Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Data
2.2. Radiopharmaceuticals and PET/CT Acquisition
2.3. Treatments and Follow-Up
2.4. Statistical Analyzes
- Pearson’s chi-square test (or Fisher’s test if necessary) for qualitative variables.
- Student’s t test (or Mann-Whitney test if necessary) for continuous variables.
- The Log-Rank test for survival data (BR-FS, ADT-FS); Kaplan-Meier curves were plotted.
3. Results
3.1. Population, Lesions, and Treatment Characteristics
3.2. Study of the Different Survivals after Treatment
3.3. Study of the PSA Response at 3–6 Months Post-RT
3.4. Analysis of Predictive Factors Related to BR-FS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | androgen deprivation treatment |
| ADT-FS | androgen deprivation treatment-free survival |
| BR | biological recurrence |
| BR-FS | biological recurrence-free survival |
| EAU | European Association of Urology |
| FCH | fluorocholine |
| FCH-PET | [18F]F-choline PET/CT |
| MDT | metastasis-directed therapies |
| PC | prostate cancer |
| PSA | prostate-specific antigen |
| PSA DT | PSA doubling time |
| PSMA | prostate-specific membrane antigen |
| PSMA-PET | [68Ga]Ga-PSMA PET/CT |
| RP | radical prostatectomy |
| RT | external radiotherapy |
| SBRT | stereotactic body radiotherapy |
References
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Rozet, F.; Mongiat-Artus, P.; Hennequin, C.; Beauval, J.B.; Beuzeboc, P.; Cormier, L.; Fromont-Hankard, G.; Mathieu, R.; Ploussard, G.; Renard-Penna, R.; et al. Recommandations françaises du Comité de cancérologie de l’AFU—Actualisation 2020–2022: Cancer de la prostate. Prog. Urol. 2020, 30, S136–S251. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; Preisser, F.; Graefen, M.; Huland, H.; Pompe, R.S. External Validation of the European Association of Urology Biochemical Recurrence Risk Groups to Predict Metastasis and Mortality After Radical Prostatectomy in a European Cohort. Eur. Urol. 2019, 75, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Partin, A.W.; Pearson, J.D.; Landis, P.K.; Carter, H.B.; Pound, C.R.; Clemens, J.Q.; Epstein, J.I.; Walsh, P.C. Evaluation of Serum Prostate-Specific Antigen Velocity after Radical Prostatectomy to Distinguish Local Recurrence from Distant Metastases. Urology 1994, 43, 649–659. [Google Scholar] [CrossRef]
- Hövels, A.M.; Heesakkers, R.A.M.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The Diagnostic Accuracy of CT and MRI in the Staging of Pelvic Lymph Nodes in Patients with Prostate Cancer: A Meta-Analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Suh, C.H.; Shinagare, A.B.; Westenfield, A.M.; Ramaiya, N.H.; Van den Abbeele, A.D.; Kim, K.W. Yield of Bone Scintigraphy for the Detection of Metastatic Disease in Treatment-Naive Prostate Cancer: A Systematic Review and Meta-Analysis. Clin. Radiol. 2018, 73, 158–167. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Huang, M.; Tian, R. The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer With Biochemical Recurrence: A Meta-Analysis. Front. Oncol. 2021, 11, 684629. [Google Scholar] [CrossRef]
- Treglia, G.; Ceriani, L.; Sadeghi, R.; Giovacchini, G.; Giovanella, L. Relationship between Prostate-Specific Antigen Kinetics and Detection Rate of Radiolabelled Choline PET/CT in Restaging Prostate Cancer Patients: A Meta-Analysis. Clin. Chem. Lab. Med. 2014, 52, 725–733. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Guttilla, A.; Saladini, G.; Zattoni, F.; Colletti, P.M.; Rubello, D. Choline PET or PET/CT and Biochemical Relapse of Prostate Cancer: A Systematic Review and Meta-Analysis. Clin. Nucl. Med. 2013, 38, 305–314. [Google Scholar] [CrossRef]
- Castellucci, P.; Ceci, F.; Graziani, T.; Schiavina, R.; Brunocilla, E.; Mazzarotto, R.; Pettinato, C.; Celli, M.; Lodi, F.; Fanti, S. Early Biochemical Relapse after Radical Prostatectomy: Which Prostate Cancer Patients May Benefit from a Restaging 11C-Choline PET/CT Scan before Salvage Radiation Therapy? J. Nucl. Med. 2014, 55, 1424–1429. [Google Scholar] [CrossRef]
- Castellucci, P.; Fuccio, C.; Nanni, C.; Santi, I.; Rizzello, A.; Lodi, F.; Franceschelli, A.; Martorana, G.; Manferrari, F.; Fanti, S. Influence of Trigger PSA and PSA Kinetics on 11C-Choline PET/CT Detection Rate in Patients with Biochemical Relapse after Radical Prostatectomy. J. Nucl. Med. 2009, 50, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET Imaging with a (68)Ga-Labelled PSMA Ligand and (18)F-Choline-Based PET/CT for the Diagnosis of Recurrent Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Schwenck, J.; Rempp, H.; Reischl, G.; Kruck, S.; Stenzl, A.; Nikolaou, K.; Pfannenberg, C.; la Fougère, C. Comparison of 68Ga-Labelled PSMA-11 and 11C-Choline in the Detection of Prostate Cancer Metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 92–101. [Google Scholar] [CrossRef]
- Caroli, P.; Sandler, I.; Matteucci, F.; De Giorgi, U.; Uccelli, L.; Celli, M.; Foca, F.; Barone, D.; Romeo, A.; Sarnelli, A.; et al. 68Ga-PSMA PET/CT in Patients with Recurrent Prostate Cancer after Radical Treatment: Prospective Results in 314 Patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2035–2044. [Google Scholar] [CrossRef]
- Alberts, I.L.; Seide, S.E.; Mingels, C.; Bohn, K.P.; Shi, K.; Zacho, H.D.; Rominger, A.; Afshar-Oromieh, A. Comparing the Diagnostic Performance of Radiotracers in Recurrent Prostate Cancer: A Systematic Review and Network Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2978–2989. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-Specific Membrane Antigen-Avid Lesions: A Systematic Review and Meta-Analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Calais, J.; Fendler, W.P.; Eiber, M.; Gartmann, J.; Chu, F.-I.; Nickols, N.G.; Reiter, R.E.; Rettig, M.B.; Marks, L.S.; Ahlering, T.E.; et al. Impact of 68Ga-PSMA-11 PET/CT on the Management of Prostate Cancer Patients with Biochemical Recurrence. J. Nucl. Med. 2018, 59, 434–441. [Google Scholar] [CrossRef]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The Impact of 68Ga-PSMA PET/CT on Management Intent in Prostate Cancer: Results of an Australian Prospective Multicenter Study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of 68Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef]
- Fendler, W.P.; Ferdinandus, J.; Czernin, J.; Eiber, M.; Flavell, R.R.; Behr, S.C.; Wu, I.-W.K.; Lawhn-Heath, C.; Pampaloni, M.H.; Reiter, R.E.; et al. Impact of 68Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J. Nucl. Med. 2020, 61, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Salaün, P.-Y.; Abgral, R.; Malard, O.; Querellou-Lefranc, S.; Quere, G.; Wartski, M.; Coriat, R.; Hindie, E.; Taieb, D.; Tabarin, A.; et al. Good Clinical Practice Recommendations for the Use of PET/CT in Oncology. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Supiot, S.; Rousseau, C. Oligometastatic Prostate Cancer: Is It Worth Targeting the Tip of the Iceberg? Transl. Cancer Res. 2019, 8, S171–S175. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Supiot, S.; Vaugier, L.; Pasquier, D.; Buthaud, X.; Magné, N.; Peiffert, D.; Sargos, P.; Crehange, G.; Pommier, P.; Loos, G.; et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-Dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur. Urol. 2021, 80, 405–414. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef]
- Siva, S.; Bressel, M.; Murphy, D.G.; Shaw, M.; Chander, S.; Violet, J.; Tai, K.H.; Udovicich, C.; Lim, A.; Selbie, L.; et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur. Urol. 2018, 74, 455–462. [Google Scholar] [CrossRef]
- Mazzola, R.; Francolini, G.; Triggiani, L.; Napoli, G.; Cuccia, F.; Nicosia, L.; Livi, L.; Magrini, S.M.; Salgarello, M.; Alongi, F. Metastasis-Directed Therapy (SBRT) Guided by PET-CT 18F-CHOLINE Versus PET-CT 68Ga-PSMA in Castration-Sensitive Oligorecurrent Prostate Cancer: A Comparative Analysis of Effectiveness. Clin. Genitourin. Cancer 2021, 19, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Deijen, C.L.; Vrijenhoek, G.L.; Schaake, E.E.; Vogel, W.V.; Moonen, L.M.F.; Pos, F.J.; van der Poel, H.G.; Borst, G.R. PSMA-11-PET/CT versus Choline-PET/CT to Guide Stereotactic Ablative Radiotherapy for Androgen Deprivation Therapy Deferral in Patients with Oligometastatic Prostate Cancer. Clin. Transl. Radiat. Oncol. 2021, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schmidt Hegemann, N.-S.; Rogowski, P.; Eze, C.; Schäfer, C.; Stief, C.; Lang, S.; Spohn, S.; Steffens, R.; Li, M.; Gratzke, C.; et al. Outcome After 68Ga-PSMA-11 versus Choline PET-Based Salvage Radiotherapy in Patients with Biochemical Recurrence of Prostate Cancer: A Matched-Pair Analysis. Cancers 2020, 12, 3395. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.A.; Beltramo, G.; Fariselli, L.; Fodor, C.; Santoro, L.; Vavassori, A.; Zerini, D.; Gherardi, F.; Ascione, C.; Bossi-Zanetti, I.; et al. Robotic Image-Guided Stereotactic Radiotherapy, for Isolated Recurrent Primary, Lymph Node or Metastatic Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 889–897. [Google Scholar] [CrossRef]
- Incerti, E.; Fodor, A.; Mapelli, P.; Fiorino, C.; Alongi, P.; Kirienko, M.; Giovacchini, G.; Busnardo, E.; Gianolli, L.; Di Muzio, N.; et al. Radiation Treatment of Lymph Node Recurrence from Prostate Cancer: Is 11C-Choline PET/CT Predictive of Survival Outcomes? J. Nucl. Med. 2015, 56, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Picchio, M.; Berardi, G.; Fodor, A.; Busnardo, E.; Crivellaro, C.; Giovacchini, G.; Fiorino, C.; Kirienko, M.; Incerti, E.; Messa, C.; et al. (11)C-Choline PET/CT as a Guide to Radiation Treatment Planning of Lymph-Node Relapses in Prostate Cancer Patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schäfer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM Standardized Reporting Guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef]
- Lawton, C.A.F.; Michalski, J.; El-Naqa, I.; Buyyounouski, M.K.; Lee, W.R.; Menard, C.; O’Meara, E.; Rosenthal, S.A.; Ritter, M.; Seider, M. RTOG GU Radiation Oncology Specialists Reach Consensus on Pelvic Lymph Node Volumes for High-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 383–387. [Google Scholar] [CrossRef]
- Sargos, P.; Guerif, S.; Latorzeff, I.; Hennequin, C.; Pommier, P.; Lagrange, J.-L.; Créhange, G.; Chapet, O.; de Crevoisier, R.; Azria, D.; et al. Definition of Lymph Node Areas for Radiotherapy of Prostate Cancer: A Critical Literature Review by the French Genito-Urinary Group and the French Association of Urology (GETUG-AFU). Cancer Treat. Rev. 2015, 41, 814–820. [Google Scholar] [CrossRef]
- Hall, W.A.; Paulson, E.; Davis, B.J.; Spratt, D.E.; Morgan, T.M.; Dearnaley, D.; Tree, A.C.; Efstathiou, J.A.; Harisinghani, M.; Jani, A.B.; et al. NRG Oncology Updated International Consensus Atlas on Pelvic Lymph Node Volumes for Intact and Postoperative Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Latorzeff, I.; Ploussard, G.; Guillotreau, J.; Jonca, F.; Labarthe, P.; Rollin, G.; Beauval, J.-B.; Pathak, A. Cardiovascular risks with prostate cancer hormonal treatment: Rationale for a department of oncocardiology. Cancer Radiother 2016, 20, 405–410. [Google Scholar] [CrossRef]
- Bastide, C.; Bruyère, F.; Karsenty, G.; Guy, L.; Rozet, F. [Hormonal treatment in prostate cancer]. Prog. Urol. 2013, 23, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Brewster, S.F. PSA Velocity and Doubling Time in Diagnosis and Prognosis of Prostate Cancer. Br. J. Med. Surg. Urol. 2012, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- De Bleser, E.; Jereczek-Fossa, B.A.; Pasquier, D.; Zilli, T.; Van As, N.; Siva, S.; Fodor, A.; Dirix, P.; Gomez-Iturriaga, A.; Trippa, F.; et al. Metastasis-Directed Therapy in Treating Nodal Oligorecurrent Prostate Cancer: A Multi-Institutional Analysis Comparing the Outcome and Toxicity of Stereotactic Body Radiotherapy and Elective Nodal Radiotherapy. Eur. Urol. 2019, 76, 732–739. [Google Scholar] [CrossRef] [PubMed]
- 46. Koerber, S.A.; Sprute, K.; Kratochwil, C.; Winter, E.; Haefner, M.F.; Katayama, S.; Schlampp, I.; Herfarth, K.; Kopka, K.; Afshar-Oromieh, A.; et al. Clinical Outcome of PSMA-Guided Radiotherapy for Patients with Oligorecurrent Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 143–151. [Google Scholar] [CrossRef]
- Oehus, A.-K.; Kroeze, S.G.C.; Schmidt-Hegemann, N.-S.; Vogel, M.M.E.; Kirste, S.; Becker, J.; Burger, I.A.; Derlin, T.; Bartenstein, P.; Eiber, M.; et al. Efficacy of PSMA Ligand PET-Based Radiotherapy for Recurrent Prostate Cancer after Radical Prostatectomy and Salvage Radiotherapy. BMC Cancer 2020, 20, 362. [Google Scholar] [CrossRef]
- Shipley, W.U.; Seiferheld, W.; Lukka, H.R.; Major, P.P.; Heney, N.M.; Grignon, D.J.; Sartor, O.; Patel, M.P.; Bahary, J.-P.; Zietman, A.L.; et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N. Engl. J. Med. 2017, 376, 417–428. [Google Scholar] [CrossRef]
- Carrie, C.; Hasbini, A.; de Laroche, G.; Richaud, P.; Guerif, S.; Latorzeff, I.; Supiot, S.; Bosset, M.; Lagrange, J.-L.; Beckendorf, V.; et al. Salvage Radiotherapy with or without Short-Term Hormone Therapy for Rising Prostate-Specific Antigen Concentration after Radical Prostatectomy (GETUG-AFU 16): A Randomised, Multicentre, Open-Label Phase 3 Trial. Lancet Oncol. 2016, 17, 747–756. [Google Scholar] [CrossRef]
- Carrie, C.; Magné, N.; Burban-Provost, P.; Sargos, P.; Latorzeff, I.; Lagrange, J.-L.; Supiot, S.; Belkacemi, Y.; Peiffert, D.; Allouache, N.; et al. Short-Term Androgen Deprivation Therapy Combined with Radiotherapy as Salvage Treatment after Radical Prostatectomy for Prostate Cancer (GETUG-AFU 16): A 112-Month Follow-up of a Phase 3, Randomised Trial. Lancet Oncol. 2019, 20, 1740–1749. [Google Scholar] [CrossRef]
- Jegadeesh, N.; Liu, Y.; Zhang, C.; Zhong, J.; Cassidy, R.J.; Gillespie, T.; Kucuk, O.; Rossi, P.; Master, V.A.; Alemozaffar, M.; et al. The Role of Adjuvant Radiotherapy in Pathologically Lymph Node-Positive Prostate Cancer. Cancer 2017, 123, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, S.G.C.; Henkenberens, C.; Schmidt-Hegemann, N.S.; Vogel, M.M.E.; Kirste, S.; Becker, J.; Burger, I.A.; Derlin, T.; Bartenstein, P.; Eiber, M.; et al. Prostate-Specific Membrane Antigen Positron Emission Tomography-Detected Oligorecurrent Prostate Cancer Treated with Metastases-Directed Radiotherapy: Role of Addition and Duration of Androgen Deprivation. Eur. Urol. Focus 2021, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
| TEP-FCH (N = 53) | TEP-PSMA (N = 70) | p-Value | |
|---|---|---|---|
| Age at RP (years) Mean (SD) Min; Max | 62.6 (6.8) 49; 78 | 63.2 (6.2) 48; 73 | 0642 |
| PSA at diagnosis (ng/mL) Mean (SD) Min; Max Missing data | 12.37 (24.57) 3.8; 181.0 0 | 9.60 (6.79) 3.4; 44.0 4 | 0.381 |
| Initial pT status (%) 2 3 4 Missing data | 19 (35.8) 32 (60.4) 2 (3.8) 0 | 28 (40.6) 41 (59.4) 0 (0.0) 1 | 0.307 |
| Initial pN status (%) 0 1 x | 38 (71.7) 5 (9.4) 10 (18.9) | 44 (62.9) 7 (10.0) 19 (27.1) | 0.583 |
| ISUP Score (%) 1 2–3 4–5 | 4 (7.5) 33 (62.3) 16 (30.2) | 1 (1.4) 56 (80.0) 13 (18.6) | 0.054 |
| R Status (%) R0 R1 Missing data | 26 (51.0) 25 (49.0) 2 | 39 (55.7) 31 (44.3) 0 | 0.712 |
| Additional treatments before imaging (%) RT prostate bed RT prostate bed + ADT RT prostate bed + elective pelvic nodes RT prostate bed + elective pelvic nodes + ADT ADT alone None | 14 (26.4) 12 (22.6) 5 (9.4) 9 (17.0) 1 (1.9) 12 (22.7) | 23 (32.9) 12 (17.1) 3 (4.3) 0 (0.0) 1 (1.4) 31 (44.3) | 0.552 0.495 0.289 <0.010 0.999 0.014 |
| The rank of recurrence on imaging (%) 1st 2nd–3rd–4th | 17 (32.1) 36 (67.9) | 35 (50.0) 35 (50.0) | 0.065 |
| PSA before imaging (ng/mL) Mean (SD) Min; Max | 1.23 (0.55) 0.12; 2.0 | 0.64 (0.46) 0.15; 1.9 | <0.001 |
| PSA DT before imaging (months) Mean (SD) Min; Max Missing data | 8.04 (6.97) 0.9; 35.0 2 | 9.44 (6.69) 1.8; 28.8 0 | 0.268 |
| EAU Risk Group at BR 1 (%) EAU low-risk EAU high-risk Missing data | 11 (21.6) 40 (78.4) 2 | 19 (27.1) 51 (72.9) 0 | 0.529 |
| TEP-FCH (N = 53) | TEP-PSMA (N = 70) | p-Value | |
|---|---|---|---|
| Number of lesions per patient (%) 1 2 3 4 5 | 25 (47.2) 19 (35.8) 7 (13.2) 2 (3.8) 0 (0.0) | 42 (60.0) 19 (27.1) 5 (7.2) 3 (4.3) 1 (1.4) | 0.199 |
| Prostate bed relapse (%) no yes | 39 (73.6) 14 (26.4) | 53 (75.7) 17 (24.3) | 0.836 |
| Pelvic lymph node lesion (%) 0 1 >1 | 22 (41.5) 19 (35.8) 12 (22.7) | 28 (40.0) 22 (31.4) 20 (28.6) | 0.737 |
| Extrapelvic lymph node lesion (%) 0 ≥1 | 44 (83.0) 9 (17.0) | 63 (90.0) 7 (10.0) | 0.288 |
| Bone lesion (%) 0 ≥1 | 38 (71.7) 15 (28.3) | 60 (85.7) 10 (14.3) | 0.071 |
| Visceral lesion (%) 0 ≥1 | 52 (98.1) 1 1 (1.9) | 70 (100.0) 0 (0.0) | 0.431 |
| TEP-FCH (N = 53) | TEP-PSMA (N = 70) | p-Value | |
|---|---|---|---|
| Radiotherapy (%) Prostate bed only (+/− boost) Elective pelvic nodes + boost Elective para-aortic nodes + boost Prostate bed SBRT Nodes SBRT Bone SBRT Nodes and bone SBRT Visceral SBRT | 3 (5.7) 9 (17.0) 1 (1.9) 4 (7.5) 21 (39.6) 8 (15.1) 6 (11.3) 1 (1.9) | 11 (15.7) 30 (42.9) 1 (1.4) 1 (1.4) 17 (24.3) 9 (12.9) 1 (1.4) 0 (0.0) | 0.082 0.002 0.999 0.164 0.068 0.722 0.042 0.431 |
| ADT concomitant intermittent (%) No Yes <6 months >6 months | 30 (56.6) 18 (34.0) 10 8 | 55 (78.5) 13 (18.6) 12 1 | 0.028 |
| Other systemic treatment concomitant 1 (%) | 5 (9.4) | 2 (2.9) | 0.139 |
| Response Criteria 1 | TEP-FCH (N = 37) 2 | TEP-PSMA (N = 57) 2 | p-Value |
|---|---|---|---|
| Complete response (%) (PSA decreased by at least 50%) | 17 (46.0) | 27 (47.4) | 0.607 |
| Partial response (%) (PSA decreased from 10% to 50%) | 5 (13.5) | 13 (22.8) | |
| Stability (%) (PSA moved by ±10%) | 5 (13.5) | 5 (8.8) | |
| Progression (%) (PSA increased by more than 10%) | 10 (27.0) | 12 (21.0) |
| RT Type (%) | PSA Response 1 (N = 62) | No PSA Response (N = 32) | p-Value |
|---|---|---|---|
| Prostate bed (n = 13) | 12 (92.3) | 1 (7.7) | 0.054 |
| Elective pelvic and extrapelvic nodes (n = 28) | 26 (92.9) | 2 (7.1) | <0.001 |
| SBRT pelvic nodes (n = 23) | 11 (47.8) | 12 (52.2) | 0.044 |
| SBRT extrapelvic nodes (n = 10) | 5 (50.0) | 5 (50.0) | 0.300 |
| Bone SBRT (n = 13) | 5 (38.5) | 8 (61.5) | 0.031 |
| Bone and nodes SBRT (n = 6) | 2 (33.3) | 4 (66.7) | 0.175 |
| Visceral SBRT (n = 1) | 1 (100.0) | 0 (0.0) | 0.999 |
| HR (95% CI) | Univariate p-Value | HR (95% CI) | Multivariate p-Value | ||
|---|---|---|---|---|---|
| Age at diagnosis | 1.01 (0.98–1.04) | 0.509 | - | - | |
| PSA at diagnosis | 1.01 (1.00–1.02) | 0.122 | - | - | |
| pT 3–4 vs. 2 | 1.23 (0.78–1.92) | 0.369 | - | - | |
| pN 1 vs. 0 | 1.21 (0.60–2.45) | 0.600 | - | - | |
| ISUP 2–3 vs. 1 4–5 vs. 1 | 1.05 (0.33–3.37) 1.50 (0.45–5.03) | 0.933 0.511 | - - | - - | |
| R Status R1 vs. R0 | 0.95 (0.62–1.46) | 0.815 | - | - | |
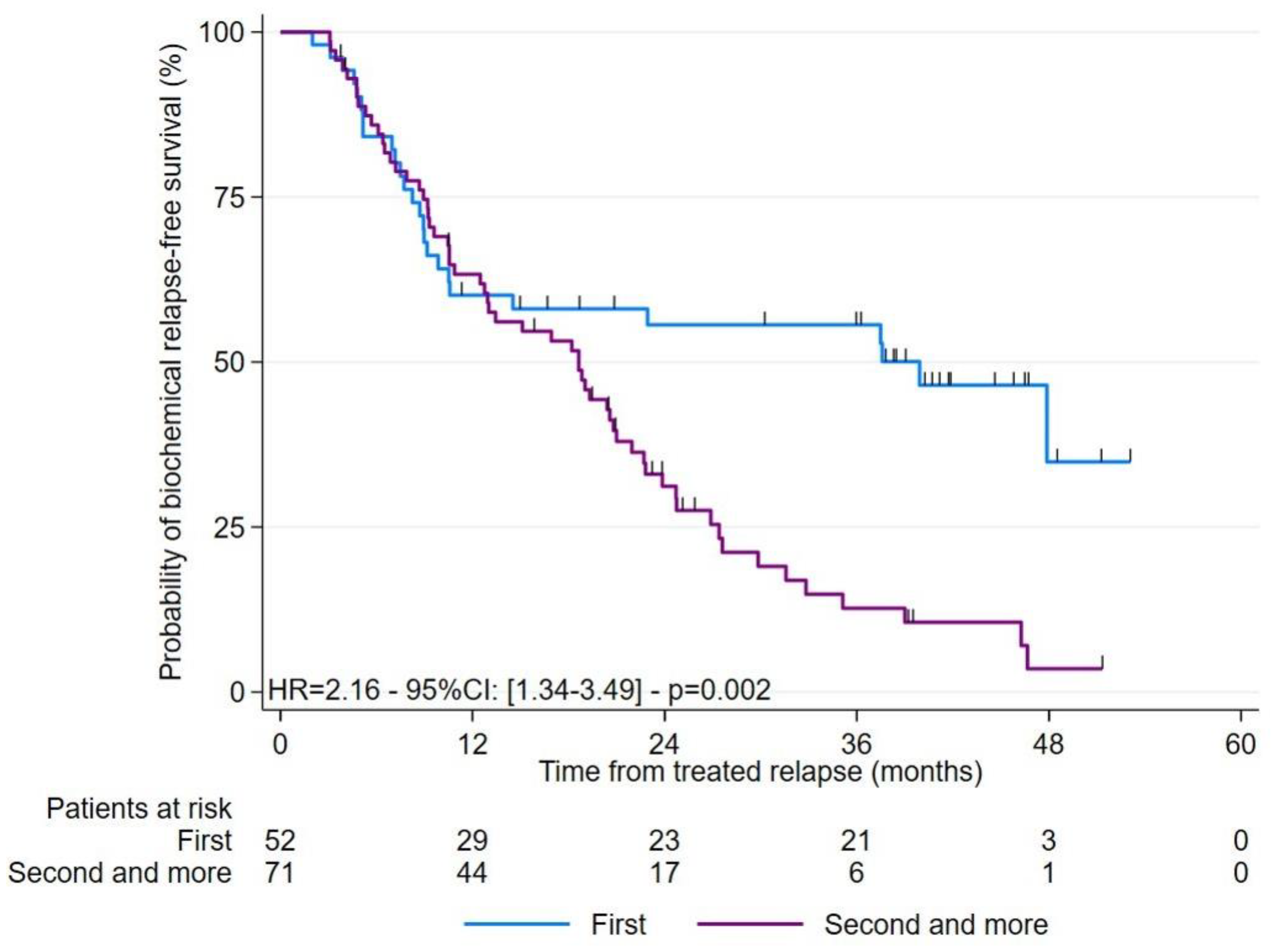
| The rank of recurrence on imaging 2–3-4 vs. 1 | 2.16 (1.34–3.49) | 0.002 | 0.73 (0.41–1.31) | 0.290 | |
| RT before imaging Yes vs. No | 2.67 (1.61–4.45) | <0.001 | 1.90 (0.82–4.39) | 0.135 | |
| RT type before imaging RT prostate bed vs. None RT prostate bed + pelvic nodes vs. None | 2.36 (1.39–3.99) 5.69 (2.82–11.48) | 0.001 <0.001 | - - | - - | |
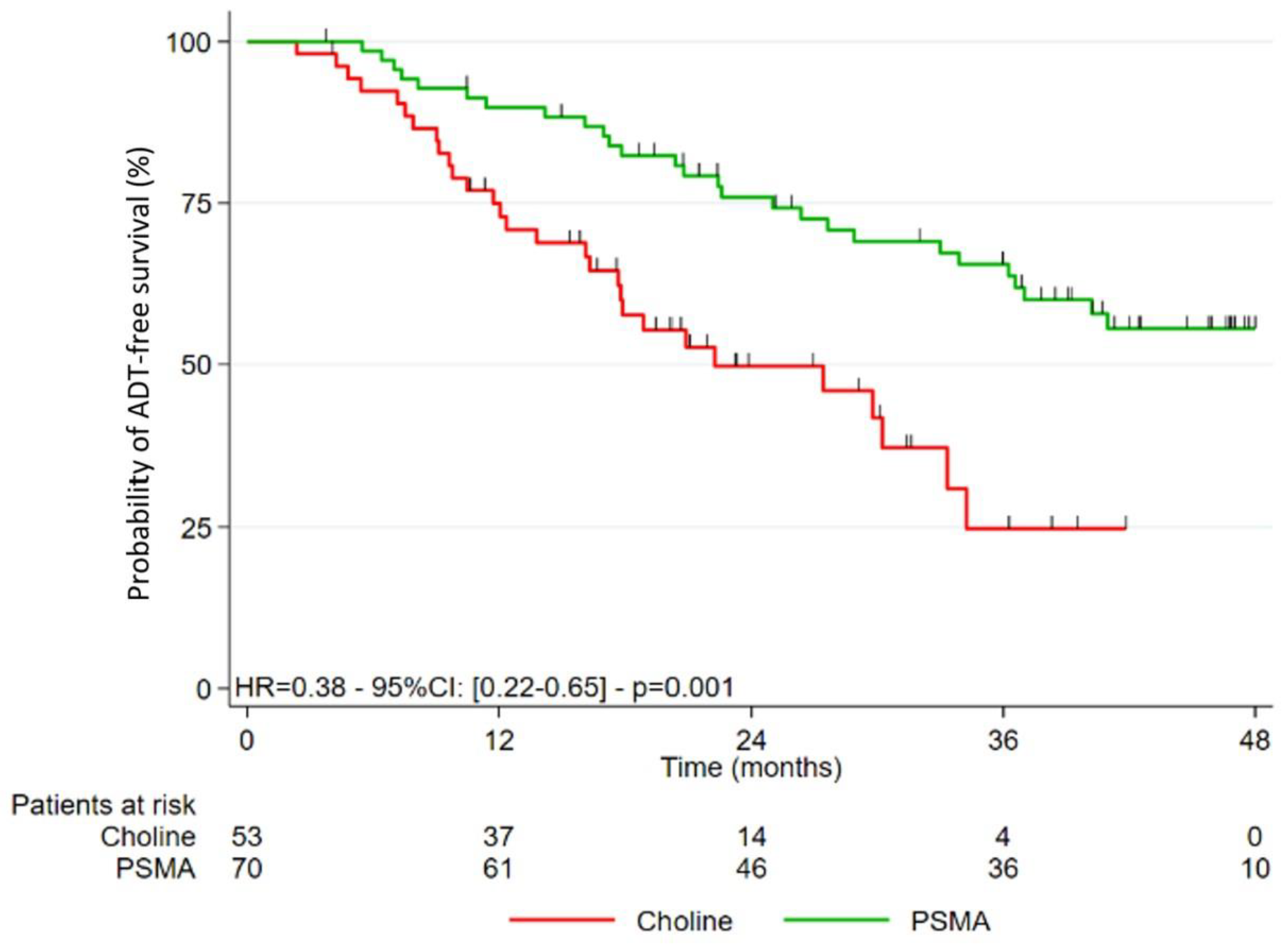
| ADT before imaging Yes vs. No | 2.19 (1.40–3.45) | 0.001 | 1.55 (0.92–2.60) | 0.097 | |
| PSA before imaging | 1.70 (1.18–2.44) | 0.004 | 1.42 (0.85–2.37) | 0.178 | |
| PSA DT before imaging | 0.96 (0.92–0.99) | 0.018 | 0.94 (0.91–0.98) | 0.005 | |
| EAU Risk Group at BR High-risk vs. Low risk | 1.90 (1.10–3.28) | 0.022 | - | - | |
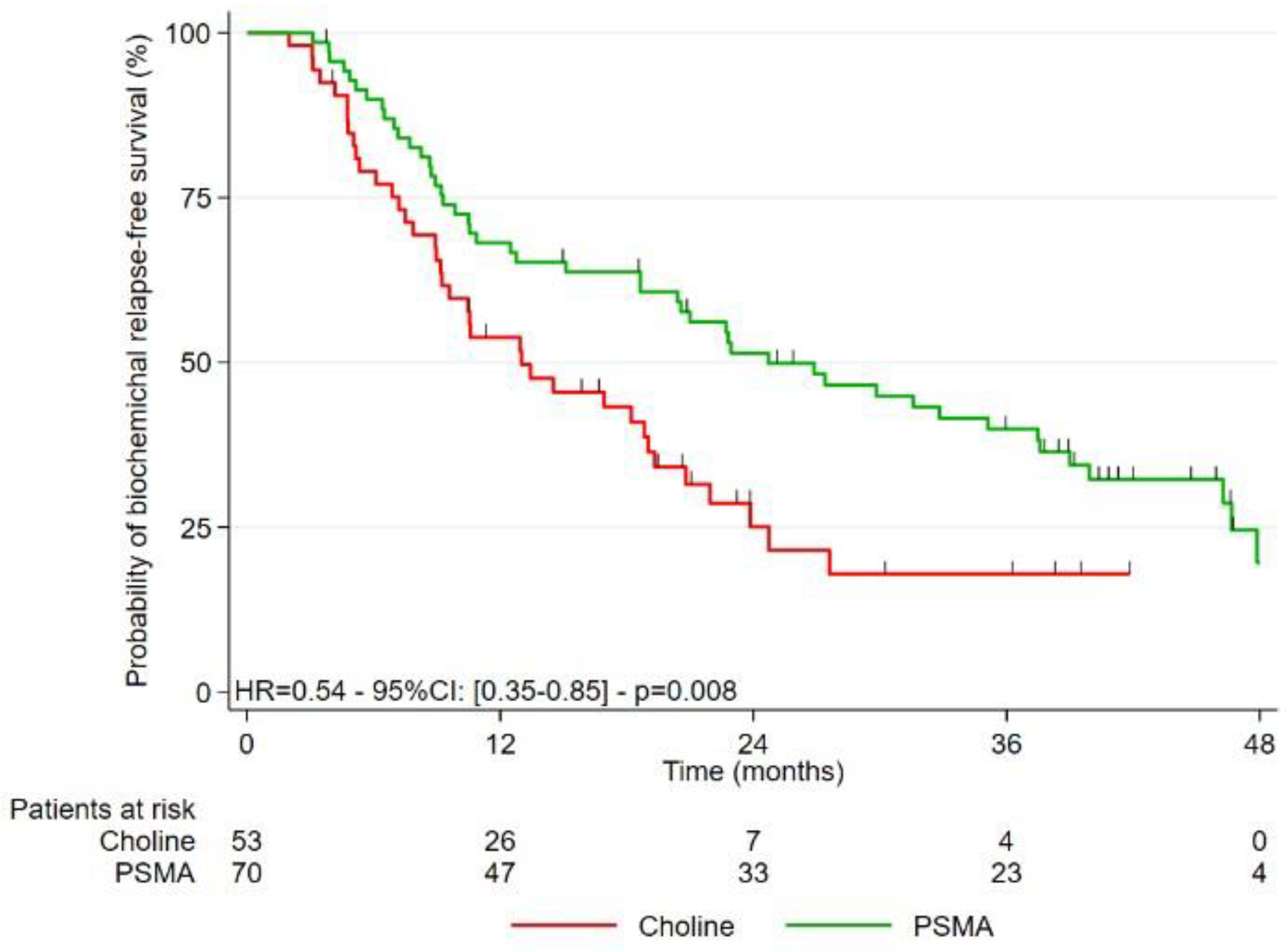
| Group PET-PSMA vs. PET-FCH | 0.54 (0.35–0.85) | 0.008 | 0.97 (0.56–1.70) | 0.924 | |
| Lesion number on PET/CT 2 or + vs. 1 | 1.22 (0.80–1.87) | 0.358 | - | - | |
| Lesion localization Pelvic node vs. Prostate bed Extrapelvic node vs. prostate bed Bone/visceral vs. prostate bed | 2.68 (1.20–5.97) 6.32 (2.51–15.95) 5.29 (2.25–12.45) | 0.016 <0.001 <0.001 | 1.75 (0.73–4.19) 2.04 (0.70–5.97) 1.25 (0.43–3.68) | 0.206 0.194 0.681 | |
| RT Type Elective pelvic node vs. prostate bed Elective para-aortic node vs. prostate bed Node SBRT vs. prostate bed Node and bone SBRT vs. prostate bed Bone SBRT vs. prostate bed | 1.37 (0.58–3.23) 4.10 (0.84–19.96) 5.14 (2.26–11.73) 4.65 (1.61–13.40) 4.09 (1.94–11.88) | 0.478 0.080 <0.001 0.004 0.001 | - - - - - | - - - - - | |
| Summary of RT type Bed prostate and/or elective pelvic node vs. SBRT | 0.32 (0.17–0.60) | <0.001 | 0.33 (0.18–0.61) | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metz, R.; Rauscher, A.; Vaugier, L.; Supiot, S.; Drouet, F.; Campion, L.; Rousseau, C. Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy. Cancers 2023, 15, 1898. https://doi.org/10.3390/cancers15061898
Metz R, Rauscher A, Vaugier L, Supiot S, Drouet F, Campion L, Rousseau C. Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy. Cancers. 2023; 15(6):1898. https://doi.org/10.3390/cancers15061898
Chicago/Turabian StyleMetz, Raphaël, Aurore Rauscher, Loïg Vaugier, Stéphane Supiot, Franck Drouet, Loic Campion, and Caroline Rousseau. 2023. "Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy" Cancers 15, no. 6: 1898. https://doi.org/10.3390/cancers15061898
APA StyleMetz, R., Rauscher, A., Vaugier, L., Supiot, S., Drouet, F., Campion, L., & Rousseau, C. (2023). Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy. Cancers, 15(6), 1898. https://doi.org/10.3390/cancers15061898








