Prognostic Impact of Pulmonary Metastasectomy in Bone Sarcoma Patients: A Retrospective, Single-Centre Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
3.1. Difference between Patients Undergoing and Not Undergoing Metastasectomy
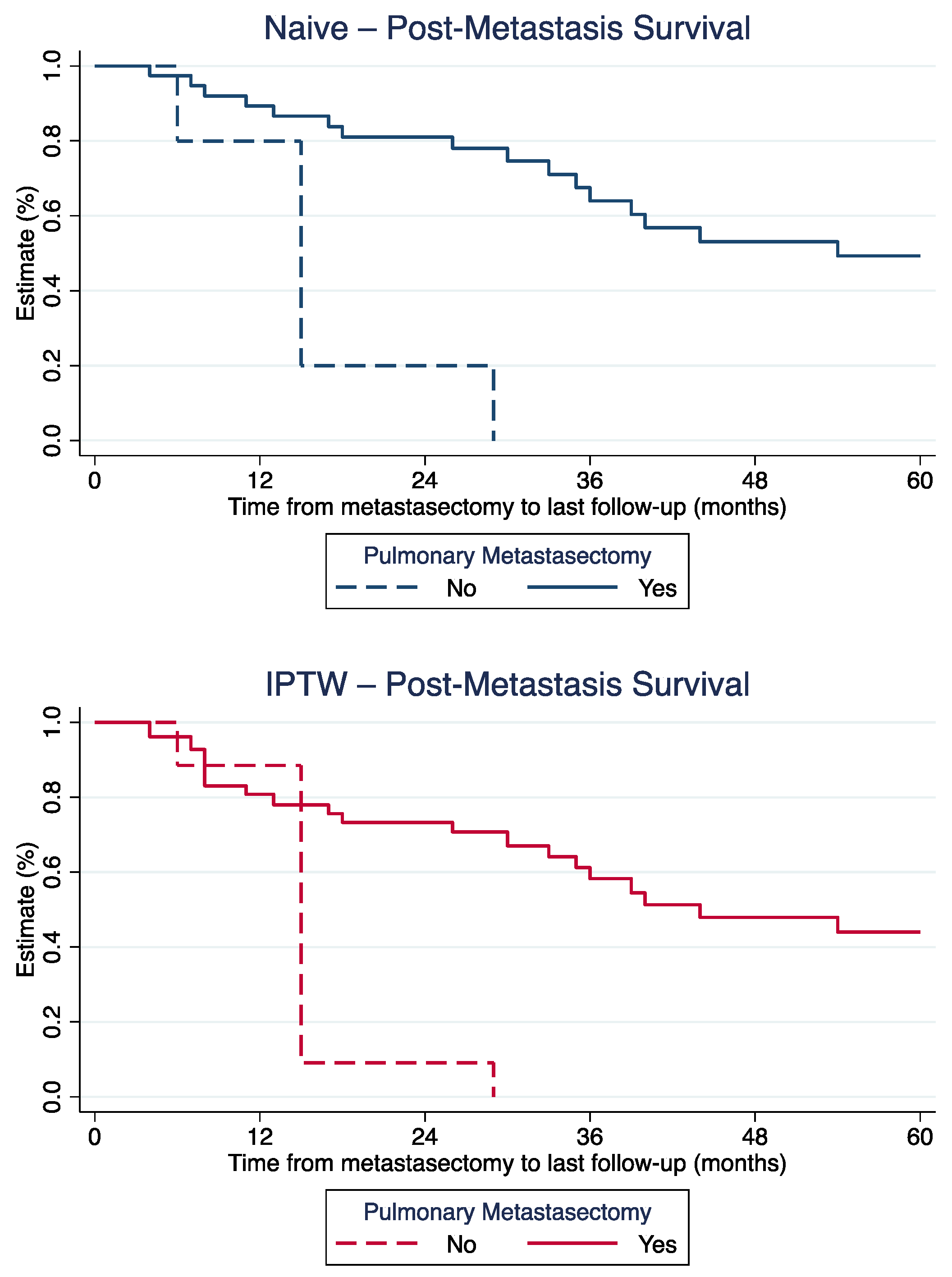
3.2. Difference in Post-Metastasis Survival
3.3. Long-Term Post-Metastasis Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bielack, S.S.; Blattmann, C.; Borkhardt, A.; Csóka, M.; Hassenpflug, W.; Kabíčková, E.; Kager, L.; Kessler, T.; Kratz, C.; Kühne, T.; et al. Osteosarcoma and causes of death: A report of 1520 deceased patients from the Cooperative Osteosarcoma Study Group (COSS). Eur. J. Cancer 2022, 176, 50–57. [Google Scholar] [CrossRef]
- Kaste, S.C.; Pratt, C.B.; Cain, A.M.; Jones-Wallace, D.J.; Rao, B.N. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: Imaging features. Cancer 1999, 86, 1602–1608. [Google Scholar] [CrossRef]
- Bacci, G.; Longhi, A.; Versari, M.; Mercuri, M.; Briccoli, A.; Picci, P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 2006, 106, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; Lowe, M.; Tsuda, Y.; Lex, J.R.; Fujiwara, T.; Almeer, G.; Gregory, J.; Stevenson, J.; Evans, S.E.; Botchu, R.; et al. How Are Indeterminate Pulmonary Nodules at Diagnosis Associated with Survival in Patients with High-Grade Osteosarcoma? Clin. Orthop. Relat. Res. 2021, 479, 298–308. [Google Scholar] [CrossRef]
- Cangir, A.; Vietti, T.J.; Gehan, E.A.; Burgert, E.O., Jr.; Thomas, P.; Tefft, M.; Nesbit, M.E.; Kissane, J.; Pritchard, D. Ewing’s sarcoma metastatic at diagnosis. Results and comparisons of two intergroup Ewing’s sarcoma studies. Cancer 1990, 66, 887–893. [Google Scholar] [CrossRef]
- The ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. 3), iii113–iii123. [Google Scholar] [CrossRef]
- Tsoi, K.M.; Tan, D.; Stevenson, J.; Evans, S.; Jeys, L.M.; Botchu, R. Indeterminate pulmonary nodules are not associated with worse overall survival in Ewing Sarcoma. J. Clin. Orthop. Trauma 2021, 16, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hecker-Nolting, S.; Maia Ferreira, A.; Bielack, S.S. Bone sarcoma: Success through interdisciplinary collaboration. J. Child. Orthop. 2021, 15, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kempf-Bielack, B.; Bielack, S.S.; Jürgens, H.; Branscheid, D.; Berdel, W.E.; Exner, G.U.; Göbel, U.; Helmke, K.; Jundt, G.; Kabisch, H.; et al. Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J. Clin. Oncol. 2005, 23, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Durer, S.; Shaikh, H. Ewing Sarcoma; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Treasure, T.; Fiorentino, F.; Scarci, M.; Møller, H.; Utley, M. Pulmonary metastasectomy for sarcoma: A systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012, 2, e001736. [Google Scholar] [CrossRef]
- Gafencu, D.A.; Welter, S.; Cheufou, D.H.; Ploenes, T.; Stamatis, G.; Stuschke, M.; Theegarten, D.; Taube, C.; Bauer, S.; Aigner, C. Pulmonary metastasectomy for sarcoma-Essen experience. J. Thorac. Dis. 2017, 9, S1278–S1281. [Google Scholar] [CrossRef] [PubMed]
- Digesu, C.S.; Wiesel, O.; Vaporciyan, A.A.; Colson, Y.L. Management of Sarcoma Metastases to the Lung. Surg. Oncol. Clin. N. Am. 2016, 25, 721–733. [Google Scholar] [CrossRef]
- Harting, M.T.; Blakely, M.L.; Jaffe, N.; Cox, C.S., Jr.; Hayes-Jordan, A.; Benjamin, R.S.; Raymond, A.K.; Andrassy, R.J.; Lally, K.P. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J. Pediatr. Surg. 2006, 41, 194–199. [Google Scholar] [CrossRef]
- Chen, F.; Miyahara, R.; Bando, T.; Okubo, K.; Watanabe, K.; Nakayama, T.; Toguchida, J.; Date, H. Prognostic factors of pulmonary metastasectomy for osteosarcomas of the extremities. Eur. J. Cardio-Thorac Surg. 2008, 34, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Hou, C.H.; Hou, S.M.; Yang, R.S. The metastasectomy and timing of pulmonary metastases on the outcome of osteosarcoma patients. Clin. Med. Oncol. 2009, 3, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Han, I.; Lee, J.S.; Cho, H.S.; Park, J.W.; Kim, H.S. Postmetastasis survival in high-grade extremity osteosarcoma: A retrospective analysis of prognostic factors in 126 patients. J. Surg. Oncol. 2018, 117, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, P.A.; Shackett, B.; Xiao, L.; Trent, J.; Tsao, K.J.; Lally, K.; Hayes-Jordan, A. Resection of pulmonary metastases in pediatric patients with Ewing sarcoma improves survival. J. Pediatr. Surg. 2011, 46, 332–335. [Google Scholar] [CrossRef]
- Salah, S.; Fayoumi, S.; Alibraheem, A.; Massad, E.; Abdel Jalil, R.; Yaser, S.; Albadainah, F.; Albaba, H.; Maakoseh, M. The influence of pulmonary metastasectomy on survival in osteosarcoma and soft-tissue sarcomas: A retrospective analysis of survival outcomes, hospitalizations and requirements of home oxygen therapy. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 296–302. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Posch, F.; Partl, R.; Doller, C.; Riedl, J.M.; Smolle, M.; Leitner, L.; Bergovec, M.; Liegl-Atzwanger, B.; Stotz, M.; Bezan, A.; et al. Benefit of Adjuvant Radiotherapy for Local Control, Distant Metastasis, and Survival Outcomes in Patients with Localized Soft Tissue Sarcoma: Comparative Effectiveness Analysis of an Observational Cohort Study. Ann. Surg. Oncol. 2018, 25, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zeng, Y.; Liu, B.; Lu, G.; Xiang, Z.; Chen, J.; Yu, Y.; Zuo, Z.; Lin, Y.; Ma, J. Risk Factors, Prognostic Factors, and Nomograms for Distant Metastasis in Patients With Newly Diagnosed Osteosarcoma: A Population-Based Study. Front. Endocrinol. 2021, 12, 672024. [Google Scholar] [CrossRef]
- Buddingh, E.P.; Anninga, J.K.; Versteegh, M.I.; Taminiau, A.H.; Egeler, R.M.; van Rijswijk, C.S.; Hogendoorn, P.C.; Lankester, A.C.; Gelderblom, H. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr. Blood Cancer 2010, 54, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yin, J.; Zhou, Q.; Yang, J.; Zeng, B.; Yeung, S.J.; Shen, J.; Cheng, C. Survival after pulmonary metastasectomy for relapsed osteosarcoma. J. Thorac. Cardiovasc. Surg. 2022, 163, 469–479.e8. [Google Scholar] [CrossRef]
- Tronc, F.; Conter, C.; Marec-Berard, P.; Bossard, N.; Remontet, L.; Orsini, A.; Gamondes, J.P.; Louis, D. Prognostic factors and long-term results of pulmonary metastasectomy for pediatric histologies. Eur. J. Cardiothorac. Surg. 2008, 34, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
| Total (%) | Metastasectomy | p-Value | SMD | SMD after IPTW | |||
|---|---|---|---|---|---|---|---|
| No (n = 8) | Yes (n = 39) | ||||||
| Gender | Male | 24 (51.1) | 3 | 21 | 0.400 | 0.32 | 0.02 |
| Female | 23 (48.9) | 5 | 18 | ||||
| Age at diagnosis of metastases (in years; median + IQR) | 21.8 [15.6–47.3] | 41.8 [22.0–65.4] | 19.3 [15.6–40.5] | 0.025 * | 0.84 | 0.34 | |
| Primary lung metastases | No | 39 (83.0) | 5 | 33 | 0.091 | 0.56 | 0.17 |
| Yes | 8 (17.0) | 3 | 5 | ||||
| Time to lung metastases * (in months; median + IQR) | 9 [3–28] | 2.5 [0–8.5] | 12 [6–37] | 0.023 * | N/A | N/A | |
| Time to lung metastases ** | <9 months | 24 (51.1) | 7 | 17 | 0.024 * | 1.01 | 0.52 |
| ≥9 months | 23 (48.9) | 1 | 22 | ||||
| Histology | Osteosarcoma | 37 (78.8) | 5 | 32 | 0.329 | 0.43 | 0.11 |
| Ewing sarcoma | 5 (10.6) | 1 | 4 | 0.07 | 0.15 | ||
| Others | 5 (10.6) | 2 | 3 | 0.46 | 0.02 | ||
| RTX *** | No | 43 (91.5) | 7 | 36 | 0.657 | 0.15 | 0.08 |
| Yes | 4 (8.5) | 1 | 3 | ||||
| CTX | No | 7 (14.9) | 3 | 4 | 0.049 * | 0.64 | 0.06 |
| Yes | 40 (85.1) | 5 | 35 | ||||
| Multiple pulmonary metastases | No | 14 (29.8) | 0 | 14 | 0.043 * | 1.04 | 0.91 |
| Yes | 33 (70.2) | 8 | 25 | ||||
| Bilateral involvement | No | 23 (48.9) | 1 | 22 | 0.024 * | 1.01 | 0.99 |
| Yes | 24 (52.1) | 7 | 17 | ||||
| HR | 95% Confidence Interval | p-Value | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Univariate | |||||
| Metastasectomy | No | 1 | 0.003 ** | ||
| Yes | 0.237 | 0.090 | 0.623 | ||
| Gender | Male | 1 | 0.360 | ||
| Female | 1.499 | 0.630 | 3.567 | ||
| Age at diagnosis of metastasis (in years) | 1.035 | 1.014 | 1.056 | 0.001 ** | |
| Time to lung metastases | <9 months | 1 | 0.001 ** | ||
| ≥9 months | 0.255 | 0.114 | 0.573 | ||
| Multiple metastases | No | 1 | 0.021 ** | ||
| Yes | 2.858 | 1.173 | 6.967 | ||
| Histology | Osteosarcoma | 1 | |||
| Ewing sarcoma | 0.522 | 0.122 | 2.224 | 0.380 | |
| Others | 2.075 | 0.609 | 7.073 | 0.243 | |
| RTX * | No | 1 | 0.557 | ||
| Yes | 0.658 | 0.163 | 2.661 | ||
| CTX | No | 1 | 0.068 | ||
| Yes | 0.347 | 0.111 | 1.083 | ||
| Multivariate | |||||
| Metastasectomy | No | 1 | 0.004 ** | ||
| Yes | 0.279 | 0.118 | 0.662 | ||
| Age at diagnosis of metastasis (in years) | 1.054 | 1.034 | 1.0.73 | <0.001 ** | |
| Time to lung metastases | <9 months | 1 | 0.003 ** | ||
| ≥9 months | 0.214 | 0.077 | 0.595 | ||
| Multiple metastases | No | 1 | 0.798 | ||
| Yes | 0.860 | 0.273 | 2.714 | ||
| 1 | Age at Lung Metastasis Diagnosis (in Years) | Diagnosis | Time from Primary Diagnosis to Metastasis (in Months) | Number of Metastases | Bilateral Involvement | Type of Metastasectomy | PMS (in Months) | 2nd Metastasis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| M | 33 | Chondrosarcoma, left distal femur | 60 | ≥5 | 1 | Thoracotomy, bilateral wedge resection | 75 | Yes, lungs | AWD |
| F | 43 | Chondrosarcoma, left hemithorax | 46 | ≥5 | 0 | Thoracotomy, unilateral wedge resection | 89 | Yes, lungs | AWD |
| M | 15 | Ewing sarcoma, right ulna | 8 | 1 | 0 | Thoracotomy, unilateral wedge resection | 123 | No | NED |
| M | 15 | Osteosarcoma, left distal femur | 0 | 2 | 0 | Sternotomy, unilateral metastasectomy | 88 | No | NED |
| F | 24 | High-grade osteosarcoma, left calcaneus | 37 | 1 | 0 | Video-assisted thoracotomy, unilateral wedge resection | 80 | No | NED |
| M | 15 | Osteosarcoma, left distal femur | 67 | 1 | 0 | Thoracotomy, unilateral wedge resection | 107 | Yes, lungs, thorax | NED |
| M | 11 | Osteosarcoma, left distal femur | 23 | 1 | 0 | Sternotomy, lobectomy | 135 | Yes, brain | AWD |
| M | 17 | Osteosarcoma, left distal femur | 26 | ≥5 | 1 | Segmental resection | 171 | No | NED |
| M | 13 | Osteosarcoma (chondroblastic), left distal femur | 6 | ≥5 | 1 | Sternotomy, bilateral metastasectomy | 109 | Yes, lungs | DOD |
| M | 16 | High-grade osteosarcoma, right femur diaphysis | 18 | 1 | 0 | Thoracotomy, unilateral wedge resection | 66 | No | NED |
| M | 19 | Osteosarcoma (osteoblastic), left distal tibia | 0 | 4 | 1 | Sternotomy, bilateral metastasectomy * | 111 | No | NED |
| M | 21 | Osteosarcoma, right distal femur | 38 | 2 | 0 | Sternotomy, unilateral metastasectomy | 83 | Yes, lungs | AWD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolle, M.A.; Kogler, A.; Andreou, D.; Scheipl, S.; Bergovec, M.; Castellani, C.; Till, H.; Benesch, M.; Posch, F.; Szkandera, J.; et al. Prognostic Impact of Pulmonary Metastasectomy in Bone Sarcoma Patients: A Retrospective, Single-Centre Study. Cancers 2023, 15, 1733. https://doi.org/10.3390/cancers15061733
Smolle MA, Kogler A, Andreou D, Scheipl S, Bergovec M, Castellani C, Till H, Benesch M, Posch F, Szkandera J, et al. Prognostic Impact of Pulmonary Metastasectomy in Bone Sarcoma Patients: A Retrospective, Single-Centre Study. Cancers. 2023; 15(6):1733. https://doi.org/10.3390/cancers15061733
Chicago/Turabian StyleSmolle, Maria Anna, Angelika Kogler, Dimosthenis Andreou, Susanne Scheipl, Marko Bergovec, Christoph Castellani, Holger Till, Martin Benesch, Florian Posch, Joanna Szkandera, and et al. 2023. "Prognostic Impact of Pulmonary Metastasectomy in Bone Sarcoma Patients: A Retrospective, Single-Centre Study" Cancers 15, no. 6: 1733. https://doi.org/10.3390/cancers15061733
APA StyleSmolle, M. A., Kogler, A., Andreou, D., Scheipl, S., Bergovec, M., Castellani, C., Till, H., Benesch, M., Posch, F., Szkandera, J., Smolle-Jüttner, F.-M., & Leithner, A. (2023). Prognostic Impact of Pulmonary Metastasectomy in Bone Sarcoma Patients: A Retrospective, Single-Centre Study. Cancers, 15(6), 1733. https://doi.org/10.3390/cancers15061733







