Systemic Anticoagulation and Inpatient Outcomes of Pancreatic Cancer: Real-World Evidence from U.S. Nationwide Inpatient Sample
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Ethics Statement
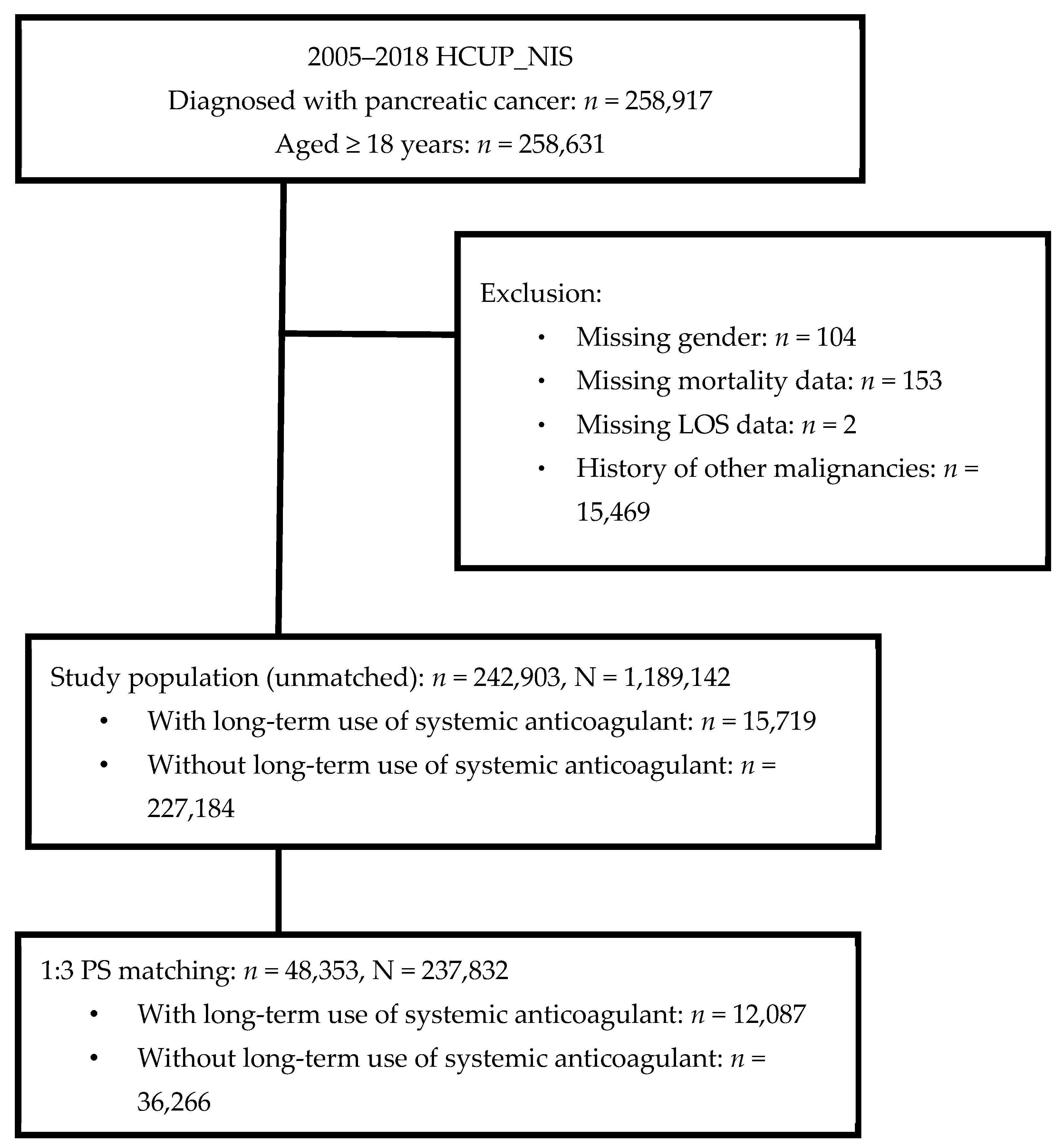
2.3. Study Population
2.4. Study Variables and Outcome Measures
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characteristics of Hospitalized Patients with Pancreatic Cancer, with or without Long-Term Systemic Anticoagulant Use
3.3. Clinical Outcomes of Hospitalized Patients with Pancreatic Cancer, with or without Long-Term Systemic Anticoagulant Use
3.4. Associations between Long-Term Systemic Anticoagulant Use, Life-Threatening Events, in-Hospital Mortality, and Prolonged LOS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, O.M.; Johnston, B.T.; Coleman, H.G. Achalasia: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2013, 19, 5806–5812. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Muller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2008, 122, 742–750. [Google Scholar] [CrossRef]
- Sener, S.F.; Fremgen, A.; Menck, H.R.; Winchester, D.P. Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J. Am. Coll Surg. 1999, 189, 1–7. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer. 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat Res. Commun. 2021, 27, 100328. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 527–536. [Google Scholar] [CrossRef]
- Frere, C. Burden of venous thromboembolism in patients with pancreatic cancer. World J. Gastroenterol. 2021, 27, 2325–2340. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Dallos, M.C.; Eisenberger, A.B.; Bates, S.E. Prevention of Venous Thromboembolism in Pancreatic Cancer: Breaking Down a Complex Clinical Dilemma. Oncologist 2020, 25, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Bournet, B.; Conroy, T.; Vicaut, E.; Rak, J.; Zogoulous, G.; Barkun, J.; Ouaissi, M.; Buscail, L.; Frere, C. Primary Thromboprophylaxis in Pancreatic Cancer Patients: Why Clinical Practice Guidelines Should Be Implemented. Cancers 2020, 12, 618. [Google Scholar] [CrossRef]
- Frere, C.; Crichi, B.; Bournet, B.; Canivet, C.; Abdallah, N.A.; Buscail, L.; Farge, D. Primary Thromboprophylaxis in Ambulatory Pancreatic Cancer Patients Receiving Chemotherapy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 2028. [Google Scholar] [CrossRef] [PubMed]
- Sandow, L.P.; Cannon, L.A.; Weston, N.R.; Aung, K.L.; Oo, T.H. The Clinical Applicability of Primary Thromboprophylaxis in Ambulatory Patients with Pancreatic Cancer. Pancreas 2021, 50, 494–499. [Google Scholar] [CrossRef]
- Healthcare Cost and Utilization Project (HCUP). Introduction to the Nationwide Inpatient Sample (NIS); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008.
- Romano, P.S.; Roos, L.L.; Jollis, J.G. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J. Clin. Epidemiol. 1993, 46, 1075–1079; discussion 1081–1090. [Google Scholar] [CrossRef]
- Parsons, L.S. Performing a 1:N Case-Control Match on Propensity Score Lori S. Parsons, Ovation Research Group, Seattle, Washington. Available online: https://support.sas.com/resources/papers/proceedings/proceedings/sugi29/165-29.pdf (accessed on 15 November 2022).
- Campello, E.; Ilich, A.; Simioni, P.; Key, N.S. The relationship between pancreatic cancer and hypercoagulability: A comprehensive review on epidemiological and biological issues. Br. J. Cancer 2019, 121, 359–371. [Google Scholar] [CrossRef]
- Toth, P.P. Considerations for long-term anticoagulant therapy in patients with venous thromboembolism in the novel oral anticoagulant era. Vasc. Health Risk Manag. 2016, 12, 23–34. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e419S–e496S. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3069. [Google Scholar]
- Rodger, M.A.; Le Gal, G. Who should get long-term anticoagulant therapy for venous thromboembolism and with what? Blood Adv. 2018, 2, 3081–3087. [Google Scholar] [CrossRef]
- Sohail, M.A.; Saif, M.W. Role of anticoagulation in the management of pancreatic cancer. JOP 2009, 10, 82–87. [Google Scholar] [PubMed]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.K.; Levine, M.N.; Kadziola, Z.; Lemoine, N.R.; Low, V.; Patel, H.K.; Rustin, G.; Thomas, M.; Quigley, M.; Williamson, R.C.N. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The fragmin advanced malignancy outcome study (FAMOUS). J. Clin. Oncol. 2004, 22, 1944–1948. [Google Scholar] [CrossRef]
- Klerk, C.P.W.; Smorenburg, S.M.; Otten, H.-M.; Lensing, A.W.A.; Prins, M.H.; Piovella, F.; Prandoni, P.; Bos, M.M.E.M.; Richel, D.J.; van Tienhoven, G.; et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J. Clin. Oncol. 2005, 23, 2130–2135. [Google Scholar] [CrossRef]
- Bao, Y.; Wan, X.; Fu, J.; Wu, B. The risk of venous thromboembolism in cancer patients receiving chemotherapy: A meta-analysis with systematic review. Ann. Transl. Med. 2021, 9, 277. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef]
- Khorana, A.A.; Dalal, M.; Lin, J.; Connolly, G.C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013, 119, 648–655. [Google Scholar] [CrossRef]
- Hanna-Sawires, R.G.; Groen, J.V.; Hamming, A.; Tollenaar, R.; Mesker, W.E.; Luelmo, S.A.C.; Vahrmeijer, A.L.; Bonsing, B.A.; Versteeg, H.H.; Klok, F.A.; et al. Incidence, timing and risk factors of venous thromboembolic events in patients with pancreatic cancer. Thromb. Res. 2021, 207, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Mandala, M.; Tondini, C. The impact of thromboprophylaxis on cancer survival: Focus on pancreatic cancer. Expert Rev. Anticancer Ther. 2011, 11, 579–588. [Google Scholar] [CrossRef]
- Maraveyas, A.; Haque, F.; Muazzam, I.A.; Ilyas, W.; Bozas, G. Increased dose primary thromboprophylaxis in ambulatory patients with advanced pancreatic ductal adenocarcinoma, a single centre cohort study. Thromb. J. 2020, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, P.; Bokas, A.; Papadimitropoulou, A.; Koustas, E.; Theocharis, S.; Papakotoulas, P.; Schizas, D.; Papalampros, A.; Felekouras, E.; Papavassiliou, A.G.; et al. Combinatorial Treatment of Tinzaparin and Chemotherapy Can Induce a Significant Antitumor Effect in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 7053. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Kahale, L.A.; Ballout, R.A.; Barba, M.; Yosuico, V.E.; van Doormaal, F.F.; Middeldorp, S.; Bryant, A.; Schunemann, H. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst. Rev. 2014, 12, CD006652. [Google Scholar]
- Grandoni, F.; Alberio, L. Direct Oral Anticoagulant Drugs: On the Treatment of Cancer-Related Venous Thromboembolism and their Potential Anti-Neoplastic Effect. Cancers 2019, 11, 46. [Google Scholar] [CrossRef]
- Kahale, L.A.; Hakoum, M.B.; Tsolakian, I.G.; Matar, C.F.; Barba, M.; Yosuico, V.E.D.; Terrenato, I.; Sperati, F.; Schunemann, H.; Akl, E.A. Oral anticoagulation in people with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst. Rev. 2017, 12, CD006466. [Google Scholar] [PubMed]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Caravaggio Investigators. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Bauersachs, R.; Brenner, B.; Campanini, M.; Cohen, A.; Connors, J.M.; Fontanella, A.; Gussoni, G.; Huisman, M.V.; et al. Caravaggio Study Investigators. Apixaban versus Dalteparin for the Treatment of Acute Venous Thromboembolism in Patients with Cancer: The Caravaggio Study. Thromb. Haemost. 2018, 118, 1668–1678. [Google Scholar]
| Characteristic | Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Long-Term Systemic Anticoagulant Use | Long-Term Systemic Anticoagulant Use | |||||||
| Total (n = 242,903) | Yes (n = 15,719) | No (n = 227,184) | p-Value | Total (n = 48,183) | Yes (n = 12,045) | No (n = 36,138) | p-Value | |
| Patients’ characteristics | ||||||||
| Age | 67.8 ± 0.05 | 69.8 ± 0.11 | 67.6 ± 0.05 | <0.001 | 70.1 ± 0.07 | 70.0 ± 0.12 | 70.1 ± 0.07 | 0.295 |
| <45 | 7485 (3.1) | 297 (1.9) | 7188 (3.2) | <0.001 | 878 (1.8) | 232 (1.9) | 646 (1.8) | 0.511 |
| 45–64 | 86,466 (35.6) | 4609 (29.3) | 81,857 (36.0) | 13,935 (28.9) | 3502 (29.0) | 10,433 (28.9) | ||
| 65–74 | 73,518 (30.3) | 5016 (31.9) | 68,502 (30.2) | 15,027 (31.2) | 3789 (31.5) | 11,238 (31.1) | ||
| 75–84 | 55,531 (22.9) | 4294 (27.3) | 51,237 (22.5) | 13,483 (28.0) | 3293 (27.4) | 10,190 (28.2) | ||
| 85+ | 19,903 (8.2) | 1503 (9.6) | 18,400 (8.1) | 4860 (10.1) | 1229 (10.2) | 3631 (10.0) | ||
| Sex | <0.001 | 0.242 | ||||||
| Male | 119,468 (49.2) | 7324 (46.6) | 112,144 (49.3) | 24,932 (51.8) | 6290 (52.2) | 18,642 (51.6) | ||
| Female | 123,435 (50.8) | 8395 (53.4) | 115,040 (50.7) | 23,251 (48.2) | 5755 (47.8) | 17,496 (48.4) | ||
| Race | <0.001 | 0.978 | ||||||
| White | 159,076 (73.1) | 11,445 (78.7) | 147,631 (72.7) | 37,405 (77.6) | 9342 (77.5) | 28,063 (77.6) | ||
| Black | 28,641 (13.2) | 1655 (11.4) | 26,986 (13.3) | 5769 (12.0) | 1443 (12.0) | 4326 (12.0) | ||
| Hispanic | 16,559 (7.6) | 808 (5.6) | 15,751 (7.7) | 2794 (5.8) | 708 (5.9) | 2086 (5.8) | ||
| Others | 13,338 (6.1) | 623 (4.3) | 12,715 (6.3) | 2215 (4.6) | 552 (4.6) | 1663 (4.6) | ||
| Missing | 25,289 | 1188 | 24,101 | |||||
| Household income | <0.001 | 0.935 | ||||||
| Quartile1 | 58,858 (24.7) | 3425 (22.2) | 55,433 (24.9) | 11,061 (23.0) | 2755 (22.9) | 8306 (23.0) | ||
| Quartile2 | 58,578 (24.6) | 3802 (24.6) | 54,776 (24.5) | 11,579 (24.0) | 2884 (23.9) | 8695 (24.0) | ||
| Quartile3 | 59,467 (24.9) | 4041 (26.1) | 55,426 (24.9) | 12,117 (25.1) | 3059 (25.4) | 9058 (25.0) | ||
| Quartile4 | 61,354 (25.8) | 4205 (27.2) | 57,149 (25.7) | 13,426 (27.8) | 3347 (27.8) | 10,079 (27.9) | ||
| Missing | 4646 | 246 | 4400 | |||||
| Insurance status | <0.001 | 0.359 | ||||||
| Medicare/Medicaid | 159,623 (65.9) | 11,218 (71.5) | 148,405 (65.5) | 34,750 (72.1) | 8653 (71.9) | 26,097 (72.2) | ||
| Private including HMO | 71,125 (29.3) | 3979 (25.3) | 67,146 (29.6) | 11,843 (24.6) | 2970 (24.6) | 8873 (24.5) | ||
| Self-pay/no-charge/other | 11,769 (4.8) | 504 (3.2) | 11,265 (5.0) | 1590 (3.3) | 422 (3.5) | 1168 (3.2) | ||
| Missing | 386 | 18 | 368 | |||||
| Admission type | <0.001 | 0.989 | ||||||
| Emergent | 192,576 (79.6) | 13,469 (86.0) | 179,107 (79.1) | 41,134 (85.4) | 10,282 (85.4) | 30,852 (85.4) | ||
| Elective | 49,722 (20.4) | 2201 (14.0) | 47,521 (20.9) | 7049 (14.6) | 1763 (14.6) | 5286 (14.6) | ||
| Missing | 605 | 49 | 556 | |||||
| Metastatic disease | 131,940 (54.3) | 9006 (57.3) | 122,934 (54.1) | <0.001 | 27,284 (56.6) | 6758 (56.1) | 20,526 (56.8) | 0.166 |
| Pancreatic cancer type | <0.001 | 0.450 | ||||||
| Head | 66,519 (27.4) | 3374 (21.5) | 63,145 (27.8) | 10,659 (22.1) | 2711 (22.5) | 7948 (22.0) | ||
| Body/Tail | 23,694 (9.8) | 1660 (10.6) | 22,034 (9.7) | 4714 (9.8) | 1162 (9.6) | 3552 (9.8) | ||
| Islets cell | 1980 (0.8) | 83 (0.5) | 1897 (0.8) | 230 (0.5) | 67 (0.6) | 163 (0.5) | ||
| unspecified | 148,519 (61.2) | 10,408 (66.2) | 138,111 (60.8) | 32,135 (66.7) | 7994 (66.4) | 24,141 (66.8) | ||
| Overlapping | 2191 (0.9) | 194 (1.2) | 1997 (0.9) | 445 (0.9) | 111 (0.9) | 334 (0.9) | ||
| Pancreatic resection | 21,505 (8.9) | 670 (4.3) | 20,835 (9.2) | <0.001 | 2280 (4.7) | 549 (4.6) | 1731 (4.8) | 0.323 |
| Long-term aspirin use | 13,859 (5.8) | 1340 (8.6) | 12,519 (5.6) | <0.001 | 3722 (7.8) | 973 (8.1) | 2749 (7.7) | 0.119 |
| Long-term antiplatelet use | 1276 (0.5) | 92 (0.6) | 1184 (0.5) | 0.301 | 340 (0.7) | 83 (0.7) | 257 (0.7) | 0.783 |
| Bleeding | ||||||||
| ICH | 685 (0.3) | 91 (0.6) | 594 (0.3) | <0.001 | 196 (0.4) | 51 (0.4) | 145 (0.4) | 0.774 |
| Upper Gastrointestinal | 10,202 (4.2) | 708 (4.5) | 9494 (4.2) | 0.067 | 2806 (5.8) | 715 (5.9) | 2091 (5.8) | 0.492 |
| Lower Gastrointestinal | 2355 (1.0) | 275 (1.8) | 2080 (0.9) | <0.001 | 643 (1.3) | 167 (1.4) | 476 (1.3) | 0.594 |
| Other | 2947 (1.2) | 365 (2.3) | 2582 (1.1) | <0.001 | 816 (1.7) | 206 (1.7) | 610 (1.7) | 0.850 |
| History | ||||||||
| MI | 9563 (3.9) | 890 (5.7) | 8673 (3.8) | <0.001 | 2526 (5.2) | 648 (5.4) | 1878 (5.2) | 0.488 |
| Valvular heart disease | 7719 (3.2) | 1025 (6.5) | 6694 (2.9) | <0.001 | 2537 (5.3) | 642 (5.3) | 1895 (5.2) | 0.681 |
| Prior PCI, CABG, or valvular surgery | 16,152 (6.6) | 1691 (10.7) | 14,461 (6.4) | <0.001 | 4481 (9.3) | 1153 (9.6) | 3328 (9.2) | 0.255 |
| CHD | 33,556 (13.8) | 2638 (16.7) | 30,918 (13.6) | <0.001 | 8091 (16.7) | 2006 (16.6) | 6085 (16.8) | 0.663 |
| AF | 27,039 (11.2) | 5748 (36.6) | 21,291 (9.4) | <0.001 | 17,128 (35.6) | 4134 (34.4) | 129,94 (36.0) | 0.002 |
| PAD | 1680 (0.7) | 200 (1.3) | 1480 (0.7) | <0.001 | 457 (1.0) | 122 (1.0) | 335 (0.9) | 0.382 |
| Circulatory diseases | 25,842 (10.7) | 7037 (44.9) | 18,805 (8.3) | <0.001 | 17,024 (35.4) | 4397 (36.6) | 12,627 (35.0) | 0.003 |
| Cerebral artery occlusion or stenosis | 1476 (0.6) | 115 (0.7) | 1361 (0.6) | 0.037 | 375 (0.8) | 91 (0.8) | 284 (0.8) | 0.717 |
| CCI | <0.001 | 0.115 | ||||||
| 0 | 42,788 (17.6) | 2005 (12.7) | 40,783 (17.9) | 6252 (13.0) | 1638 (13.6) | 4614 (12.7) | ||
| 1–3 | 60,146 (24.8) | 3989 (25.4) | 56,157 (24.7) | 12,436 (25.8) | 3098 (25.7) | 9338 (25.8) | ||
| 4–6 | 63,825 (26.2) | 4136 (26.3) | 59,689 (26.2) | 12,379 (25.7) | 3087 (25.6) | 9292 (25.7) | ||
| 7+ | 76,144 (31.4) | 5589 (35.6) | 70,555 (31.1) | 17,116 (35.6) | 4222 (35.1) | 12,894 (35.7) | ||
| Hospitals’ characteristics | ||||||||
| Hospital bed size | <0.001 | 0.882 | ||||||
| Small | 31,073 (12.6) | 2190 (13.8) | 28,883 (12.5) | 6447 (13.2) | 1628 (13.4) | 4819 (13.2) | ||
| Medium | 55,771 (23.2) | 3716 (23.8) | 52,055 (23.1) | 11,703 (24.4) | 2930 (24.4) | 8773 (24.4) | ||
| Large | 155,326 (64.2) | 9788 (62.4) | 145,538 (64.3) | 30,033 (62.4) | 7487 (62.2) | 22,546 (62.4) | ||
| Missing | 733 | 25 | 708 | |||||
| Hospital location/teaching status | 0.855 | 0.779 | ||||||
| Rural | 18,707 (7.7) | 1209 (7.7) | 17,498 (7.7) | 3331 (6.9) | 842 (7.0) | 2489 (6.9) | ||
| Urban nonteaching | 71,226 (29.2) | 4579 (28.9) | 66,647 (29.2) | 14,518 (29.9) | 3595 (29.6) | 10,923 (30.0) | ||
| Urban teaching | 152,237 (63.1) | 9906 (63.4) | 142,331 (63.1) | 30,334 (63.2) | 7608 (63.4) | 22,726 (63.1) | ||
| Missing | 733 | 25 | 708 | |||||
| Hospital region | <0.001 | 0.914 | ||||||
| Northeast | 53,941 (22.4) | 3266 (21.0) | 50,675 (22.5) | 11,303 (23.6) | 2847 (23.9) | 8456 (23.6) | ||
| Midwest | 55,368 (22.8) | 4284 (27.2) | 51,084 (22.5) | 10,095 (21.0) | 2524 (21.0) | 7571 (21.0) | ||
| South | 87,268 (35.8) | 5043 (32.1) | 82,225 (36.1) | 17,177 (35.6) | 4262 (35.3) | 12,915 (35.7) | ||
| West | 46,326 (18.9) | 3126 (19.7) | 43,200 (18.9) | 9608 (19.8) | 2412 (19.9) | 7196 (19.7) | ||
| Characteristic | Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Long-Term Systemic Anticoagulation | Long-Term Systemic Anticoagulation | |||||||
| Total (n = 242,903) | Yes (n = 15,719) | No (n = 227,184) | p-Value | Total (n = 48,183) | Yes (n = 12,045) | No (n = 36,138) | p-Value | |
| Life-threatening events | 68,509 (28.3) | 4687 (29.9) | 63,822 (28.2) | <0.001 | 15,611 (32.5) | 3611 (30.1) | 12,000 (33.3) | <0.001 |
| AMI | 4479 (1.8) | 334 (2.1) | 4145 (1.8) | 0.007 | 1173 (2.4) | 266 (2.2) | 907 (2.5) | 0.053 |
| AHF | 2382 (1.0) | 276 (1.8) | 2106 (0.9) | <0.001 | 826 (1.7) | 204 (1.7) | 622 (1.7) | 0.882 |
| Sepsis | 41,444 (17.1) | 2527 (16.1) | 38,917 (17.2) | 0.001 | 9053 (18.8) | 1978 (16.5) | 7075 (19.6) | <0.001 |
| Shock | 7142 (2.9) | 356 (2.3) | 6786 (3.0) | <0.001 | 1569 (3.3) | 260 (2.2) | 1309 (3.6) | <0.001 |
| AKI | 34,127 (14.1) | 2381 (15.2) | 31,746 (14.1) | <0.001 | 8162 (17.0) | 1815 (15.1) | 6347 (17.7) | <0.001 |
| In-hospital mortality | 20,577 (8.5) | 1000 (6.3) | 19,577 (8.6) | <0.001 | 4285 (8.9) | 761 (6.3) | 3524 (9.7) | <0.001 |
| Prolonged LOS a,b | 62,022 (27.8) | 3459 (23.5) | 58,563 (28.1) | <0.001 | 11,778 (26.8) | 2705 (23.9) | 9073 (27.8) | <0.001 |
| Outcomes | Long-Term Systemic Anticoagulant Use | Multivariable | |
|---|---|---|---|
| aOR (95% CI) | p-Value | ||
| Life-threatening events | |||
| AMI | Yes vs. No | 0.88 (0.76, 1.01) | 0.071 |
| AHF | Yes vs. No | 1.01 (0.86, 1.18) | 0.894 |
| Sepsis | Yes vs. No | 0.81 (0.77, 0.86) | <0.001 |
| Shock | Yes vs. No | 0.59 (0.52, 0.68) | <0.001 |
| AKI | Yes vs. No | 0.84 (0.79, 0.89) | <0.001 |
| In-hospital mortality | Yes vs. No | 0.63 (0.58, 0.68) | <0.001 |
| Prolonged LOS a,b,c | Yes vs. No | 0.82 (0.78, 0.86) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-M.; Shih, H.-J.; Chen, Y.-C.; Hsieh, T.-Y.; Ou, C.-W.; Su, P.-H.; Chen, S.-M.; Zheng, Y.-C.; Hsu, L.-S. Systemic Anticoagulation and Inpatient Outcomes of Pancreatic Cancer: Real-World Evidence from U.S. Nationwide Inpatient Sample. Cancers 2023, 15, 1985. https://doi.org/10.3390/cancers15071985
Huang Y-M, Shih H-J, Chen Y-C, Hsieh T-Y, Ou C-W, Su P-H, Chen S-M, Zheng Y-C, Hsu L-S. Systemic Anticoagulation and Inpatient Outcomes of Pancreatic Cancer: Real-World Evidence from U.S. Nationwide Inpatient Sample. Cancers. 2023; 15(7):1985. https://doi.org/10.3390/cancers15071985
Chicago/Turabian StyleHuang, Yen-Min, Hsuan-Jen Shih, Yi-Chan Chen, Tsan-Yu Hsieh, Che-Wei Ou, Po-Hsu Su, Shih-Ming Chen, Yun-Cong Zheng, and Li-Sung Hsu. 2023. "Systemic Anticoagulation and Inpatient Outcomes of Pancreatic Cancer: Real-World Evidence from U.S. Nationwide Inpatient Sample" Cancers 15, no. 7: 1985. https://doi.org/10.3390/cancers15071985
APA StyleHuang, Y.-M., Shih, H.-J., Chen, Y.-C., Hsieh, T.-Y., Ou, C.-W., Su, P.-H., Chen, S.-M., Zheng, Y.-C., & Hsu, L.-S. (2023). Systemic Anticoagulation and Inpatient Outcomes of Pancreatic Cancer: Real-World Evidence from U.S. Nationwide Inpatient Sample. Cancers, 15(7), 1985. https://doi.org/10.3390/cancers15071985





