Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Overview
1.2. Genetic Characteristics of Chondrosarcoma
1.3. Chondrosarcoma Standard Treatment
1.3.1. Chondrosarcoma Chemotherapy
1.3.2. Chondrosarcoma Radiotherapy
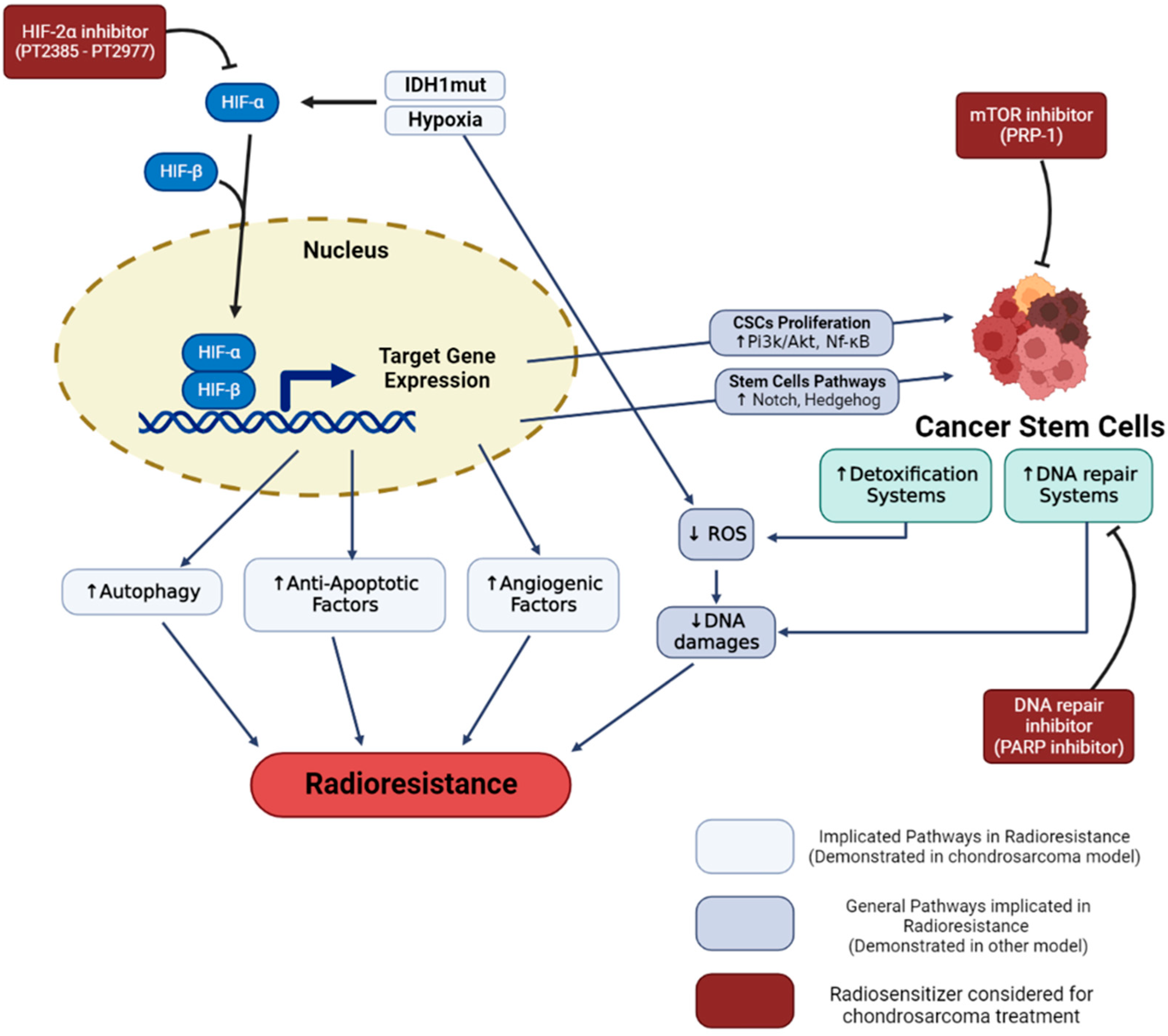
2. Radiation Resistance of Chondrosarcoma: Microenvironment, Molecular and Cellular Consequences
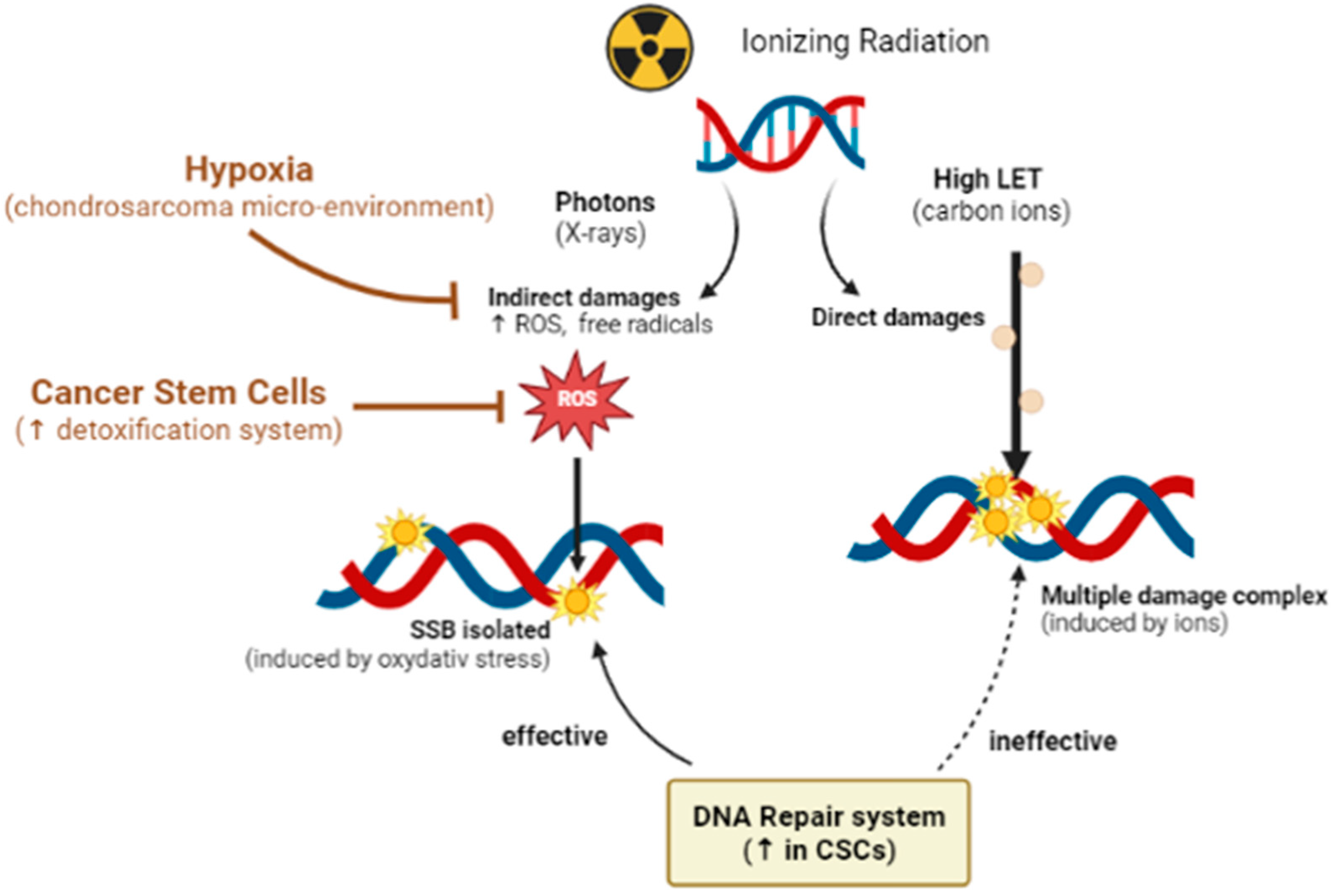
2.1. Hypoxia-Related Radiation Resistance
2.2. Radioresistance Links with CSCs
3. Hadrontherapy and Combined Therapy of Chondrosarcoma
3.1. Hadrontherapy
3.1.1. Physical Advantage of Hadrontherapy: The Bragg Peak
3.1.2. Biological Advantage of Hadrontherapy: High LET and RBE
3.1.3. State of Art of Hadrontherapy in Clinical Practice
3.2. Combined Approaches in Chondrosarcoma Control Strategy
3.2.1. IDH Inhibitors
3.2.2. PARP Inhibitors
3.2.3. Targeting the Hypoxic Microenvironment
3.2.4. Targeting CSCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nazeri, E.; Gouran Savadkoohi, M.; Majidzadeh-A, K.; Esmaeili, R. Chondrosarcoma: An Overview of Clinical Behavior, Molecular Mechanisms Mediated Drug Resistance and Potential Therapeutic Targets. Crit. Rev. Oncol./Hematol. 2018, 131, 102–109. [Google Scholar] [CrossRef]
- Dai, X.; Ma, W.; He, X.; Jha, R.K. Review of Therapeutic Strategies for Osteosarcoma, Chondrosarcoma, and Ewing’s Sarcoma. Med. Sci. Monit. 2011, 17, RA177-190. [Google Scholar] [CrossRef]
- David, E.; Blanchard, F.; Heymann, M.F.; De Pinieux, G.; Gouin, F.; Rédini, F.; Heymann, D. The Bone Niche of Chondrosarcoma: A Sanctuary for Drug Resistance, Tumour Growth and Also a Source of New Therapeutic Targets. Sarcoma 2011, 2011, 932451. [Google Scholar] [CrossRef]
- Puri, A. Chondrosarcomas in Children and Adolescents. EFORT Open Rev. 2020, 5, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, T.D.; Shultz, B.N.; Munger, A.M.; Amick, M.; Toombs, C.S.; Friedaender, G.E.; Grauer, J.N. Chondrosarcoma Patient Characteristics, Management, and Outcomes Based on over 5000 Cases from the National Cancer Database (NCDB). PLoS ONE 2022, 17, e0268215. [Google Scholar] [CrossRef]
- Chou, P.; Mehta, S.; Gonzalez-Crussi, F. Chondrosarcoma of the head in children. Pediatr. Pathol. 1990, 10, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Tosun, I.; Naderi, S. Approach to Primary Vertebral Tumors in the Light of the 2020 Updated World Health Organization Classification of Bone Tumors. Turk. Neurosurg. 2021. [Google Scholar] [CrossRef]
- Zoccali, C.; Baldi, J.; Attala, D.; Rossi, B.; Anelli, V.; Annovazzi, A.; Ferraresi, V. Intralesional vs. Extralesional Procedures for Low-Grade Central Chondrosarcoma: A Systematic Review of the Literature. Arch. Orthop. Trauma Surg. 2018, 138, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.; Unni, K.K.; Mertens, F. Pathology and Genetics of Tumours of Soft Tissue and Bone; International Agency for Research on Cancer: Lyon, France, 2002; Volume 177, ISBN 92-832-2413-2. [Google Scholar]
- Girard, N.; Lhuissier, E.; Aury-Landas, J.; Cauvard, O.; Lente, M.; Boittin, M.; Baugé, C.; Boumédiene, K. Heterogeneity of Chondrosarcomas Response to Irradiations with X-rays and Carbon Ions: A Comparative Study on Five Cell Lines. J. Bone Oncol. 2020, 22, 100283. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.; Pacheco, M.; Cocchi, S.; Asioli, S.; Gambarotti, M.; Donati, D.M.; Evangelista, A.; Gnoli, M.; Locatelli, M.; Mordenti, M.; et al. Secondary Peripheral Chondrosarcoma Arising in Solitary Osteochondroma: Variables Influencing Prognosis and Survival. Orphanet. J. Rare Dis. 2022, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, S.; Bouaziz, M.C.; Drissi, C.; Abid, L.; Ladeb, M.F. Periosteal Chondrosarcoma. Am. J. Roentgenol. 2009, 192, W1–W6. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.A. Chondrosarcoma: Biology, Genetics, and Epigenetics [Version 1; Referees: 2 Approved]. F1000Research 2018, 7, 1826. [Google Scholar] [CrossRef]
- Strach, M.C.; Grimison, P.S.; Hong, A.; Boyle, R.; Stalley, P.; Karim, R.; Connolly, E.A.; Bae, S.; Desai, J.; Crowe, P.; et al. Mesenchymal Chondrosarcoma: An Australian Multi-Centre Cohort Study. Cancer Med. 2022, 12, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Tauscher, F.; Birkenmaier, C.; Baur-Melnyk, A.; Knösel, T.; Jansson, V.; Dürr, H.R. Clear Cell Chondrosarcoma Is an Underestimated Tumor: Report of 7 Cases and Meta-Analysis of the Literature. J. Bone Oncol. 2019, 19, 100267. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Baldi, G.G.; Morosi, C.; Gronchi, A.; Maestro, R. Extraskeletal Myxoid Chondrosarcoma: State of the Art and Current Research on Biology and Clinical Management. Cancers 2020, 12, 2703. [Google Scholar] [CrossRef] [PubMed]
- Cozma, G.V.; Sima, L.V.; Cloşca, R.M.; Baderca, F.; Horhat, I.D.; Balica, N.C.; Tischer, A.A.; Moţ, I.C.; Maliţa, D.C.; Marin, A.; et al. Conventional Grade 1 Chondrosarcoma: A Challenging Diagnosis with Important Implications on Therapy and Prognosis. Rom. J. Morphol. Embryol. 2021, 62, 605–613. [Google Scholar] [CrossRef]
- Hosseini, A.; Mirzaei, A.; Salimi, V.; Jamshidi, K.; Babaheidarian, P.; Fallah, S.; Rampisheh, Z.; Khademian, N.; Abdolvahabi, Z.; Bahrabadi, M.; et al. The Local and Circulating SOX9 as a Potential Biomarker for the Diagnosis of Primary Bone Cancer. J. Bone Oncol. 2020, 23, 100300. [Google Scholar] [CrossRef]
- Zhang, Q.; Xi, Y.; Li, D.; Yuan, Z.; Dong, J. The Utility of 18F-FDG PET and PET/CT in the Diagnosis and Staging of Chondrosarcoma: A Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 229. [Google Scholar] [CrossRef]
- Amary, M.F.; Bacsi, K.; Maggiani, F.; Damato, S.; Halai, D.; Berisha, F.; Pollock, R.; O’Donnell, P.; Grigoriadis, A.; Diss, T.; et al. IDH1 and IDH2 Mutations Are Frequent Events in Central Chondrosarcoma and Central and Periosteal Chondromas but Not in Other Mesenchymal Tumours. J. Pathol. 2011, 224, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-Associated IDH1 Mutations Produce 2-Hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Lu, C.; Ward, P.S.; Kapoor, G.S.; Rohle, D.; Turcan, S.; Abdel-Wahab, O.; Edwards, C.R.; Khanin, R.; Figueroa, M.E.; Melnick, A.; et al. IDH Mutation Impairs Histone Demethylation and Results in a Block to Cell Differentiation. Nature 2012, 483, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Knobbe, C.B.; Itsumi, M.; Elia, A.J.; Harris, I.S.; Chio, I.I.C.; Cairns, R.A.; McCracken, S.; Wakeham, A.; Haight, J.; et al. D-2-Hydroxyglutarate Produced by Mutant IDH1 Perturbs Collagen Maturation and Basement Membrane Function. Genes Dev. 2012, 26, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lin, Y.; Xu, W.; Jiang, W.; Zha, Z.; Wang, P.; Yu, W.; Li, Z.; Gong, L.; Peng, Y.; et al. Glioma-Derived Mutations in IDH1 Dominantly Inhibit IDH1 Catalytic Activity and Induce HIF-1alpha. Science 2009, 324, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, L.; Eid, J.E.; Liu, C.; Yu, J.; Yue, J.; Trent, J.C. IDH1 Mutation Induces HIF-1α and Confers Angiogenic Properties in Chondrosarcoma JJ012 Cells. Dis. Mrk. 2022, 2022, 7729968. [Google Scholar] [CrossRef]
- Kim, H.; Cho, Y.; Kim, H.-S.; Kang, D.; Cheon, D.; Kim, Y.-J.; Chang, M.J.; Lee, K.M.; Chang, C.B.; Kang, S.-B.; et al. A System-Level Approach Identifies HIF-2α as a Critical Regulator of Chondrosarcoma Progression. Nat. Commun. 2020, 11, 5023. [Google Scholar] [CrossRef]
- Tlemsani, C.; Larousserie, F.; De Percin, S.; Audard, V.; Hadjadj, D.; Chen, J.; Biau, D.; Anract, P.; Terris, B.; Goldwasser, F.; et al. Biology and Management of High-Grade Chondrosarcoma: An Update on Targets and Treatment Options. Int. J. Mol. Sci. 2023, 24, 1361. [Google Scholar] [CrossRef]
- Ho, X.D.; Nguyen, H.G.; Trinh, L.H.; Reimann, E.; Prans, E.; Kõks, G.; Maasalu, K.; Le, V.Q.; Nguyen, V.H.; Le, N.T.N.; et al. Analysis of the Expression of Repetitive DNA Elements in Osteosarcoma. Front. Genet. 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Ho, X.D.; Phung, P.; Le, V.Q.; Nguyen, V.H.; Reimann, E.; Prans, E.; Kõks, G.; Maasalu, K.; Le, N.T.; Trinh, L.H.; et al. Whole Transcriptome Analysis Identifies Differentially Regulated Networks between Osteosarcoma and Normal Bone Samples. Exp. Biol. Med. 2017, 242, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Hogendoorn, P.C.W.; Athanasou, N.; Bielack, S.; Alava, E.D.; Tos, A.P.D.; Ferrari, S.; Gelderblom, H.; Grimer, R.; Hall, K.S.; Hassan, B.; et al. Bone Sarcomas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2010, 21, v204–v213. [Google Scholar] [CrossRef]
- Jeong, W.; Kim, H.J. Biomarkers of Chondrosarcoma. J. Clin. Pathol. 2018, 71, 579–583. [Google Scholar] [CrossRef]
- Boehme, K.A.; Schleicher, S.B.; Traub, F.; Rolauffs, B. Chondrosarcoma: A Rare Misfortune in Aging Human Cartilage? The Role of Stem and Progenitor Cells in Proliferation, Malignant Degeneration and Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Monga, V.; Mani, H.; Hirbe, A.; Milhem, M. Non-Conventional Treatments for Conventional Chondrosarcoma. Cancers 2020, 12, 1962. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.S.; Davis, L.E. Osteosarcoma, Chondrosarcoma, and Chordoma. J. Clin. Oncol. 2018, 36, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gallego, B.; Murillo, D.; Rey, V.; Huergo, C.; Estupiñán, Ó.; Rodríguez, A.; Tornín, J.; Rodríguez, R. Addressing Doxorubicin Resistance in Bone Sarcomas Using Novel Drug-Resistant Models. Int. J. Mol. Sci. 2022, 23, 6425. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Lin, C.Y.; Kuo, S.J.; Su, C.M.; Tang, C.H. An Update on Current and Future Treatment Options for Chondrosarcoma. Expert Rev. Anticancer Ther. 2019, 19, 773–786. [Google Scholar] [CrossRef]
- Shah, F.H.; Kim, S.J. Identification of Medicinal Compounds as Potential Inhibitors for Mutated Isocitrate Dehydrogenases against Chondrosarcoma. Saudi J. Biol. Sci. 2022, 29, 161–167. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Therapeutic Targets and Emerging Treatments in Advanced Chondrosarcoma. Int. J. Mol. Sci. 2022, 23, 1096. [Google Scholar] [CrossRef]
- Fan, B.; Mellinghoff, I.K.; Wen, P.Y.; Lowery, M.A.; Goyal, L.; Tap, W.D.; Pandya, S.S.; Manyak, E.; Jiang, L.; Liu, G.; et al. Clinical Pharmacokinetics and Pharmacodynamics of Ivosidenib, an Oral, Targeted Inhibitor of Mutant IDH1, in Patients with Advanced Solid Tumors. Investig. New Drugs 2020, 38, 433–444. [Google Scholar] [CrossRef]
- Khurshed, M.; Molenaar, R.J.; van Linde, M.E.; Mathôt, R.A.; Struys, E.A.; van Wezel, T.; van Noorden, C.J.F.; Klümpen, H.J.; Bovée, J.V.M.G.; Wilmink, J.W. A Phase Ib Clinical Trial of Metformin and Chloroquine in Patients with Idh1-Mutated Solid Tumors. Cancers 2021, 13, 2474. [Google Scholar] [CrossRef]
- Jones, R.L.; Katz, D.; Loggers, E.T.; Davidson, D.; Rodler, E.T.; Pollack, S.M. Clinical Benefit of Antiangiogenic Therapy in Advanced and Metastatic Chondrosarcoma. Med. Oncol. 2017, 34, 167. [Google Scholar] [CrossRef]
- Chow, W.; Frankel, P.; Ruel, C.; Araujo, D.M.; Milhem, M.; Okuno, S.; Hartner, L.; Undevia, S.; Staddon, A. Results of a Prospective Phase 2 Study of Pazopanib in Patients with Surgically Unresectable or Metastatic Chondrosarcoma. Cancer 2020, 126, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yu, C.; Wang, Z.; Mu, H.; Cai, Z. PLCD1-Induced DNA Damage Inhibits the Tumor Growth via Downregulating CDKs in Chondrosarcoma. J. Oncol. 2022, 2022, 4488640. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan Coyne, G.; Kummar, S.; Hu, J.; Ganjoo, K.; Chow, W.A.; Do, K.T.; Zlott, J.; Bruns, A.; Rubinstein, L.; Foster, J.C.; et al. Clinical Activity of Single-Agent Cabozantinib (XL184), a Multi-Receptor Tyrosine Kinase Inhibitor, in Patients with Refractory Soft-Tissue Sarcomas. Clin. Cancer Res. 2022, 28, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yan, T.; Guo, W.; Niu, J.; Zhao, Z.; Sun, K.; Zhang, H.; Yu, Y.; Ren, T. Constitutive GLI1 Expression in Chondrosarcoma Is Regulated by Major Vault Protein via MTOR/S6K1 Signaling Cascade. Cell Death Differ. 2021, 28, 2221–2237. [Google Scholar] [CrossRef]
- van Oosterwijk, J.G.; Anninga, J.K.; Gelderblom, H.; Cleton-Jansen, A.M.; Bovée, J.V.M.G. Update on Targets and Novel Treatment Options for High-Grade Osteosarcoma and Chondrosarcoma. Hematol./Oncol. Clin. North Am. 2013, 27, 1021–1048. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.A.; Xie, H.; Costa, F.F.; Vanin, E.F.; Seftor, E.A.; Sredni, S.T.; Bischof, J.; Wang, D.; Bonaldo, M.F.; Hendrix, M.J.C.; et al. Global Demethylation of Rat Chondrosarcoma Cells after Treatment with 5-Aza-2′-Deoxycytidine Results in Increased Tumorigenicity. PLoS ONE 2009, 4, e8340. [Google Scholar] [CrossRef]
- Subramanian, S.; Bates, S.E.; Wright, J.J.; Espinoza-Delgado, I.; Piekarz, R.L. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals 2010, 3, 2751–2767. [Google Scholar] [CrossRef]
- Sheng, W.; Zhang, Z.-C.; Shi, D.-Y.; Wang, B.-C.; Wu, Q.; Shao, Z.-W.; Yang, S.-H.; He, T.-C.; Liu, J.-X. Epigenetic Silencing of SFRP5 Promotes the Metastasis and Invasion of Chondrosarcoma by Expression Inhibition and Wnt Signaling Pathway Activation. Chem.-Biol. Interact. 2018, 296, 1–8. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in Immune Checkpoint Inhibitors for Bone Sarcoma Therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Ricciotti, R.W.; Mantilla, J.; Loggers, E.T.; Pollack, S.M.; Cranmer, L.D. Response to PD1 Inhibition in Conventional Chondrosarcoma. J. Immunother. Cancer 2018, 6, 94. [Google Scholar] [CrossRef]
- Chen, S.; Fritchie, K.; Wei, S.; Ali, N.; Curless, K.; Shen, T.; Brini, A.T.; Latif, F.; Sumathi, V.; Siegal, G.P.; et al. Diagnostic Utility of IDH1/2 Mutations to Distinguish Dedifferentiated Chondrosarcoma from Undifferentiated Pleomorphic Sarcoma of Bone. Hum. Pathol. 2017, 65, 239–246. [Google Scholar] [CrossRef]
- de Jong, Y.; Ingola, M.; Briaire-de Bruijn, I.H.; Kruisselbrink, A.B.; Venneker, S.; Palubeckaite, I.; Heijs, B.P.A.M.; Cleton-Jansen, A.-M.; Haas, R.L.M.; Bovée, J.V.M.G. Radiotherapy Resistance in Chondrosarcoma Cells; a Possible Correlation with Alterations in Cell Cycle Related Genes. Clin. Sarcoma Res. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Amichetti, M.; Amelio, D.; Cianchetti, M.; Giacomelli, I.; Scartoni, D. The Treatment of Chordoma and Chondrosarcoma of the Skull Base with Particular Attention to Radiotherapy. Clin. Oncol. 2017, 2, 1195. [Google Scholar]
- Catanzano, A.A.; Kerr, D.L.; Lazarides, A.L.; Dial, B.L.; Lane, W.O.; Blazer, D.G.; Larrier, N.A.; Kirsch, D.G.; Brigman, B.E.; Eward, W.C. Revisiting the Role of Radiation Therapy in Chondrosarcoma: A National Cancer Database Study. Sarcoma 2019, 2019, 4878512. [Google Scholar] [CrossRef]
- Mehta, S.R.; Suhag, V.; Semwal, M.; Sharma, N. Radiotherapy: Basic Concepts and Recent Advances. Med. J. Armed Forces India 2010, 66, 158–162. [Google Scholar] [CrossRef]
- Lepleux, C.; Marie-Brasset, A.; Temelie, M.; Boulanger, M.; Brotin, É.; Goldring, M.B.; Hirtz, C.; Varès, G.; Nakajima, T.; Saintigny, Y.; et al. Bystander Effectors of Chondrosarcoma Cells Irradiated at Different LET Impair Proliferation of Chondrocytes. J. Cell Commun. Signal. 2019, 13, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Her, S.; Jaffray, D.A. Radiotherapy for Cancer: Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 1–2. [Google Scholar] [CrossRef]
- Mercado, C.E.; Holtzman, A.L.; Rotondo, R.; Rutenberg, M.S.; Mendenhall, W.M. Proton Therapy for Skull Base Tumors: A Review of Clinical Outcomes for Chordomas and Chondrosarcomas. Head Neck 2019, 41, 536–541. [Google Scholar] [CrossRef]
- DeLaney, T.F.; Liebsch, N.J.; Pedlow, F.X.; Adams, J.; Weyman, E.A.; Yeap, B.Y.; Depauw, N.; Nielsen, G.P.; Harmon, D.C.; Yoon, S.S.; et al. Long-Term Results of Phase II Study of High Dose Photon/Proton Radiotherapy in the Management of Spine Chordomas, Chondrosarcomas, and Other Sarcomas. J. Surg. Oncol. 2014, 110, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Niranjan, A.; Dade Lunsford, L. Radiosurgery for Chordoma and Chondrosarcoma. Prog. Neurol. Surg. 2019, 34, 207–214. [Google Scholar] [CrossRef]
- Gelderblom, H.; Hogendoorn, P.C.W.; Dijkstra, S.D.; van Rijswijk, C.S.; Krol, A.D.; Taminiau, A.H.M.; Bovée, J.V.M.G. The Clinical Approach towards Chondrosarcoma. The Oncologist 2008, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, H.; Van De Gucht, M.; De Ridder, M. Hypoxic Radioresistance: Can ROS Be the Key to Overcome It? Cancers 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, J.; Chen, W.; Shan, B.; Guo, Y.; Xu, J.; Wang, L.; Guo, P.; Zhang, Y. Hypoxia-Induced Autophagy as an Additional Mechanism in Human Osteosarcoma Radioresistance. J. Bone Oncol. 2016, 5, 67–73. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Yakimova, A.O. Hypoxia-Induced Cancer Cell Responses Driving Radioresistance of Hypoxic Tumors: Approaches to Targeting and Radiosensitizing. Cancers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; McGough, R.; Aswad, B.; Block, J.A.; Terek, R. Hypoxia Induces HIF-1Œ± and VEGF Expression in Chondrosarcoma Cells and Chondrocytes. J. Orthop. Res. 2004, 22, 1175–1181. [Google Scholar] [CrossRef]
- McGough, R.L.; Aswad, B.I.; Terek, R.M. Pathologic Neovascularization in Cartilage Tumors. Clin. Orthop. Relat. Res. 2002, 397, 76–82. [Google Scholar] [CrossRef]
- Kubo, T.; Sugita, T.; Shimose, S.; Matsuo, T.; Arihiro, K.; Ochi, M. Expression of Hypoxia-Inducible Factor-1alpha and Its Relationship to Tumour Angiogenesis and Cell Proliferation in Cartilage Tumours. J. Bone Jt. Surg. Br. 2008, 90, 364–370. [Google Scholar] [CrossRef]
- Boeuf, S.; Bovée, J.V.M.G.; Lehner, B.; Hogendoorn, P.C.W.; Richter, W. Correlation of Hypoxic Signalling to Histological Grade and Outcome in Cartilage Tumours. Histopathology 2010, 56, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, H.; Wei, F.; Jiang, L.; Liu, X.; Liu, Z.; Ma, Q. Increased Levels of Hypoxia-Inducible Factor-1α Are Associated with Bcl-XL Expression, Tumor Apoptosis, and Clinical Outcome in Chondrosarcoma: Increased Levels Of HIF-1α in Chondrosarcoma. J. Orthop. Res. 2011, 29, 143–151. [Google Scholar] [CrossRef]
- Sun, X.; Lv, X.; Yan, Y.; Zhao, Y.; Ma, R.; He, M.; Wei, M. Hypoxia-Mediated Cancer Stem Cell Resistance and Targeted Therapy. Biomed. Pharm. 2020, 130, 110623. [Google Scholar] [CrossRef]
- Marhold, M.; Tomasich, E.; El-Gazzar, A.; Heller, G.; Spittler, A.; Horvat, R.; Krainer, M.; Horak, P. HIF1α Regulates MTOR Signaling and Viability of Prostate Cancer Stem Cells. Mol. Cancer Res. 2015, 13, 556–564. [Google Scholar] [CrossRef]
- Wu, S.-L.; Li, Y.-J.; Liao, K.; Shi, L.; Zhang, N.; Liu, S.; Hu, Y.-Y.; Li, S.-L.; Wang, Y. 2-Methoxyestradiol Inhibits the Proliferation and Migration and Reduces the Radioresistance of Nasopharyngeal Carcinoma CNE-2 Stem Cells via NF-ΚB/HIF-1 Signaling Pathway Inactivation and EMT Reversal. Oncol. Rep. 2017, 37, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Rankin, E.B. Hypoxia-Induced Phenotypes That Mediate Tumor Heterogeneity. Adv. Exp. Med. Biol. 2019, 1136, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, F.; Han, L.; Zhao, L.; Chen, J.; Olopade, O.I.; He, M.; Wei, M. HIF-2α Promotes Conversion to a Stem Cell Phenotype and Induces Chemoresistance in Breast Cancer Cells by Activating Wnt and Notch Pathways. J. Exp. Clin. Cancer Res. 2018, 37, 256. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-N.; Yang, L.; Lv, Y.-F.; Shi, Y.; Shen, L.-L.; Yao, X.-H.; Guo, Q.-N.; Zhang, P.; Cui, Y.-H.; Zhang, X.; et al. Endothelial Cells Promote Stem-like Phenotype of Glioma Cells through Activating the Hedgehog Pathway. J. Pathol. 2014, 234, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Harris, P.J.; Warren, R.Q.; Ivy, S.P. Targeting Cancer Stem Cells by Inhibiting Wnt, Notch, and Hedgehog Pathways. Nat. Rev. Clin. Oncol. 2011, 8, 97–106. [Google Scholar] [CrossRef]
- Kuşoğlu, A.; Biray Avcı, Ç. Cancer Stem Cells: A Brief Review of the Current Status. Gene 2019, 681, 80–85. [Google Scholar] [CrossRef]
- Kim, W.-T.; Ryu, C.J. Cancer Stem Cell Surface Markers on Normal Stem Cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef]
- Vermeulen, L.; Sprick, M.R.; Kemper, K.; Stassi, G.; Medema, J.P. Cancer Stem Cells—Old Concepts, New Insights. Cell Death Differ. 2008, 15, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.-M.; Estecio, M.R.; Lin, S.-H.; Zacharias, N.M. Stem Cell Theory of Cancer: Rude Awakening or Bad Dream from Cancer Dormancy? Cancers 2022, 14, 655. [Google Scholar] [CrossRef]
- Vares, G.; Ahire, V.; Sunada, S.; Ho Kim, E.; Sai, S.; Chevalier, F.; Romeo, P.-H.; Yamamoto, T.; Nakajima, T.; Saintigny, Y. A Multimodal Treatment of Carbon Ions Irradiation, MiRNA-34 and MTOR Inhibitor Specifically Control High-Grade Chondrosarcoma Cancer Stem Cells. Radiother. Oncol. 2020, 150, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ma, S.; Cao, K.; Zhou, S.; Zhao, A.; Li, M.; Qian, F.; Zhu, C. Therapeutic Approaches Targeting Cancer Stem Cells. J. Cancer Res. Ther. 2018, 14, 1469–1475. [Google Scholar] [CrossRef]
- Brown, H.K.; Tellez-Gabriel, M.; Heymann, D. Cancer Stem Cells in Osteosarcoma. Cancer Lett. 2017, 386, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tirino, V.; Desiderio, V.; Paino, F.; De Rosa, A.; Papaccio, F.; Fazioli, F.; Pirozzi, G.; Papaccio, G. Human Primary Bone Sarcomas Contain CD133+ Cancer Stem Cells Displaying High Tumorigenicity in Vivo. FASEB J. 2011, 25, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Rey, V.; Menendez, S.T.; Estupiñan, O.; Rodriguez, A.; Santos, L.; Tornin, J.; Martinez-Cruzado, L.; Castillo, D.; Ordoñez, G.R.; Costilla, S.; et al. New Chondrosarcoma Cell Lines with Preserved Stem Cell Properties to Study the Genomic Drift During In Vitro/In Vivo Growth. JCM 2019, 8, 455. [Google Scholar] [CrossRef]
- Greco, N.; Schott, T.; Mu, X.; Rothenberg, A.; Voigt, C.; McGough III, R.L.; Goodman, M.; Huard, J.; Weiss, K.R. ALDH Activity Correlates with Metastatic Potential in Primary Sarcomas of Bone. JCT 2014, 05, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Barzegar Behrooz, A.; Syahir, A.; Ahmad, S. CD133: Beyond a Cancer Stem Cell Biomarker. J. Drug Target. 2019, 27, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Korn, P.; Kampmann, A.; Spalthoff, S.; Jehn, P.; Tavassol, F.; Lentge, F.; Gellrich, N.-C.; Zimmerer, R. Suitability of CD133 as a Marker for Cancer Stem Cells in Melanoma. Asian Pac. J. Cancer Prev 2021, 22, 1591–1597. [Google Scholar] [CrossRef]
- Wang, K. Targeting Cancer Stem Cells by Disulfiram and Copper Sensitizes Radioresistant Chondrosarcoma to Radiation. Cancer Lett. 2021, 505, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Menendez, S.T.; Rey, V.; Martinez-Cruzado, L.; Gonzalez, M.V.; Morales-Molina, A.; Santos, L.; Blanco, V.; Alvarez, C.; Estupiñan, O.; Allonca, E.; et al. SOX2 Expression and Transcriptional Activity Identifies a Subpopulation of Cancer Stem Cells in Sarcoma with Prognostic Implications. Cancers 2020, 12, 964. [Google Scholar] [CrossRef]
- Thariat, J.; Valable, S.; Laurent, C.; Haghdoost, S.; Pérès, E.A.; Bernaudin, M.; Sichel, F.; Lesueur, P.; Césaire, M.; Petit, E.; et al. Hadrontherapy Interactions in Molecular and Cellular Biology. Int. J. Mol. Sci. 2019, 21, E133. [Google Scholar] [CrossRef] [PubMed]
- Durante, M. New Challenges in High-Energy Particle Radiobiology. Br. J. Radiol. 2014, 87, 20130626. [Google Scholar] [CrossRef]
- Jiang, G.-L. Particle Therapy for Cancers: A New Weapon in Radiation Therapy. Front. Med. 2012, 6, 165–172. [Google Scholar] [CrossRef]
- Durante, M.; Debus, J. Heavy Charged Particles: Does Improved Precision and Higher Biological Effectiveness Translate to Better Outcome in Patients? Semin. Radiat. Oncol. 2018, 28, 160–167. [Google Scholar] [CrossRef]
- Bassler, N.; Jäkel, O.; Søndergaard, C.S.; Petersen, J.B. Dose- and LET-Painting with Particle Therapy. Acta Oncol. 2010, 49, 1170–1176. [Google Scholar] [CrossRef]
- Suzuki, M.; Kase, Y.; Yamaguchi, H.; Kanai, T.; Ando, K. Relative Biological Effectiveness for Cell-Killing Effect on Various Human Cell Lines Irradiated with Heavy-Ion Medical Accelerator in Chiba (HIMAC) Carbon-Ion Beams. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.A.; Nazaryan, V.; Davis, L.K.; Klein, S.B.; Nichiporov, D.; Mendonca, M.S.; Wolanski, M.; Nie, X.; George, J.; Keppel, C. Variations in the RBE for Cell Killing along the Depth-Dose Profile of a Modulated Proton Therapy Beam. Radiat. Res. 2013, 179, 21–28. [Google Scholar] [CrossRef]
- Kanemoto, A.; Hirayama, R.; Moritake, T.; Furusawa, Y.; Sun, L.; Sakae, T.; Kuno, A.; Terunuma, T.; Yasuoka, K.; Mori, Y.; et al. RBE and OER within the Spread-out Bragg Peak for Proton Beam Therapy: In Vitro Study at the Proton Medical Research Center at the University of Tsukuba. J. Radiat. Res. 2014, 55, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, O.; Sishc, B.J.; Saha, J.; Pompos, A.; Rahimi, A.; Story, M.D.; Davis, A.J.; Kim, D.W.N. Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair. Cancers 2017, 9, 66. [Google Scholar] [CrossRef]
- Chevalier, F.; Hamdi, D.H.; Lepleux, C.; Temelie, M.; Nicol, A.; Austry, J.B.; Lesueur, P.; Vares, G.; Savu, D.; Nakajima, T.; et al. High LET Radiation Overcomes In Vitro Resistance to X-rays of Chondrosarcoma Cell Lines. Technol. Cancer Res. Treat. 2019, 18, 153303381987130. [Google Scholar] [CrossRef]
- Césaire, M.; Ghosh, U.; Austry, J.-B.; Muller, E.; Cammarata, F.P.; Guillamin, M.; Caruso, M.; Castéra, L.; Petringa, G.; Cirrone, G.A.P.; et al. Sensitization of Chondrosarcoma Cells with PARP Inhibitor and High-LET Radiation. J. Bone Oncol. 2019, 17, 100246. [Google Scholar] [CrossRef]
- Lohberger, B.; Barna, S.; Glänzer, D.; Eck, N.; Kerschbaum-Gruber, S.; Stasny, K.; Leithner, A.; Georg, D. Cellular and Molecular Biological Alterations after Photon, Proton, and Carbon Ions Irradiation in Human Chondrosarcoma Cells Linked with High-Quality Physics Data. Int. J. Mol. Sci. 2022, 23, 11464. [Google Scholar] [CrossRef]
- Valable, S.; Gérault, A.N.; Lambert, G.; Leblond, M.M.; Anfray, C.; Toutain, J.; Bordji, K.; Petit, E.; Bernaudin, M.; Pérès, E.A. Impact of Hypoxia on Carbon Ion Therapy in Glioblastoma Cells: Modulation by LET and Hypoxia-Dependent Genes. Cancers 2020, 12, 2019. [Google Scholar] [CrossRef] [PubMed]
- Antonovic, L.; Brahme, A.; Furusawa, Y.; Toma-Dasu, I. Radiobiological Description of the LET Dependence of the Cell Survival of Oxic and Anoxic Cells Irradiated by Carbon Ions. J. Radiat. Res. 2013, 54, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone Sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Badiyan, S.; Malyapa, R.; Albertini, F.; Bolsi, A.; Lomax, A.J.; Schneider, R. Long-Term Outcomes and Prognostic Factors of Skull-Base Chondrosarcoma Patients Treated with Pencil-Beam Scanning Proton Therapy at the Paul Scherrer Institute. Neuro Oncol. 2016, 18, 236–243. [Google Scholar] [CrossRef]
- Weber, D.C.; Murray, F.; Combescure, C.; Calugaru, V.; Alapetite, C.; Albertini, F.; Bolle, S.; Goudjil, F.; Pica, A.; Walser, M.; et al. Long Term Outcome of Skull-Base Chondrosarcoma Patients Treated with High-Dose Proton Therapy with or without Conventional Radiation Therapy. Radiother. Oncol. 2018, 129, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, A.L.; Rotondo, R.L.; Rutenberg, M.S.; Indelicato, D.J.; Mercado, C.E.; Rao, D.; Tavanaiepour, D.; Morris, C.G.; Louis, D.; Flampouri, S.; et al. Proton Therapy for Skull-Base Chondrosarcoma, a Single-Institution Outcomes Study. J. Neurooncol. 2019, 142, 557–563. [Google Scholar] [CrossRef]
- Mattke, M.; Vogt, K.; Bougatf, N.; Welzel, T.; Oelmann-Avendano, J.; Hauswald, H.; Jensen, A.; Ellerbrock, M.; Jäkel, O.; Haberer, T.; et al. High Control Rates of Proton- and Carbon-Ion–Beam Treatment with Intensity-Modulated Active Raster Scanning in 101 Patients with Skull Base Chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer 2018, 124, 2036–2044. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Rotondo, R.L.; Begosh-Mayne, D.; Scarborough, M.T.; Gibbs, C.P.; Morris, C.G.; Mendenhall, W.M. A Prospective Outcomes Study of Proton Therapy for Chordomas and Chondrosarcomas of the Spine. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Mizumoto, M.; Onoe, T.; Nakamura, N.; Kikuchi, Y.; Shibata, T.; Okimoto, T.; Sakurai, H.; Akimoto, T.; Ono, K.; et al. Proton Beam Therapy for Bone Sarcomas of the Skull Base and Spine: A Retrospective Nationwide Multicenter Study in Japan. Cancer Sci. 2017, 108, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Balosso, J.; Febvey-Combes, O.; Iung, A.; Lozano, H.; Alloh, A.S.; Cornu, C.; Hervé, M.; Akkal, Z.; Lièvre, M.; Plattner, V.; et al. A Randomized Controlled Phase III Study Comparing Hadrontherapy with Carbon Ions versus Conventional Radiotherapy—Including Photon and Proton Therapy—For the Treatment of Radioresistant Tumors: The ETOILE Trial. BMC Cancer 2022, 22, 575. [Google Scholar] [CrossRef] [PubMed]
- Nikoghosyan, A.V.; Rauch, G.; Münter, M.W.; Jensen, A.D.; Combs, S.E.; Kieser, M.; Debus, J. Randomised Trial of Proton vs. Carbon Ion Radiation Therapy in Patients with Low and Intermediate Grade Chondrosarcoma of the Skull Base, Clinical Phase III Study. BMC Cancer 2010, 10, 606. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Nikoghosyan, A.; Thilmann, C.; Haberer, T.; Jäkel, O.; Karger, C.; Scholz, M.; Kraft, G.; Wannenmacher, M.; Debus, J. Carbon Ion Radiotherapy for Chordomas and Low-Grade Chondrosarcomas of the Skull Base. Results in 67 Patients. Strahlenther. Onkol. 2003, 179, 598–605. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Nikoghosyan, A.; Hof, H.; Didinger, B.; Combs, S.E.; Jäkel, O.; Karger, C.P.; Edler, L.; Debus, J. Carbon Ion Radiotherapy of Skull Base Chondrosarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 171–177. [Google Scholar] [CrossRef]
- Uhl, M.; Mattke, M.; Welzel, T.; Oelmann, J.; Habl, G.; Jensen, A.D.; Ellerbrock, M.; Haberer, T.; Herfarth, K.K.; Debus, J. High Control Rate in Patients with Chondrosarcoma of the Skull Base after Carbon Ion Therapy: First Report of Long-Term Results. Cancer 2014, 120, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Imai, R.; Kamada, T.; Araki, N.; WORKING GROUP FOR BONE and SOFT-TISSUE SARCOMAS. Clinical Efficacy of Carbon Ion Radiotherapy for Unresectable Chondrosarcomas. Anticancer Res. 2017, 37, 6959–6964. [Google Scholar] [CrossRef]
- Riva, G.; Cavallo, I.; Gandini, S.; Ingargiola, R.; Pecorilla, M.; Imparato, S.; Rossi, E.; Mirandola, A.; Ciocca, M.; Orlandi, E.; et al. Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy. Cancers 2021, 13, 4423. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Fiore, M.R.; Barcellini, A.; Iannalfi, A.; Vischioni, B.; Ronchi, S.; Bonora, M.; Riva, G.; Vai, A.; Facoetti, A.; et al. Outcome and Toxicity of Carbon Ion Radiotherapy for Axial Bone and Soft Tissue Sarcomas. Anticancer Res. 2020, 40, 2853–2859. [Google Scholar] [CrossRef]
- Demizu, Y.; Jin, D.; Sulaiman, N.S.; Nagano, F.; Terashima, K.; Tokumaru, S.; Akagi, T.; Fujii, O.; Daimon, T.; Sasaki, R.; et al. Particle Therapy Using Protons or Carbon Ions for Unresectable or Incompletely Resected Bone and Soft Tissue Sarcomas of the Pelvis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 367–374. [Google Scholar] [CrossRef]
- Tap, W.D.; Villalobos, V.M.; Cote, G.M.; Burris, H.; Janku, F.; Mir, O.; Beeram, M.; Wagner, A.J.; Jiang, L.; Wu, B.; et al. Phase I Study of the Mutant IDH1 Inhibitor Ivosidenib: Safety and Clinical Activity in Patients With Advanced Chondrosarcoma. J. Clin. Oncol. 2020, 38, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Paz, A.C.; Wilky, B.A.; Johnson, B.; Galoian, K.; Rosenberg, A.; Hu, G.; Tinoco, G.; Bodamer, O.; Trent, J.C. Treatment with a Small Molecule Mutant IDH1 Inhibitor Suppresses Tumorigenic Activity and Decreases Production of the Oncometabolite 2-Hydroxyglutarate in Human Chondrosarcoma Cells. PLoS ONE 2015, 10, e0133813. [Google Scholar] [CrossRef]
- Suijker, J.; Oosting, J.; Koornneef, A.; Struys, E.A.; Salomons, G.S.; Schaap, F.G.; Waaijer, C.J.F.; Wijers-Koster, P.M.; Briaire-de Bruijn, I.H.; Haazen, L.; et al. Inhibition of Mutant IDH1 Decreases D-2-HG Levels without Affecting Tumorigenic Properties of Chondrosarcoma Cell Lines. Oncotarget 2015, 6, 12505–12519. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, X.; Eid, J.E.; Rosenberg, A.E.; Wilky, B.A.; Ban, Y.; Sun, X.; Galoian, K.; DeSalvo, J.; Yue, J.; et al. Mutant IDH1 Depletion Downregulates Integrins and Impairs Chondrosarcoma Growth. Cancers 2020, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Nakatani, F.; Matsunaga, H.; Seki, T.; Endo, M.; Ogawara, Y.; Machida, Y.; Katsumoto, T.; Yamagata, K.; Hattori, A.; et al. Selective Inhibition of Mutant IDH1 by DS-1001b Ameliorates Aberrant Histone Modifications and Impairs Tumor Activity in Chondrosarcoma. Oncogene 2019, 38, 6835–6849. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, C.M.; O’Shaughnessy, J. Poly (ADP-Ribose) Polymerase as a Novel Therapeutic Target in Cancer. Clin. Cancer Res. 2010, 16, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A. A Synthetic Lethal Therapeutic Approach: Poly(ADP) Ribose Polymerase Inhibitors for the Treatment of Cancers Deficient in DNA Double-Strand Break Repair. JCO 2008, 26, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Dungey, F.A.; Löser, D.A.; Chalmers, A.J. Replication-Dependent Radiosensitization of Human Glioma Cells by Inhibition of Poly(ADP-Ribose) Polymerase: Mechanisms and Therapeutic Potential. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, P.L.; Corso, C.D.; Robinson, N.D.; Scanlon, S.E.; Purshouse, K.R.; Bai, H.; Liu, Y.; Sundaram, R.K.; Hegan, D.C.; Fons, N.R.; et al. 2-Hydroxyglutarate Produced by Neomorphic IDH Mutations Suppresses Homologous Recombination and Induces PARP Inhibitor Sensitivity. Sci. Transl. Med. 2017, 9, eaal2463. [Google Scholar] [CrossRef]
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K.; Koboldt, D.C.; Fulton, R.S.; Delehaunty, K.D.; McGrath, S.D.; et al. Recurring Mutations Found by Sequencing an Acute Myeloid Leukemia Genome. N. Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Venneker, S.; Kruisselbrink, A.B.; Briaire-de Bruijn, I.H.; de Jong, Y.; van Wijnen, A.J.; Danen, E.H.J.; Bovée, J.V.M.G. Inhibition of PARP Sensitizes Chondrosarcoma Cell Lines to Chemo- and Radiotherapy Irrespective of the IDH1 or IDH2 Mutation Status. Cancers 2019, 11, 1918. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Infante, J.R.; Lam, E.T.; Figlin, R.A.; Rini, B.I.; Brugarolas, J.; Zojwalla, N.J.; Lowe, A.M.; Wang, K.; Wallace, E.M.; et al. Phase I Dose-Escalation Trial of PT2385, a First-in-Class Hypoxia-Inducible Factor-2α Antagonist in Patients With Previously Treated Advanced Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2018, 36, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.; Hoyt, A.; Moran, A.; Becker, B.; Sedani, A.; Saigh, S.; Conway, S.; Brown, J.; Galoian, K. Cancer Stem Cells as a Therapeutic Target in 3D Tumor Models of Human Chondrosarcoma: An Encouraging Future for Proline Rich Polypeptide-1. Mol. Med. Rep. 2020, 22, 3747–3758. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Hoyt, A.; Sedani, A.; Granger, C.; Saigh, S.; Blonska, M.; Zhao-Ju, L.; Conway, S.A.; Pretell, J.; Brown, J.; et al. Proline-rich Polypeptide-1 Decreases Cancer Stem Cell Population by Targeting BAFF Chromatin-remodeling Complexes in Human Chondrosarcoma JJ012 Cells. Oncol. Rep. 2020, 44, 393–403. [Google Scholar] [CrossRef]
| Reference | Patients (n) | Indication | Dose | Efficacy | Tolerance |
|---|---|---|---|---|---|
| [115] | 23 with low-grade chondrosarcoma | R2 or Biopsied only patients | 60 GyRBE using a weekly fractionation of 7 × 3.0 GyRBE. | 100% local-control rates at 3 years | 9% of grade III late-effects |
| [116] | 54 with grade I–II skull base chondrosarcoma | R2 or Biopsied only patients (including recurrent tumors) | 60 GyRBE using a weekly fractionation of 7 × 3.0 GyRBE. | local-control rates were 96.2% at 3 years and 89.8% at 4 years | 2% of grade III late effects |
| [117] | 79 patients (64% grade I, 35% grade II, 1% grade III) with skull base chondrosarocma | Recurrences, R2, or Biopsied only patients | 60 GyRBE at 3 GyE per fraction | CI, 88.8–100%) and 89.8% at 4 years | No grade III effects reported |
| [118] | 73 patients (20% grade I, 70% grade II, 5% grade III, 5% dedifferenciated). Extracranial only. | Biopsied only patients (75%), Recurrence or metastatic (25%) | 70.4 GyRBE, 16 fractions, 4 consecutive days a week, 4 weeks | 5-year local-control, overall survival, and disease-free survival rates were 53%, 53%, and 34% | 11% of grade III late effects |
| [119] | 16 patients with skull base chondrosarcoma (75% of grade II–III) | R2 or Biopsied only patients | 70.4 GyRBE, 16 fractions, 4 consecutive days a week, 4 weeks | 3-year LC rate of 94% | 12.5% of grade III late effects |
| [120] | 21 patients with chondrosarcoma. Extracranial only | Not available | 73.6 Gy(RBE) delivered in 16 fractions (4 fractions per week) | not available | <5% of grade III late effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert, A.; Tudor, M.; Montanari, J.; Commenchail, K.; Savu, D.I.; Lesueur, P.; Chevalier, F. Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions. Cancers 2023, 15, 1962. https://doi.org/10.3390/cancers15071962
Gilbert A, Tudor M, Montanari J, Commenchail K, Savu DI, Lesueur P, Chevalier F. Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions. Cancers. 2023; 15(7):1962. https://doi.org/10.3390/cancers15071962
Chicago/Turabian StyleGilbert, Antoine, Mihaela Tudor, Juliette Montanari, Kevin Commenchail, Diana Iulia Savu, Paul Lesueur, and François Chevalier. 2023. "Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions" Cancers 15, no. 7: 1962. https://doi.org/10.3390/cancers15071962
APA StyleGilbert, A., Tudor, M., Montanari, J., Commenchail, K., Savu, D. I., Lesueur, P., & Chevalier, F. (2023). Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions. Cancers, 15(7), 1962. https://doi.org/10.3390/cancers15071962








