstract
Simple Summary
Abstract
1. Introduction
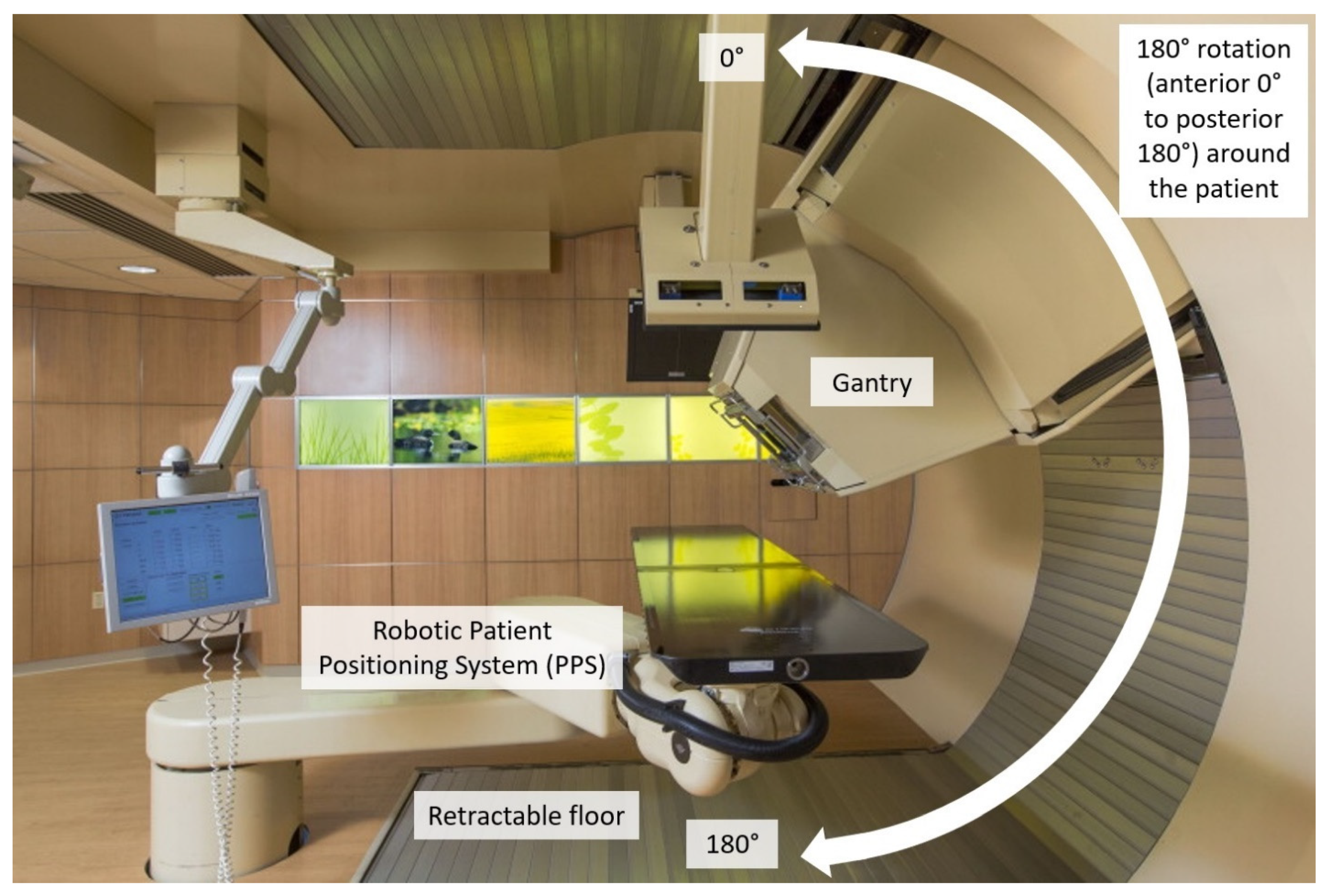
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Particle Therapy Co-Operative Group. Particle Therapy Facilities in Clinical Operation; Particle Therapy Co-Operative Group: Villigen, Switzerland, 2021. [Google Scholar]
- Particle Therapy Co-Operative Group. Particle Therapy Facilities under Construction; Particle Therapy Co-Operative Group: Villigen, Switzerland, 2021. [Google Scholar]
- Particle Therapy Co-Operative Group. Particle Therapy Facilities in a Planning Stage; Particle Therapy Co-Operative Group: Villigen, Switzerland, 2019. [Google Scholar]
- Weber, U.; Kraft, G. Comparison of carbon ions versus protons. Cancer J. 2009, 15, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Haberer, T.; Debus, J.; Eickhoff, H.; Jakel, O.; Schulz-Ertner, D.; Weber, U. The Heidelberg Ion Therapy Center. Radiother. Oncol. 2004, 73 (Suppl. S2), S186–S190. [Google Scholar] [CrossRef] [PubMed]
- Koom, W.S.; Mori, S.; Furuich, W.; Yamada, S. Beam direction arrangement using a superconducting rotating gantry in carbon ion treatment for pancreatic cancer. Br. J. Radiol. 2019, 92, 20190101. [Google Scholar] [CrossRef] [PubMed]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Koto, M.; Ikawa, H.; Hayashi, K.; Hagiwara, Y.; Makishima, H.; Kasuya, G.; Yamamoto, N.; Kamada, T.; Tsuji, H. First prospective feasibility study of carbon-ion radiotherapy using compact superconducting rotating gantry. Br. J. Radiol. 2019, 92, 20190370. [Google Scholar] [CrossRef]
- Kim, J.-I.; Park, J.M.; Wu, H.-G. Carbon Ion Therapy: A Review of an Advanced Technology. Prog. Med. Physics. 2020, 31, 71–80. [Google Scholar] [CrossRef]
- Iwata, Y.; Fujimoto, T.; Matsuba, S.; Fujita, T.; Sato, S.; Furukawa, T.; Hara, Y. Recent progress of a superconducting rotating-gantry for carbon-ion radiotherapy. Nucl. Instrum. Methods Phys. Res. B 2017, 406, 338–348. [Google Scholar] [CrossRef]
- Takayama, S.; Yazawa, T.; Asano, M.; Misawa, M.; Nagamoto, Y.; Amano, S.; Orikasa, T.; Hirata, Y.; Kanai, T.; Lee, S.H.; et al. Design and Magnetic Field Measurement of the Superconducting Magnets for the Next-Generation Rotating Gantry. IEEE Trans. Appl. Supercond. 2022, 32, 1–4. [Google Scholar] [CrossRef]
- Cao, W.; Lim, G.J.; Li, Y.; Zhu, X.R.; Zhang, X. Improved beam angle arrangement in intensity modulated proton therapy treatment planning for localized prostate cancer. Cancers 2015, 7, 574–584. [Google Scholar] [CrossRef]
- Kosaki, K.; Ecker, S.; Habermehl, D.; Rieken, S.; Jakel, O.; Herfarth, K.; Debus, J.; Combs, S.E. Comparison of intensity modulated radiotherapy (IMRT) with intensity modulated particle therapy (IMPT) using fixed beams or an ion gantry for the treatment of patients with skull base meningiomas. Radiat. Oncol. 2012, 7, 44. [Google Scholar] [CrossRef]
- Yan, S.; Lu, H.M.; Flanz, J.; Adams, J.; Trofimov, A.; Bortfeld, T. Reassessment of the Necessity of the Proton Gantry: Analysis of Beam Orientations From 4332 Treatments at the Massachusetts General Hospital Proton Center Over the Past 10 Years. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, O.; Debus, J. Selection of beam angles for radiotherapy of skull base tumours using charged particles. Phys. Med. Biol. 2000, 45, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Tsujii, H.; Blakely, E.A.; Debus, J.; De Neve, W.; Durante, M.; Jakel, O.; Mayer, R.; Orecchia, R.; Potter, R.; et al. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. Lancet Oncol. 2015, 16, e93–e100. [Google Scholar] [CrossRef]
- Wan Chan Tseung, H.; Ma, J.; Beltran, C. A fast GPU-based Monte Carlo simulation of proton transport with detailed modeling of nonelastic interactions. Med. Phys. 2015, 42, 2967–2978. [Google Scholar] [CrossRef]
- Beltran, C.; Tseung, H.W.C.; Augustine, K.E.; Bues, M.; Mundy, D.W.; Walsh, T.J.; Herman, M.G.; Laack, N.N. Clinical Implementation of a Proton Dose Verification System Utilizing a GPU Accelerated Monte Carlo Engine. Int. J. Part Ther. 2016, 3, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Beltran, C.; Seum Wan Chan Tseung, H.; Herman, M.G. A GPU-accelerated and Monte Carlo-based intensity modulated proton therapy optimization system. Med. Phys. 2014, 41, 121707. [Google Scholar] [CrossRef]
- Ma, J.; Wan Chan Tseung, H.S.; Herman, M.G.; Beltran, C. A robust intensity modulated proton therapy optimizer based on Monte Carlo dose calculation. Med. Phys. 2018, 45, 4045–4054. [Google Scholar] [CrossRef]
- Wan Chan Tseung, H.S.; Ma, J.; Kreofsky, C.R.; Ma, D.J.; Beltran, C. Clinically applicable Monte Carlo-based biological dose optimization for the treatment of head and neck cancers with spot-scanning proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Muller, O.M.; Shiraishi, S.; Harper, M.; Amundson, A.C.; Wong, W.W.; McGee, L.A.; Rwigema, J.-C.M.; Schild, S.E.; Bues, M.; et al. Empirical Relative Biological Effectiveness (RBE) for Mandible Osteoradionecrosis (ORN) in Head and Neck Cancer Patients Treated With Pencil-Beam-Scanning Proton Therapy (PBSPT): A Retrospective, Case-Matched Cohort Study. Front. Oncol. 2022, 12, 843175. [Google Scholar] [CrossRef]
- Yang, Y.; Patel, S.H.; Bridhikitti, J.; Wong, W.W.; Halyard, M.Y.; McGee, L.A.; Rwigema, J.M.; Schild, S.E.; Vora, S.A.; Liu, T.; et al. Exploratory study of seed spots analysis to characterize dose and linear-energy-transfer effect in adverse event initialization of pencil-beam-scanning proton therapy. Med. Phys. 2022, 49, 6237–6252. [Google Scholar] [CrossRef]
- Yang, Y.; Vargas, C.E.; Bhangoo, R.S.; Wong, W.W.; Schild, S.E.; Daniels, T.B.; Keole, S.R.; Rwigema, J.-C.M.; Glass, J.L.; Shen, J.; et al. Exploratory Investigation of Dose-Linear Energy Transfer (LET) Volume Histogram (DLVH) for Adverse Events Study in Intensity Modulated Proton Therapy (IMPT). Int. J. Radiat. Oncol. 2021, 110, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Bronk, L.; Kerr, M.; Sobieski, M.; Chen, M.; Geng, C.; You, J.; Wang, X.; Sahoo, N.; Cao, W.; et al. Exploring the advantages of intensity-modulated proton therapy: Experimental validation of biological effects using two different beam intensitymodulation patterns. Sci. Rep. 2020, 10, 3199. [Google Scholar] [CrossRef] [PubMed]
- Kooy, H.M.; Grassberger, C. Intensity modulated proton therapy. Br. J. Radiol. 2015, 88, 20150195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.R.; Sahoo, N.; Zhang, X.; Robertson, D.; Li, H.; Choi, S.; Lee, A.K.; Gillin, M.T. Intensity modulated proton therapy treatment planning using single-field optimization: The impact of monitor unit constraints on plan quality. Med. Phys. 2010, 37, 1210–1219. [Google Scholar] [CrossRef]
- An, Y.; Shan, J.; Patel, S.H.; Wong, W.; Schild, S.E.; Ding, X.; Bues, M.; Liu, W. Robust intensity-modulated proton therapy to reduce high linear energy transfer in organs at risk. Med. Phys. 2017, 44, 6138–6147. [Google Scholar] [CrossRef]
- Liu, C.; Patel, S.H.; Shan, J.; Schild, S.E.; Vargas, C.E.; Wong, W.W.; Ding, X.; Bues, M.; Liu, W. Robust optimization for intensity modulated proton therapy to redistribute high linear energy transfer from nearby critical organs to tumors in head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 181–193. [Google Scholar] [CrossRef]
- Garant, A.; Whitaker, T.J.; Spears, G.M.; Routman, D.M.; Harmsen, W.S.; Wilhite, T.J.; Ashman, J.B.; Sio, T.T.; Rule, W.G.; Neben Wittich, M.A.; et al. A Comparison of Patient-Reported Health-Related Quality of Life During Proton Versus Photon Chemoradiation Therapy for Esophageal Cancer. Pr. Radiat. Oncol. 2019, 9, 410–417. [Google Scholar] [CrossRef]
- Routman, D.M.; Garant, A.; Lester, S.C.; Day, C.N.; Harmsen, W.S.; Sanheuza, C.T.; Yoon, H.H.; Neben-Wittich, M.A.; Martenson, J.A.; Haddock, M.G.; et al. A Comparison of Grade 4 Lymphopenia With Proton Versus Photon Radiation Therapy for Esophageal Cancer. Adv. Radiat. Oncol. 2019, 4, 63–69. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, J.; Wang, W.; Stuart, B.; Kong, L.; Gao, J.; You, D.; Wu, X. Performance of a 6D Treatment Chair for Patient Positioning in an Upright Posture for Fixed Ion Beam Lines. Front. Oncol. 2020, 10, 122. [Google Scholar] [CrossRef]
- Rahim, S.; Korte, J.; Hardcastle, N.; Hegarty, S.; Kron, T.; Everitt, S. Upright Radiation Therapy-A Historical Reflection and Opportunities for Future Applications. Front. Oncol. 2020, 10, 213. [Google Scholar] [CrossRef]
- McCarroll, R.E.; Beadle, B.M.; Fullen, D.; Balter, P.A.; Followill, D.S.; Stingo, F.C.; Yang, J.; Court, L.E. Reproducibility of patient setup in the seated treatment position: A novel treatment chair design. J. Appl. Clin. Med. Phys. 2017, 18, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Duisters, C.; Beurskens, H.; Nijsten, S.; Starmans, M.; Wanders, S.; Verschueren, T.; Lambin, P.; Minken, A.; De Ruysscher, D. Palliative chest irradiation in sitting position in patients with bulky advanced lung cancer. Radiother. Oncol. 2006, 79, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, N.; Ding, X.; Li, Z. Fixed beamlines can replace gantries for particle therapy. Med. Phys. 2022, 49, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Furuichi, W.; Mori, S. Evaluation of patient positional reproducibility on the treatment couch and its impact on dose distribution using rotating gantry system in scanned carbon-ion beam therapy. Phys. Med. 2019, 57, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Tsujii, H.; Mizoe, J.E.; Matsuoka, Y.; Tsuji, H.; Osaka, Y.; Minohara, S.; Miyahara, N.; Endo, M.; Kanai, T. A horizontal CT system dedicated to heavy-ion beam treatment. Radiother. Oncol. 1999, 50, 235–237. [Google Scholar] [CrossRef]
- Botchu, R.; Bharath, A.; Davies, A.M.; Butt, S.; James, S.L. Current concept in upright spinal MRI. Eur. Spine J. 2018, 27, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.G.; Rai, R.; Liney, G.P.; A Dowling, J.; Holloway, L.C.; E Metcalfe, P.; Keall, P.J. Anatomical deformation due to horizontal rotation: Towards gantry-free radiation therapy. Phys. Med. Biol. 2019, 64, 175014. [Google Scholar] [CrossRef]
- Yamada, M.; Sato, H.; Ieko, Y.; Miyasaka, Y.; Kanai, T.; Yano, N.; Ono, T.; Akamatsu, H.; Harada, M.; Ichikawa, M.; et al. In silico comparison of the dosimetric impacts of a greater omentum spacer for abdominal and pelvic tumors in carbon-ion, proton and photon radiotherapy. Radiat. Oncol. 2019, 14, 207. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakamura, K.; Shioyama, Y.; Sasaki, T.; Ohga, S.; Yamaguchi, T.; Yoshitake, T.; Asai, K.; Kakiuchi, G.; Honda, H. Treatment Planning Comparison for Carbon Ion Radiotherapy, Proton Therapy and Intensity-modulated Radiotherapy for Spinal Sarcoma. Anticancer Res. 2015, 35, 4083–4089. [Google Scholar]
- Dreher, C.; Habermehl, D.; Ecker, S.; Brons, S.; El-Shafie, R.; Jäkel, O.; Debus, J.; Combs, S.E. Optimization of carbon ion and proton treatment plans using the raster-scanning technique for patients with unresectable pancreatic cancer. Radiat. Oncol. 2015, 10, 237. [Google Scholar] [CrossRef]
- Georg, D.; Hopfgartner, J.; Gòra, J.; Kuess, P.; Kragl, G.; Berger, D.; Hegazy, N.; Goldner, G.; Georg, P. Dosimetric considerations to determine the optimal technique for localized prostate cancer among external photon, proton, or carbon-ion therapy and high-dose-rate or low-dose-rate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 715–722. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
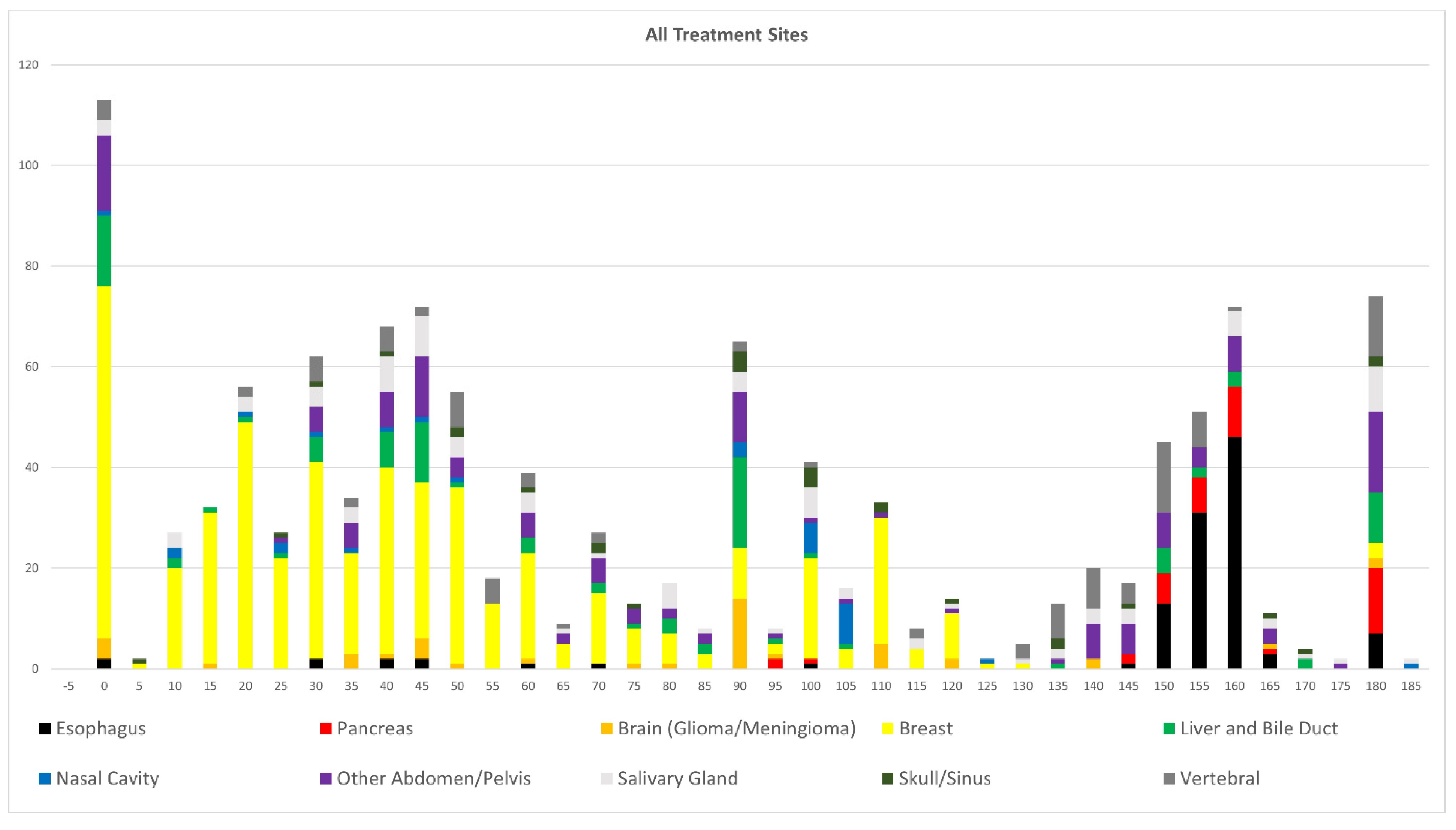
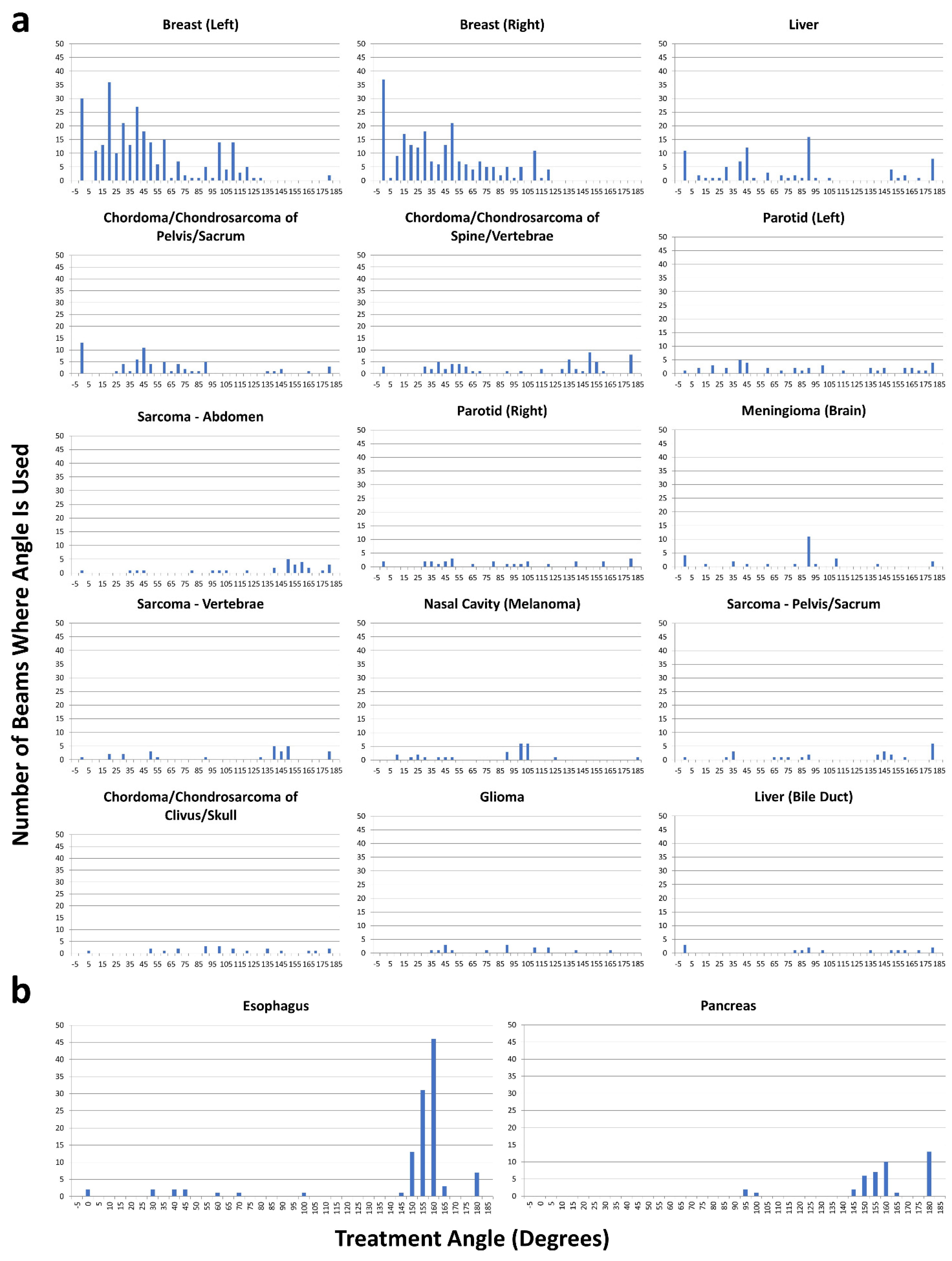
| Patients | Initial Plans | Number of Beams | Range of Angles (°) | |
|---|---|---|---|---|
| Total | 467 | 484 | 1196 | (0,185) |
| Adenoid Cystic Carcinoma (Nasal Cavity) | 1 | 1 | 4 | (0,105) |
| Adenoid Cystic Carcinoma (Oral Cavity) | 1 | 1 | 3 | (60,115) |
| Breast (Bilateral) | 2 | 2 | 9 | (0,180) |
| Breast (Left) | 128 | 128 | 276 | (0,180) |
| Breast (Right) | 102 | 102 | 217 | (0,120) |
| Chordoma/Chondrosarcoma of Chest Wall | 1 | 1 | 3 | (135,180) |
| Chordoma/Chondrosarcoma of Clivus/Skull | 6 | 6 | 22 | (5,180) |
| Chordoma/Chondrosarcoma of Pelvis/Sacrum | 24 | 24 | 67 | (0,180) |
| Chordoma/Chondrosarcoma of Spine/Vertebrae | 17 | 20 | 66 | (0,180) |
| Esophagus | 54 | 55 | 120 | (0,180) |
| Glioma | 5 | 5 | 16 | (35,165) |
| Kidney | 5 | 5 | 12 | (90,180) |
| Liver | 28 | 32 | 84 | (0,180) |
| Liver (Bile Duct) | 6 | 6 | 15 | (0,180) |
| Meningioma (Brain) | 9 | 9 | 28 | (0,180) |
| Nasal Cavity (Melanoma) | 8 | 8 | 26 | (10,185) |
| Pancreas | 19 | 19 | 45 | (95,180) |
| Parotid (Left) | 13 | 14 | 44 | (0,180) |
| Parotid (Right) | 7 | 9 | 29 | (0,180) |
| Sarcoma—Abdomen | 11 | 11 | 30 | (0,180) |
| Sarcoma—Pelvis/Sacrum | 8 | 9 | 26 | (0,180) |
| Sarcoma—Sinus/Skull | 2 | 2 | 6 | (25,100) |
| Sarcoma—Thorax | 1 | 1 | 3 | (70,155) |
| Sarcoma—Vertebrae | 7 | 8 | 27 | (0,180) |
| Submandibular Gland (Left) | 4 | 4 | 13 | (10,185) |
| Submandibular Gland (Right) | 1 | 1 | 3 | (45,180) |
| Uterus | 1 | 1 | 2 | (140,140) |
| Number of Beams | Range of Angles (°) | |
|---|---|---|
| Total | 995 | (5,185) |
| Adenoid Cystic Carcinoma (Nasal Cavity) | 3 | (35,105) |
| Adenoid Cystic Carcinoma (Oral Cavity) | 3 | (60,115) |
| Breast (Bilateral) | 5 | (40,100) |
| Breast (Left) | 244 | (10,130) |
| Breast (Right) | 180 | (5,120) |
| Chordoma/Chondrosarcoma of Chest Wall | 2 | (135,155) |
| Chordoma/Chondrosarcoma of Clivus/Skull | 20 | (5,170) |
| Chordoma/Chondrosarcoma of Pelvis/Sacrum | 51 | (25,165) |
| Chordoma/Chondrosarcoma of Spine/Vertebrae | 55 | (30,160) |
| Esophagus | 103 | (30,165) |
| Glioma | 16 | (35,165) |
| Kidney | 8 | (90,160) |
| Liver | 65 | (10,170) |
| Liver (Bile Duct) | 10 | (80,170) |
| Meningioma (Brain) | 22 | (15,140) |
| Nasal Cavity (Melanoma) | 26 | (10,185) |
| Pancreas | 29 | (95,165) |
| Parotid (Left) | 39 | (10,175) |
| Parotid (Right) | 23 | (30,160) |
| Sarcoma—Abdomen | 25 | (35,175) |
| Sarcoma—Pelvis/Sacrum | 18 | (30,160) |
| Sarcoma—Sinus/Skull | 6 | (25,100) |
| Sarcoma—Thorax | 3 | (70,155) |
| Sarcoma—Vertebrae | 23 | (20,150) |
| Submandibular Gland (Left) | 12 | (10,185) |
| Submandibular Gland (Right) | 2 | (45,45) |
| Uterus | 2 | (140,140) |
| Median Number of Beam Angles Per Patient | |
|---|---|
| All Sites | 2 |
| Adenoid Cystic Carcinoma (Nasal Cavity) | 3 |
| Adenoid Cystic Carcinoma (Oral Cavity) | 3 |
| Breast (Bilateral) | 3 |
| Breast (Left) | 2 |
| Breast (Right) | 2 |
| Chordoma/Chondrosarcoma of Chest Wall | 3 |
| Chordoma/Chondrosarcoma of Clivus/Skull | 3 |
| Chordoma/Chondrosarcoma of Pelvis/Sacrum | 2 |
| Chordoma/Chondrosarcoma of Spine/Vertebrae | 2 |
| Esophagus | 2 |
| Glioma | 3 |
| Kidney | 2 |
| Liver | 3 |
| Liver (Bile Duct) | 2.5 |
| Meningioma (Brain) | 3 |
| Nasal Cavity (Melanoma) | 2.5 |
| Pancreas | 2 |
| Parotid (Left) | 3 |
| Parotid (Right) | 3 |
| Sarcoma—Abdomen | 3 |
| Sarcoma—Pelvis/Sacrum | 2.5 |
| Sarcoma—Sinus/Skull | 3 |
| Sarcoma—Thorax | 3 |
| Sarcoma—Vertebrae | 2 |
| Submandibular Gland (Left) | 3 |
| Submandibular Gland (Right) | 2 |
| Uterus | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinniah, S.; Deisher, A.J.; Herman, M.G.; Johnson, J.E.; Mahajan, A.; Foote, R.L. Rotating Gantries Provide Individualized Beam Arrangements for Charged Particle Therapy. Cancers 2023, 15, 2044. https://doi.org/10.3390/cancers15072044
Chinniah S, Deisher AJ, Herman MG, Johnson JE, Mahajan A, Foote RL. Rotating Gantries Provide Individualized Beam Arrangements for Charged Particle Therapy. Cancers. 2023; 15(7):2044. https://doi.org/10.3390/cancers15072044
Chicago/Turabian StyleChinniah, Siven, Amanda J. Deisher, Michael G. Herman, Jedediah E. Johnson, Anita Mahajan, and Robert L. Foote. 2023. "Rotating Gantries Provide Individualized Beam Arrangements for Charged Particle Therapy" Cancers 15, no. 7: 2044. https://doi.org/10.3390/cancers15072044
APA StyleChinniah, S., Deisher, A. J., Herman, M. G., Johnson, J. E., Mahajan, A., & Foote, R. L. (2023). Rotating Gantries Provide Individualized Beam Arrangements for Charged Particle Therapy. Cancers, 15(7), 2044. https://doi.org/10.3390/cancers15072044







