A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Extraction and Clinical Endpoints
2.3. Statistics
3. Results
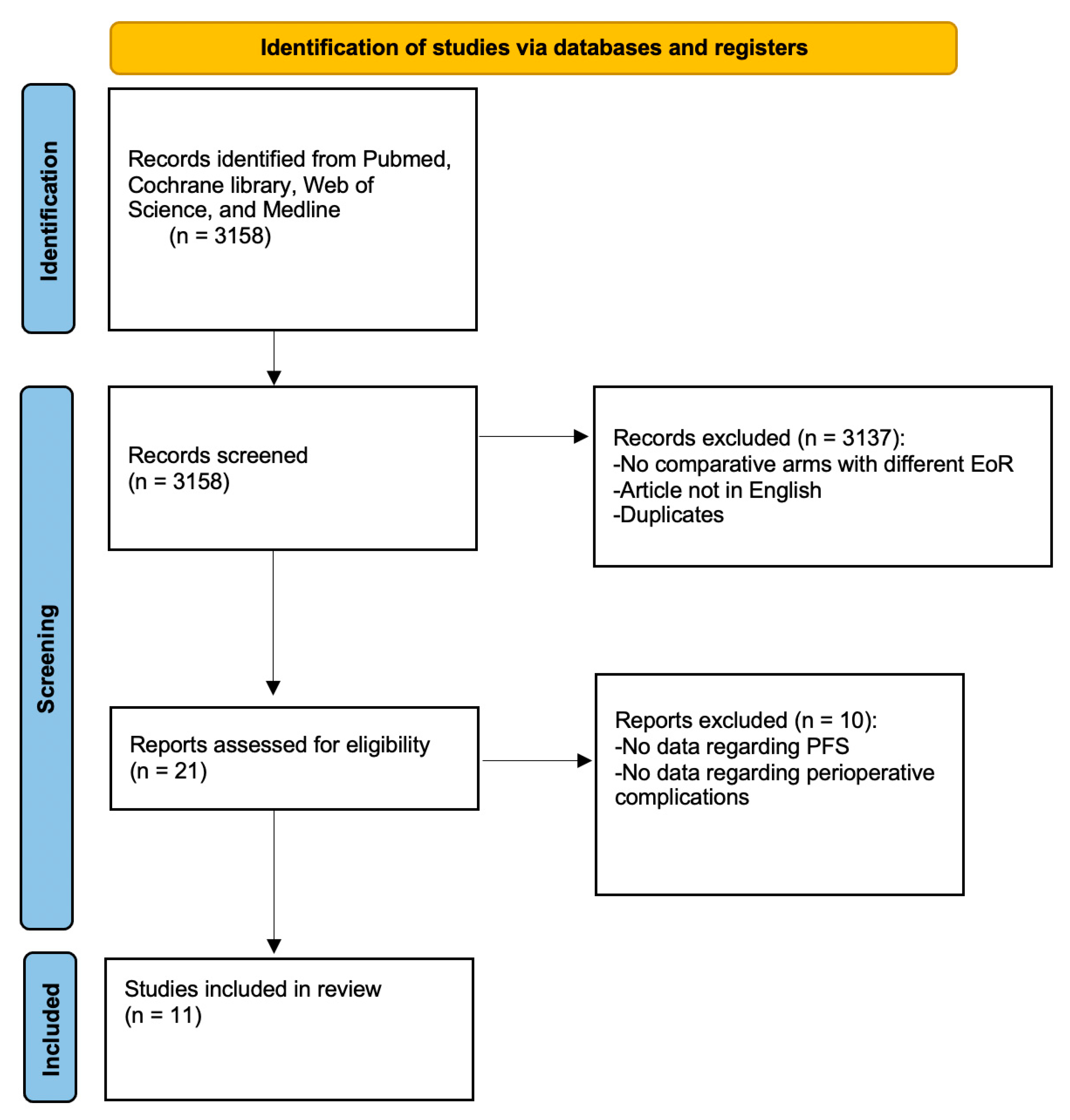
3.1. Literature Search
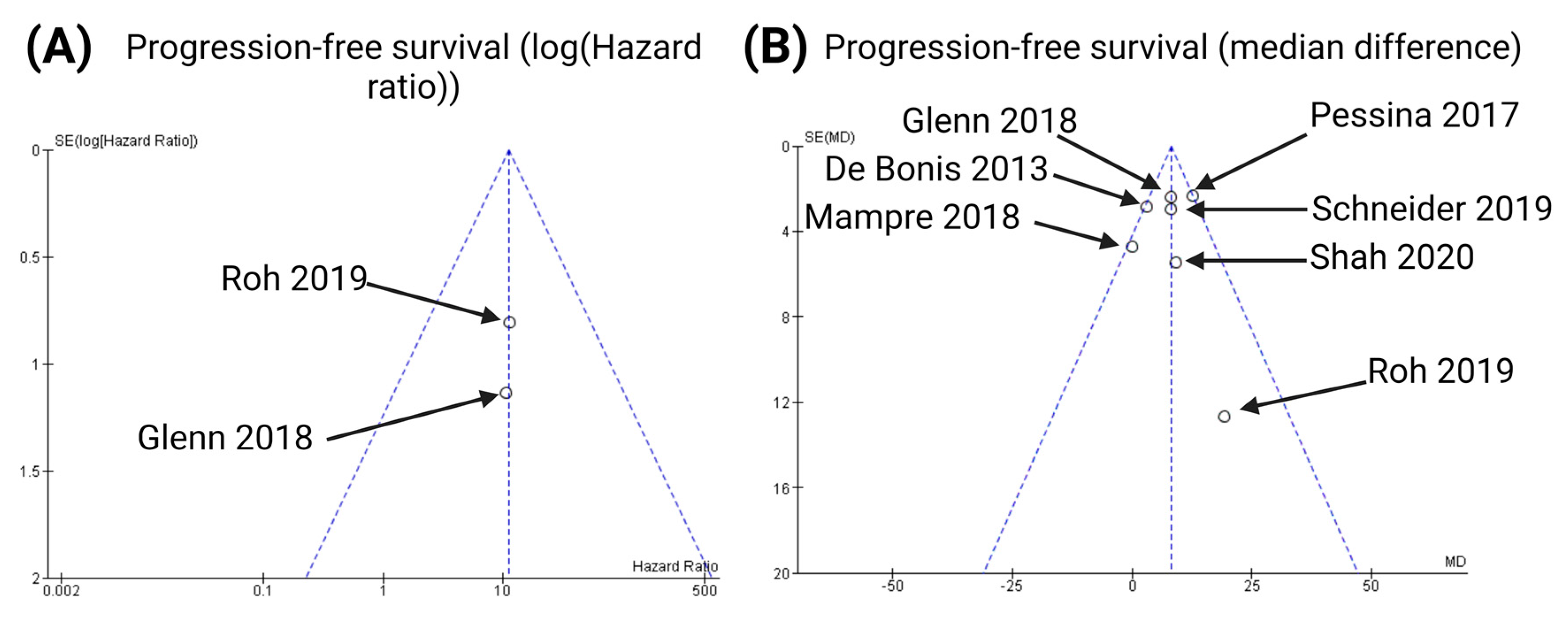
3.2. Characteristics of Included Studies Regarding the Analysis of Progression-Free Survival
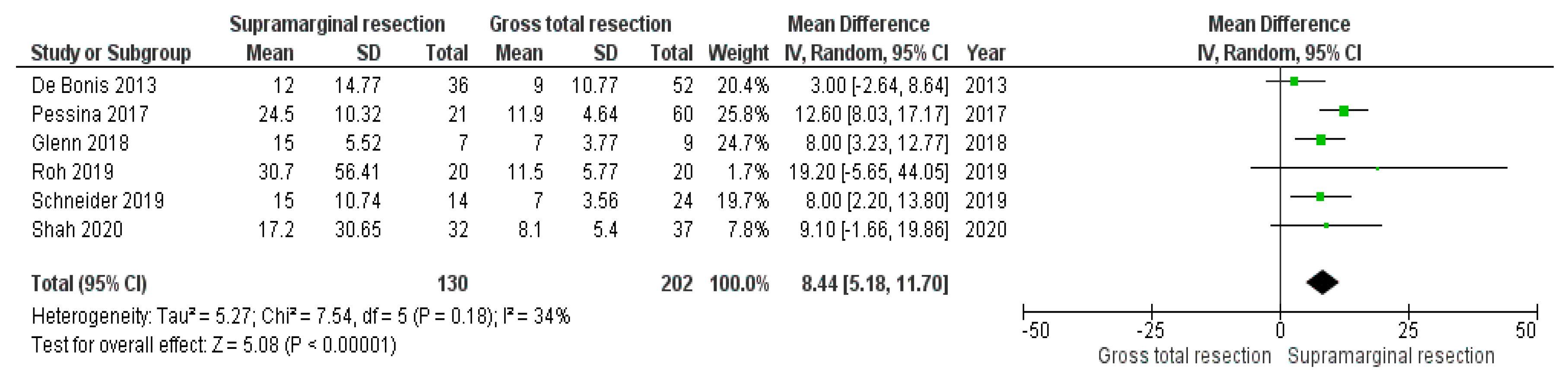
3.3. Impact of Supramarginal Resection on Progression-Free Survival in Glioblastoma
3.4. Characteristics of Included Studies Regarding the Analysis of Perioperative Complications
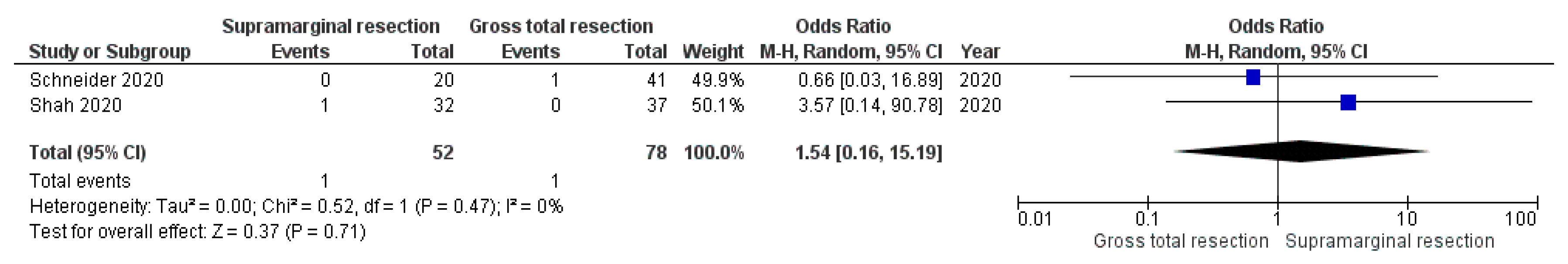
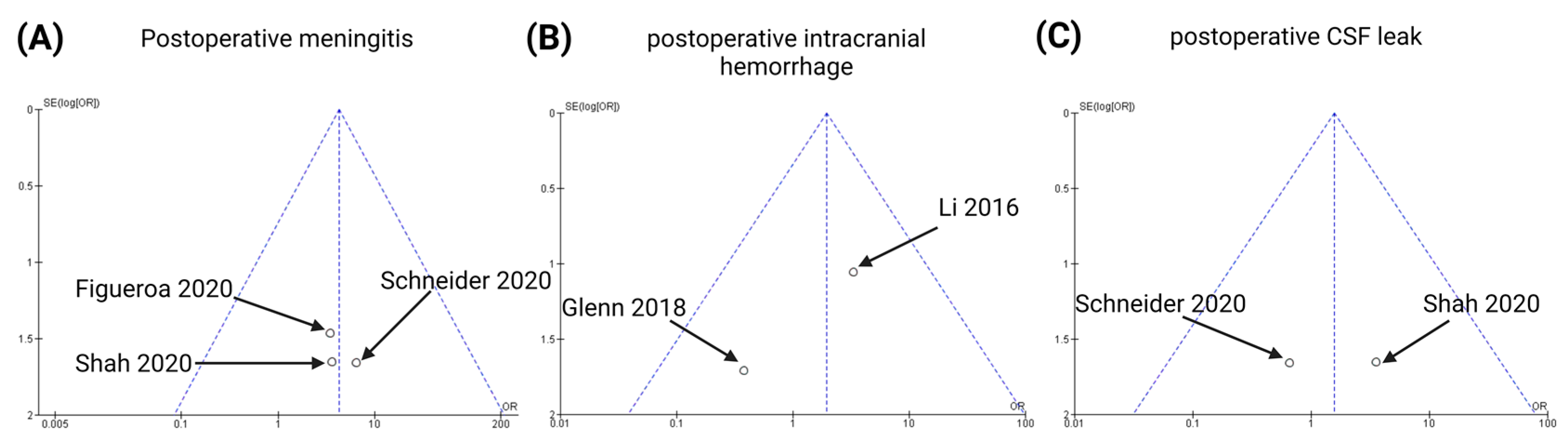
3.5. Impact of Supramarginal Resection on Postoperative Complications
3.5.1. Postoperative Meningitis
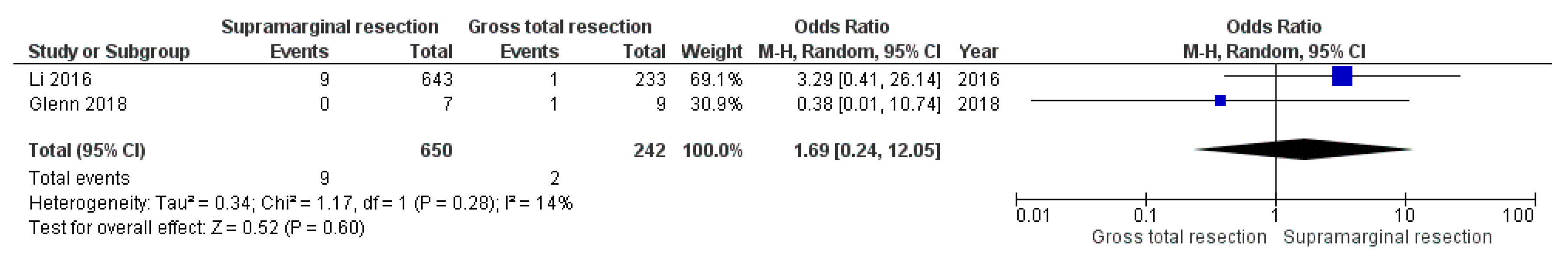
3.5.2. Postoperative Intracranial Hemorrhage
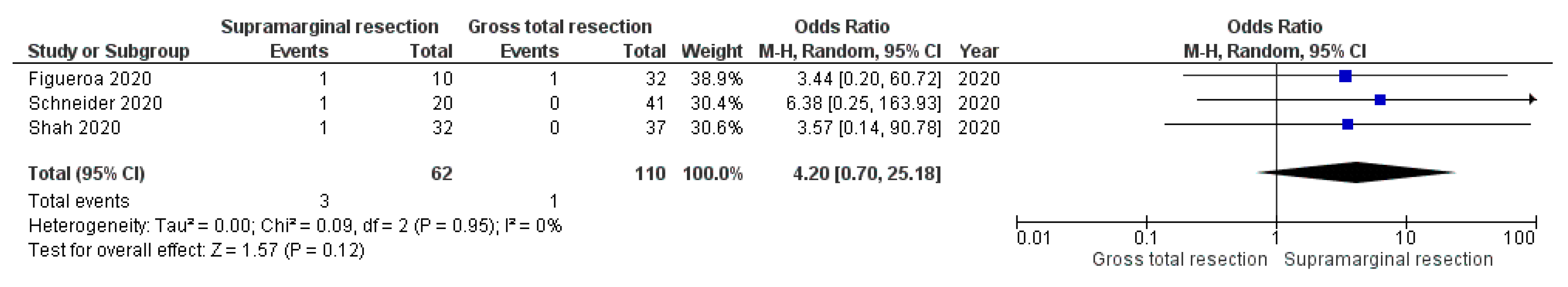
3.5.3. Postoperative CSF Leaks
3.6. Publication Bias
4. Discussion
4.1. Supramarginal Resection and Postoperative Complications
4.2. Supramarginal Resection and Probability of Progression-Free Survival
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Omuro, A.; Brandes, A.A.; Carpentier, A.F.; Idbaih, A.; Reardon, D.A.; Cloughesy, T.; Sumrall, A.; Baehring, J.; van den Bent, M.; Bähr, O.; et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro Oncol. 2023, 25, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Lassman, A.B.; Pugh, S.L.; Wang, T.J.C.; Aldape, K.; Gan, H.K.; Preusser, M.; Vogelbaum, M.A.; Sulman, E.P.; Won, M.; Zhang, P.; et al. Depatuxizumab-mafodotin in EGFR-amplified newly diagnosed glioblastoma: A phase III randomized clinical trial. Neuro Oncol. 2022, 15, noac173. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Capper, D.; Jeibmann, A.; Habel, A.; Paulus, W.; Troost, D.; von Deimling, A. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch. Neurol. 2012, 69, 523–526. [Google Scholar] [PubMed]
- Sherriff, J.; Tamangani, J.; Senthil, L.; Cruickshank, G.; Spooner, D.; Jones, B.; Brookes, C.; Sanghera, P. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br. J. Radiol. 2013, 86, 20120414. [Google Scholar] [CrossRef] [PubMed]
- Wallner, K.E.; Galicich, J.H.; Krol, G.; Arbit, E.; Malkin, M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Hoelzinger, D.B.; Demuth, T.; Berens, M.E. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J. Natl. Cancer Inst. 2007, 99, 1583–1593. [Google Scholar] [CrossRef]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef]
- Tripathi, S.; Vivas-Buitrago, T.; Domingo, R.A.; Biase, G.; Brown, D.; Akinduro, O.O.; Ramos-Fresnedo, A.; Sherman, W.; Gupta, V.; Middlebrooks, E.H.; et al. IDH-wild-type glioblastoma cell density and infiltration distribution influence on supramarginal resection and its impact on overall survival: A mathematical model. J. Neurosurg. 2021, 136, 1567–1575. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Choi, J.; Khalafallah, A.M.; Price, C.; Bettegowda, C.; Lim, M.; Gallia, G.; Weingart, J.; Brem, H.; Mukherjee, D. A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J. Neurooncol. 2020, 148, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Surgery for Malignant Brain Gliomas: Fluorescence-Guided Resection or Functional-Based Resection? Front. Surg. 2019, 6, 21. [Google Scholar] [CrossRef]
- Guerrini, F.; Roca, E.; Spena, G. Supramarginal Resection for Glioblastoma: It Is Time to Set Boundaries! A Critical Review on a Hot Topic. Brain Sci. 2022, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Tiek, D.; Song, X.; Huang, T.; Hu, B.; Cheng, S.Y. The Many Facets of Therapy Resistance and Tumor Recurrence in Glioblastoma. Cells 2021, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Sagberg, L.M.; Iversen, D.H.; Fyllingen, E.H.; Jakola, A.S.; Reinertsen, I.; Solheim, O. Brain atlas for assessing the impact of tumor location on perioperative quality of life in patients with high-grade glioma: A prospective population-based cohort study. Neuroimage Clin. 2019, 21, 101658. [Google Scholar] [CrossRef]
- Sagberg, L.M.; Solheim, O.; Jakola, A.S. Quality of survival the 1st year with glioblastoma: A longitudinal study of patient-reported quality of life. J. Neurosurg. 2016, 124, 989–997. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022): Cochrane. 2022. Available online: https://training.cochrane.org/handbook/current (accessed on 30 November 2022).
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Glenn, C.A.; Baker, C.M.; Conner, A.K.; Burks, J.D.; Bonney, P.A.; Briggs, R.G.; Smitherman, A.D.; Battiste, J.D.; Sughrue, M.E. An Examination of the Role of Supramaximal Resection of Temporal Lobe Glioblastoma Multiforme. World Neurosurg. 2018, 114, e747–e755. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Lauriola, L.; Maira, G.; Mangiola, A. The influence of surgery on recurrence pattern of glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 37–43. [Google Scholar] [CrossRef]
- Schneider, M.; Potthoff, A.L.; Keil, V.C.; Güresir, A.; Weller, J.; Borger, V.; Hamed, M.; Waha, A.; Vatter, H.; Güresir, E.; et al. Surgery for temporal glioblastoma: Lobectomy outranks oncosurgical-based gross-total resection. J. Neurooncol. 2019, 145, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Pessina, F.; Navarria, P.; Cozzi, L.; Ascolese, A.M.; Simonelli, M.; Santoro, A.; Clerici, E.; Rossi, M.; Scorsetti, M.; Bello, L. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: Is it useful and safe? A single institution retrospective experience. J. Neurooncol. 2017, 135, 129–139. [Google Scholar] [CrossRef]
- Roh, T.H.; Kang, S.G.; Moon, J.H.; Sung, K.S.; Park, H.H.; Kim, S.H.; Kim, E.H.; Hong, C.K.; Suh, C.O.; Chang, J.H. Survival benefit of lobectomy over gross-total resection without lobectomy in cases of glioblastoma in the noneloquent area: A retrospective study. J. Neurosurg. 2019, 132, 895–901. [Google Scholar] [CrossRef]
- Shah, A.H.; Mahavadi, A.; Di, L.; Sanjurjo, A.; Eichberg, D.G.; Borowy, V.; Figueroa, J.; Luther, E.; de la Fuente, M.I.; Semonche, A.; et al. Survival benefit of lobectomy for glioblastoma: Moving towards radical supramaximal resection. J. Neurooncol. 2020, 148, 501–508. [Google Scholar] [CrossRef]
- Mampre, D.; Ehresman, J.; Pinilla-Monsalve, G.; Osorio, M.A.G.; Olivi, A.; Quinones-Hinojosa, A.; Chaichana, K.L. Extending the resection beyond the contrast-enhancement for glioblastoma: Feasibility, efficacy, and outcomes. Br. J. Neurosurg. 2018, 32, 528–535. [Google Scholar] [CrossRef]
- Hamada, S.M.; Ahmed, H.A. Anatomical resection in glioblastoma: Extent of resection and its impact on duration of survival. Egypt J. Neurol. Psychiatry Neurosurg. 2016, 53, 135–145. [Google Scholar] [CrossRef]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Ilic, I.; Potthoff, A.L.; Hamed, M.; Schäfer, N.; Velten, M.; Güresir, E.; Herrlinger, U.; Borger, V.; Vatter, H.; et al. Safety metric profiling in surgery for temporal glioblastoma: Lobectomy as a supra-total resection regime preserves perioperative standard quality-rates. J. Neurooncol. 2020, 149, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.; Morell, A.; Bowory, V.; Shah, A.H.; Eichberg, D.; Buttrick, S.S.; Richardson, A.; Sarkiss, C.; Ivan, M.E.; Komotar, R.J. Minimally invasive keyhole temporal lobectomy approach for supramaximal glioma resection: A safety and feasibility study. J. Clin. Neurosci. 2020, 72, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Merkow, R.P.; Bilimoria, K.Y.; Tomlinson, J.S.; Paruch, J.L.; Fleming, J.B.; Talamonti, M.S.; Ko, C.Y.; Bentrem, D.J. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann. Surg. 2014, 260, 372–377. [Google Scholar] [CrossRef]
- Weber, L.; Padevit, L.; Müller, T.; Velz, J.; Vasella, F.; Voglis, S.; Gramatzki, D.; Weller, M.; Regli, L.; Sarnthein, J.; et al. Association of perioperative adverse events with subsequent therapy and overall survival in patients with WHO grade III and IV gliomas. Front. Oncol. 2022, 12, 959072. [Google Scholar] [CrossRef]
- Rahman, M.; Abbatematteo, J.; De Leo, E.K.; Kubilis, P.S.; Vaziri, S.; Bova, F.; Sayour, E.; Mitchell, D.; Quinones-Hinojosa, A. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J. Neurosurg. 2017, 127, 123–131. [Google Scholar] [CrossRef]
- De la Garza-Ramos, R.; Kerezoudis, P.; Tamargo, R.J.; Brem, H.; Huang, J.; Bydon, M. Surgical complications following malignant brain tumor surgery: An analysis of 2002–2011 data. Clin. Neurol. Neurosurg. 2016, 140, 6–10. [Google Scholar] [CrossRef]
- Jackson, C.; Westphal, M.; Quinones-Hinojosa, A. Complications of glioma surgery. Handb. Clin. Neurol. 2016, 134, 201–208. [Google Scholar]
- McGirt, M.J.; Mukherjee, D.; Chaichana, K.L.; Than, K.D.; Weingart, J.D.; Quinones-Hinojosa, A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 2009, 65, 463–469. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef]
- Incekara, F.; Koene, S.; Vincent, A.J.P.E.; van den Bent, M.J.; Smits, M. Association Between Supratotal Glioblastoma Resection and Patient Survival: A Systematic Review and Meta-Analysis. World Neurosurg. 2019, 127, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Vivas-Buitrago, T.; Domingo, R.A.; Tripathi, S.; De Biase, G.; Brown, D.; Akinduro, O.O.; Ramos-Fresnedo, A.; Sabsevitz, D.S.; Bendok, B.R.; Sherman, W.; et al. Influence of supramarginal resection on survival outcomes after gross-total resection of IDH-wild-type glioblastoma. J. Neurosurg. 2021, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Surgical oncology for gliomas: The state of the art. Nat. Rev. Clin. Oncol. 2018, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, E.; Baumfalk, A.E.; van Zandvoort, M.J.E.; Robe, P.A.; Snijders, T.J. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: A systematic review of neurocognitive functioning prior to anti-tumor treatment. J. Neurooncol. 2017, 134, 9–18. [Google Scholar] [CrossRef]
- Campanella, F.; Mondani, M.; Skrap, M.; Shallice, T. Semantic access dysphasia resulting from left temporal lobe tumors. Brain 2009, 132 Pt 1, 87–102. [Google Scholar] [CrossRef]
- You, G.; Sha, Z.; Jiang, T. Clinical Diagnosis and Perioperative Management of Glioma-Related Epilepsy. Front. Oncol. 2021, 10, 550353. [Google Scholar] [CrossRef]
- Borger, V.; Hamed, M.; Ilic, I.; Potthoff, A.L.; Racz, A.; Schäfer, N.; Güresir, E.; Surges, R.; Herrlinger, U.; Vatter, H.; et al. Seizure outcome in temporal glioblastoma surgery: Lobectomy as a supratotal resection regime outclasses conventional gross-total resection. J. Neurooncol. 2021, 152, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ung, T.H.; Ney, D.E.; Damek, D.; Rusthoven, C.G.; Youssef, A.S.; Lillehei, K.O.; Ormond, D.R. The Neurologic Assessment in Neuro-Oncology (NANO) Scale as an Assessment Tool for Survival in Patients with Primary Glioblastoma. Neurosurgery 2019, 84, 687–695. [Google Scholar] [CrossRef]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.M.; Wildrick, D.M. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 1998, 42, 1044–1055; discussion 1055–1056. [Google Scholar] [CrossRef]

| Study | Country | Hierarchy of Evidence | Study Design | Number of Patients | Supramarginal Resection | Gross Total Resection | Age | Female: Male Ratio | Completeness of Adjuvant Radiochemotherapy | Duration of Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Glenn et al., 2018 [24] | USA | IV | Retrospective | 32 (16 patients with subtotal resection) | 7 | 9 | SMR: 56.3 GTR: 58.1 | SMR: 1:6 GTR: 1:3.5 | NA | NA |
| De Bonis et al., 2013 [25] | Italy | IV | Retrospective | 88 | 36 | 52 | 57.5 (mean age in entire cohort) | 1:1.15 (in entire cohort) | NA | NA |
| Schneider et al., 2019 [26] | Germany | IV | Retrospective | 38 | 14 | 24 | SMR: 63 (mean) GTR: 68 (mean) | SMR: 1:2.5 GTR: 1:2.18 | NA | NA |
| Pessina et al., 2017 [27] | Italy | IV | Retrospective | 282 (143 patients with subtotal resection and 58 patients with biopsy) | 21 | 60 | 61 (median age in entire cohort) | 1:1.69 (in entire cohort) | Interruption of chemotherapy in 6 patients (2.1%) and delay/reduction in 14 patients (5.0%) | 13.8 months (median f/u for entire cohort) |
| Roh et al., [28] 2019 | Republic of Korea | IV | Retrospective | 40 | 20 | 20 | SMR: 62 (median) GTR: 60 (median) | SMR: 1:2.33 GTR: 1:1.86 | NA | 46.1 months (median) |
| Shah et al., 2020 [29] | USA | IV | Retrospective | 69 | 32 | 37 | SMR: 60 (median) GTR: 65 (median) | SMR: 1:3.6 GTR: 1:0.68 | 61 (89.7%) completed radiochemotherapy. Not stratified by EoR and no data regarding interruption or delay | SMR: 12.4 (median) GTR: 6 (median) |
| Mampre et al., 2018 [30] | USA | IV | Retrospective | 245 (161 patients with subtotal resection) | 11 | 84 | 59.8 (mean) | 1:1.55 | Chemotherapy only in 16 (7%) patients or radiation only in 8 (3%) patients. Not stratified by EoR and no data regarding interruption or delay | 12.1 (median time for all surviving patients) |
| Hamada et al., 2016 [31] | Egypt | IV | Prospective | 59 (14 patients with subtotal resection and 4 patients with debulking) | 20 | 21 | 48.57 (mean) | 1:2.69 | NA | NA |
| Study | Supramarginal Resection Technique | Supramarginal Resection and PFS (Months) | Gross Total Resection and PFS (Months) | Method of Statistical Comparison | Available Multivariate Statistical Results | Study Limitations |
|---|---|---|---|---|---|---|
| Glenn et al., 2018 [24] | Resection extended beyond the T1 contrast-enhancement margin to include at least 1 cm of surrounding brain. Tumors with T1 contrast-enhancement less than 1 cm from the temporal cortex were included in the supramaximal resection group when the resection included the overlying cortex, as well as at least a 1 cm brain margin in all other directions. | 15 (median) | 7 (median) | Multivariate Cox proportional hazard model | Cox regression (including hazard ratios, 95% CI, p-values) | Retrospective design |
| De Bonis et al., 2013 [25] | Extent of resection was classified into two groups: “border resection” (resection margins at the level of tumor border (= contrast-enhanced peripheral areas of tumors) or “extended resection” (ER, resection margins beyond tumor borders, i.e., in the apparently normal white matter, 1–2 cm far from tumor border). | 12 (median) | 11 (median) | Log-rank test | Not available | Retrospective design, no multivariate Cox regression analysis of PFS |
| Schneider et al., 2019 [26] | Gross total resection of contrast-enhancing tumor portion of temporal GB was compared with patients who underwent temporal tumor resection with additional anterior temporal lobectomy (from the temporal tip to posterior margin of resection at nondominant side: 5–6 cm and 4–5 cm on the dominant hemisphere | 15 (median) | 7 (median) | Log-rank test, multivariate logistic regression analysis | Multivariate logistic regression analysis | Retrospective design, no multivariate Cox regression analysis of PFS |
| Pessina et al., 2017 [27] | SMR was defined as surgical resection of 100% of contrast-enhanced and 100% of FLAIR-altered tumor areas. | 24.5 (median) | 11.9 (median) | Log-rank test, p-values of multivariate Cox model | Multivariate Cox regression analysis (only p-values available) | Retrospective design, no hazard ratios or confidence intervals of multivariate Cox regression analysis |
| Roh et al., 2019 [28] | SMR: temporal lobectomy for temporal GB with the posterior margin of resection approximately 5–6 cm from the temporal pole. Anterior portion of superior temporal gyrus was also removed. Frontal lobectomy was performed for frontal GB. Corpus callosum was resected if it was invaded. The posterior margin of frontal lobectomy was just beneath the coronal suture, which is considered to be 1–2 cm anterior to the precentral sulcus. | 30.7 (median) | 11.5 (median) | Log-rank test, multivariate Cox model | Multivariate Cox regression (including hazard ratios, 95% CI, p-values) | Retrospective design |
| Shah et al., 2020 [29] | SMR: temporal lobectomy for temporal GB with the posterior margin of resection approximately 5–6 cm from the temporal pole. Anterior portion of superior temporal gyrus was also removed. Frontal lobectomy was performed for frontal GB. Corpus callosum was resected if it was invaded. The posterior margin of frontal lobectomy was just beneath the coronal suture, which is considered to be 1–2 cm anterior to the precentral sulcus. Occipital lobectomies were also performed. | 17.2 (median) | 8.1 (median) | Log-rank test, multivariate Cox regression | Multivariate Cox regression model (only p-values available) | Retrospective design, only p-values of Cox regression available |
| Mampre et al., 2018 [30] | FLAIR resection was performed if resection was possible without causing iatrogenic deficits. | NA | NA | Log-rank tests, multivariate Cox regression analysis | Multivariate Cox regression analysis (including hazard ratios, 95% CI, p-values) | Retrospective design; postoperative FLAIR volume and no extent of resection was analyzed regarding PFS. No mean or median times to PFS stratified by GTR and SMR |
| Hamada et al., 2016 [31] | Anatomical resection (AR) of the involved entire gyrus was performed if it was classified as noneloquent. | Frontal AR: 10.75 (mean) Occipital AR: 7.5 (mean) Parietal AR: not performed Temporal AR: 12.25 (mean) | Frontal GTR: 8.5 (mean) Occipital GTR: 6 (mean) Parietal GTR: 4.67 (mean) Temporal GTR: 9.43 (mean) | Not available | Not available | No statistical comparison of EoR regarding PFS |
| Study | Mortality | Meningitis | Intracranial Hemorrhage | CSF Leak |
|---|---|---|---|---|
| Li et al., 2016 [32] | NA | NA | SMR: 9/643 GTR: 1/233 | NA |
| Glenn et al., 2018 [24] | NA | SMR: 0/7 GTR: 0/9 | SMR: 0/7 GTR: 1/9 | NA |
| Schneider et al., 2020 [33] | NA | SMR: 1/20 GTR: 0/41 | NA | SMR: 0/20 GTR: 1/41 |
| Pessina et al., 2017 [27] | SMR: 0/21 GTR: 0/60 | NA | NA | NA |
| Shah et al., 2020 [29] | NA | SMR: 1/32 GTR: 0/37 | NA | SMR: 1/32 GTR: 0/37 |
| Figueroa et al., 2020 [34] | NA | SMR: 1/10 GTR: 1/32 | NA | 1 CSF leak (not stratified by EoR) |
| Hamada et al., 2016 [31] | SMR: 0/16 GTR: 0/16 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wach, J.; Vychopen, M.; Kühnapfel, A.; Seidel, C.; Güresir, E. A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety? Cancers 2023, 15, 1772. https://doi.org/10.3390/cancers15061772
Wach J, Vychopen M, Kühnapfel A, Seidel C, Güresir E. A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety? Cancers. 2023; 15(6):1772. https://doi.org/10.3390/cancers15061772
Chicago/Turabian StyleWach, Johannes, Martin Vychopen, Andreas Kühnapfel, Clemens Seidel, and Erdem Güresir. 2023. "A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety?" Cancers 15, no. 6: 1772. https://doi.org/10.3390/cancers15061772
APA StyleWach, J., Vychopen, M., Kühnapfel, A., Seidel, C., & Güresir, E. (2023). A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety? Cancers, 15(6), 1772. https://doi.org/10.3390/cancers15061772








