A Feasibility Study of Functional Lung Volume Preservation during Stereotactic Body Radiotherapy Guided by Gallium-68 Perfusion PET/CT
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. PET/CT Protocol
2.3. Radiation Therapy
2.4. Data and Statistical Analysis
3. Results
3.1. Study Population
3.2. Dosimetric Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. PET/CT Protocol
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.; Mai, G.T.; Vinod, S.; Babington, S.; Ruben, J.; Kron, T.; Chesson, B.; Herschtal, A.; Vanevski, M.; Rezo, A.; et al. Stereotactic Ablative Radiotherapy versus Standard Radiotherapy in Stage 1 Non-Small-Cell Lung Cancer (TROG 09.02 CHISEL): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet Oncol. 2019, 20, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Temin, S.; Baker, S.; Blanchard, E.; Brahmer, J.R.; Celano, P.; Duma, N.; Ellis, P.M.; Elkins, I.B.; Haddad, R.Y.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline. J. Clin. Oncol. 2022, 40, 3323–3343. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Katz, A.W.; Schell, M.C.; Philip, A.; Okunieff, P. Descriptive Analysis of Oligometastatic Lesions Treated with Curative-Intent Stereotactic Body Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1516–1522. [Google Scholar] [CrossRef]
- Milano, M.T.; Katz, A.W.; Zhang, H.; Okunieff, P. Oligometastases Treated with Stereotactic Body Radiotherapy: Long-Term Follow-up of Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 878–886. [Google Scholar] [CrossRef]
- Salama, J.K.; Hasselle, M.D.; Chmura, S.J.; Malik, R.; Mehta, N.; Yenice, K.M.; Villaflor, V.M.; Stadler, W.M.; Hoffman, P.C.; Cohen, E.E.W.; et al. Stereotactic Body Radiotherapy for Multisite Extracranial Oligometastases: Final Report of a Dose Escalation Trial in Patients with 1 to 5 Sites of Metastatic Disease. Cancer 2012, 118, 2962–2970. [Google Scholar] [CrossRef]
- Binkley, M.S.; Trakul, N.; Jacobs, L.R.; von Eyben, R.; Le, Q.-T.; Maxim, P.G.; Loo, B.W.; Shultz, D.B.; Diehn, M. Colorectal Histology Is Associated With an Increased Risk of Local Failure in Lung Metastases Treated With Stereotactic Ablative Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 1044–1052. [Google Scholar] [CrossRef]
- Harrow, S.; Palma, D.A.; Olson, R.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): Extended Long-Term Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 611–616. [Google Scholar] [CrossRef]
- Kong, F.-M.S.; Moiseenko, V.; Zhao, J.; Milano, M.T.; Li, L.; Rimner, A.; Das, S.; Li, X.A.; Miften, M.; Liao, Z.; et al. Organs at Risk Considerations for Thoracic Stereotactic Body Radiation Therapy: What Is Safe for Lung Parenchyma? Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 172–187. [Google Scholar] [CrossRef]
- Diez, P.; Hanna, G.G.; Aitken, K.L.; van As, N.; Carver, A.; Colaco, R.J.; Conibear, J.; Dunne, E.M.; Eaton, D.J.; Franks, K.N.; et al. UK 2022 Consensus on Normal Tissue Dose-Volume Constraints for Oligometastatic, Primary Lung and Hepatocellular Carcinoma Stereotactic Ablative Radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2022, 34, 288–300. [Google Scholar] [CrossRef]
- Bucknell, N.W.; Hardcastle, N.; Bressel, M.; Hofman, M.S.; Kron, T.; Ball, D.; Siva, S. Functional Lung Imaging in Radiation Therapy for Lung Cancer: A Systematic Review and Meta-Analysis. Radiother. Oncol. 2018, 129, 196–208. [Google Scholar] [CrossRef]
- Lucia, F.; Rehn, M.; Blanc-Béguin, F.; Le Roux, P.-Y. Radiation Therapy Planning of Thoracic Tumors: A Review of Challenges Associated With Lung Toxicities and Potential Perspectives of Gallium-68 Lung PET/CT Imaging. Front. Med. 2021, 8, 723748. [Google Scholar] [CrossRef]
- Le Roux, P.-Y.; Siva, S.; Steinfort, D.P.; Callahan, J.; Eu, P.; Irving, L.B.; Hicks, R.J.; Hofman, M.S. Correlation of 68Ga Ventilation-Perfusion PET/CT with Pulmonary Function Test Indices for Assessing Lung Function. J. Nucl. Med. 2015, 56, 1718–1723. [Google Scholar] [CrossRef]
- Le Roux, P.-Y.; Robin, P.; Salaun, P.-Y. New Developments and Future Challenges of Nuclear Medicine and Molecular Imaging for Pulmonary Embolism. Thromb. Res. 2018, 163, 236–241. [Google Scholar] [CrossRef]
- Blanc-Béguin, F.; Hennebicq, S.; Robin, P.; Tripier, R.; Salaün, P.-Y.; Le Roux, P.-Y. Radiopharmaceutical Labelling for Lung Ventilation/Perfusion PET/CT Imaging: A Review of Production and Optimization Processes for Clinical Use. Pharmaceuticals 2022, 15, 518. [Google Scholar] [CrossRef]
- Le Roux, P.-Y.; Hicks, R.J.; Siva, S.; Hofman, M.S. PET/CT Lung Ventilation and Perfusion Scanning Using Galligas and Gallium-68-MAA. Semin. Nucl. Med. 2019, 49, 71–81. [Google Scholar] [CrossRef]
- Hicks, R.J.; Hofman, M.S. Is There Still a Role for SPECT-CT in Oncology in the PET-CT Era? Nat. Rev. Clin. Oncol. 2012, 9, 712–720. [Google Scholar] [CrossRef]
- Siva, S.; Devereux, T.; Ball, D.L.; MacManus, M.P.; Hardcastle, N.; Kron, T.; Bressel, M.; Foroudi, F.; Plumridge, N.; Steinfort, D.; et al. Ga-68 MAA Perfusion 4D-PET/CT Scanning Allows for Functional Lung Avoidance Using Conformal Radiation Therapy Planning. Technol. Cancer Res. Treat. 2016, 15, 114–121. [Google Scholar] [CrossRef]
- Noël, G.; Antoni, D. Organs at Risk Radiation Dose Constraints. Cancer Radiother. 2022, 26, 59–75. [Google Scholar] [CrossRef]
- Decramer, M.; Janssens, W.; Miravitlles, M. Chronic Obstructive Pulmonary Disease. Lancet 2012, 379, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yamazaki, H.; Kimoto, T.; Suzuki, G.; Yamada, K. Repeated Stereotactic Body Radiotherapy for Lung Malignancies: Toxicity Can Be Reduced by Sparing Lung Irradiation. Anticancer Res. 2022, 42, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Béguin, F.; Damien, P.; Floch, R.; Kerleguer, K.; Hennebicq, S.; Robin, P.; Salaün, P.-Y.; Le Roux, P.-Y. Radiation Exposure to Nuclear Medicine Technologists Performing a V/Q PET: Comparison with Conventional V/Q Scintigraphy, [18F]FDG PET and [68Ga]Ga DOTATOC PET Procedures. Front. Med. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
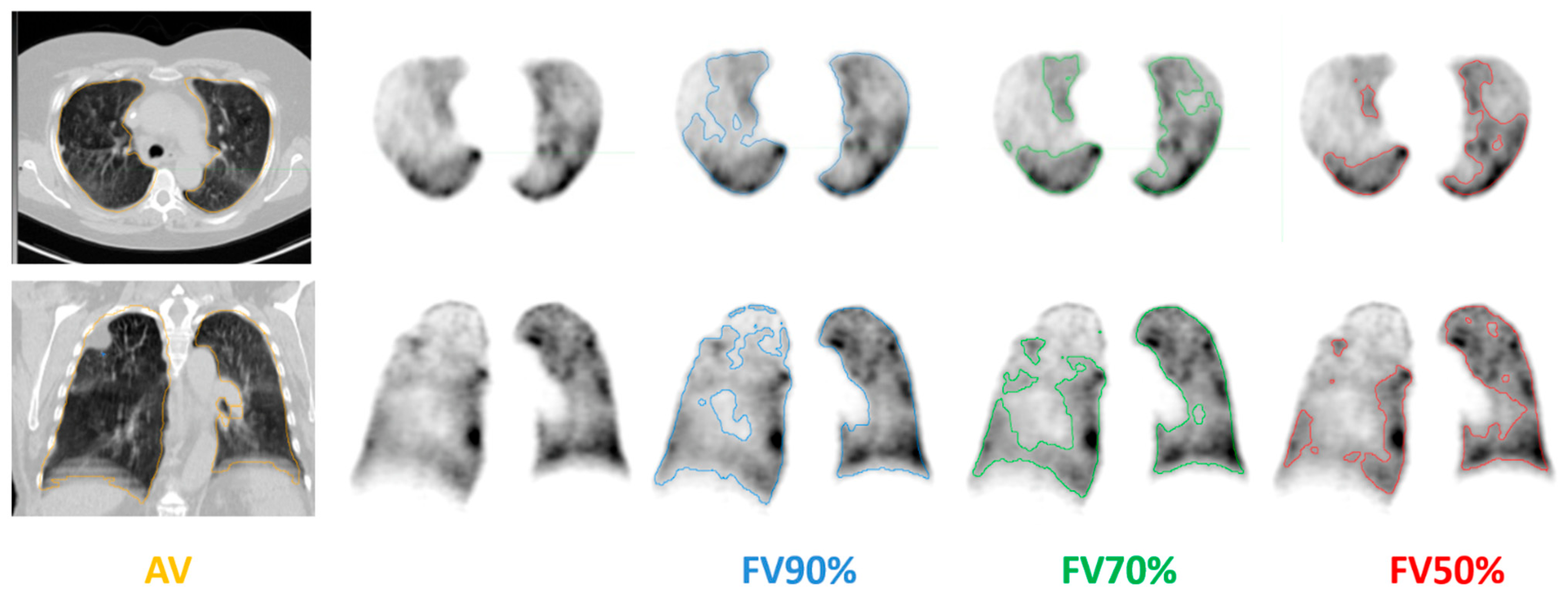

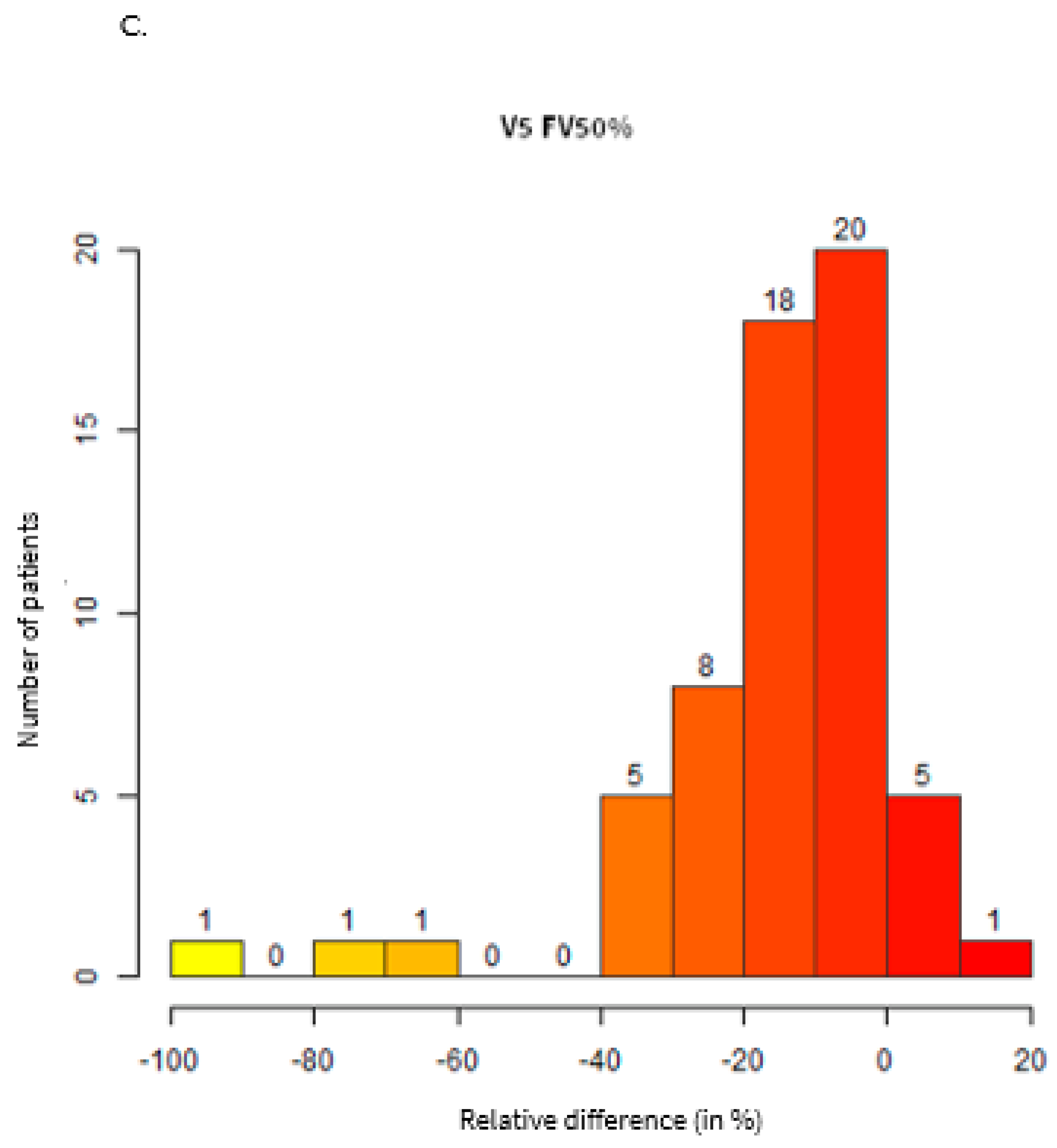
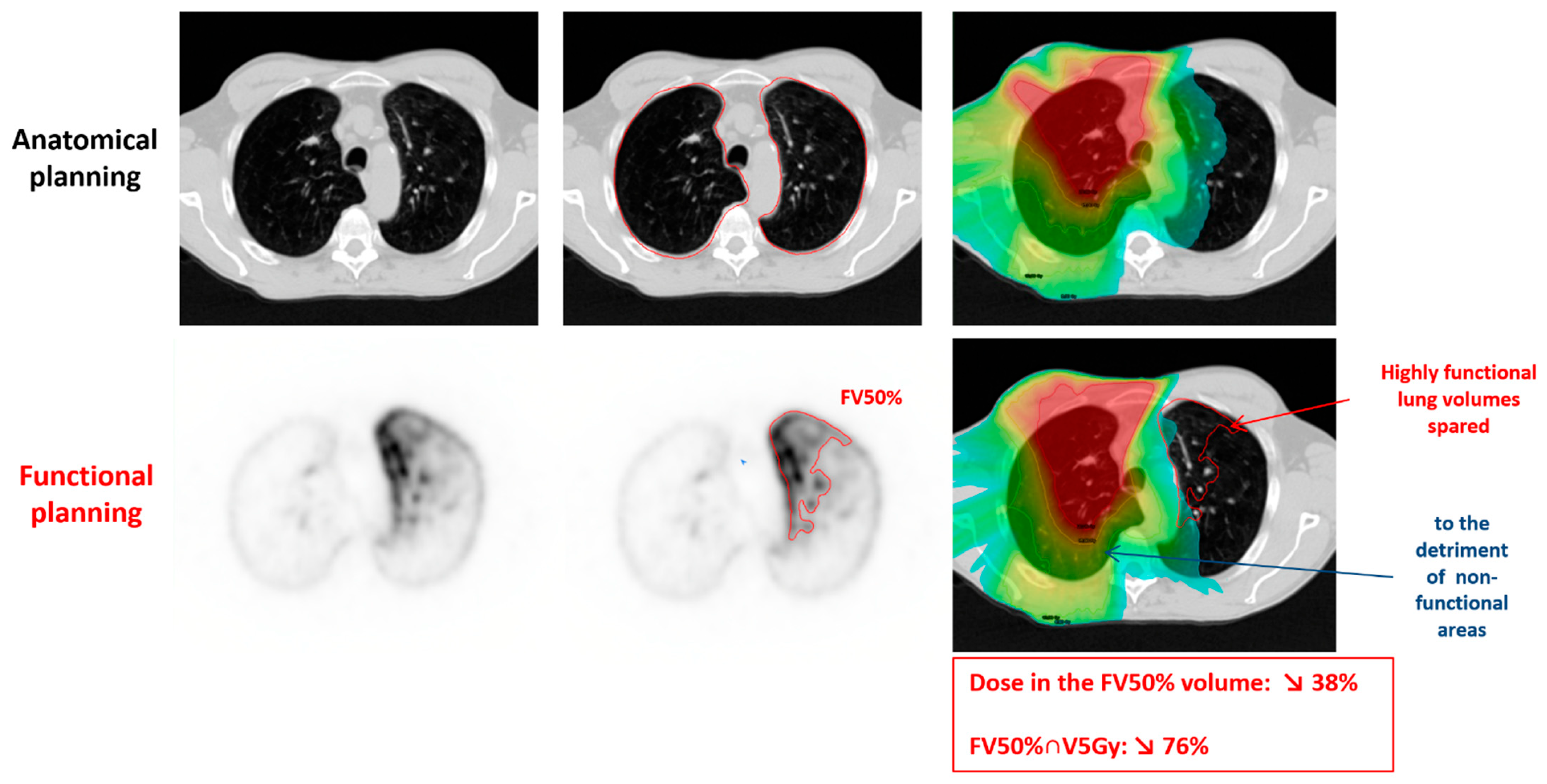
| Group | ||
|---|---|---|
| N = 60 | % | |
| Gender | ||
| 31 29 | 52 48 |
| Age median (range) | 69 (51–84) | |
| Histology | ||
| 25 23 12 | 42 38 20 |
| Current smokers | ||
| 19 41 | 32 68 |
| Current or previous smoker | ||
| 43 17 | 72 28 |
| Prior thoracic RT | ||
| 19 41 | 32 68 |
| Number of lesions | ||
| 51 9 | 85 15 |
| Prior thoracic surgery | ||
| 14 46 | 23 77 |
| PS | ||
| 27 25 8 | 45 42 13 |
| FEV1 median (range) | 66 (11–94) | |
| 10 | 17 |
| FEV1/FVC median (range) | 67 (24–100) | |
| 10 | |
| DLCO median (range) | 56 (9–119) | |
| 19 | 32 |
| ITV volume median (range) | 6.4 cc (1.0–83.4) | |
| PTV volume median (range) | 14.4 cc (3.2–139.7) | |
| Anatomical Plan | Functional Plan | Functional Plan Minus Anatomical Plan | Functional Plan Minus Anatomical Plan | Difference (p-Value) | |
|---|---|---|---|---|---|
| Absolute Difference | Relative Difference | ||||
| Median (Range) | Median (Range) | Median Gy (Range) | Median % (Range) | ||
| FV50% | 3.1 (0.2–12.7) | 3.0 (0.2–12.4) | −0.2 (−1.1 to 0.1) | −8.0 (−43.0 to 1.2) | <0.0001 |
| FV70% | 3.3 (0.3–10.7) | 3.0 (0.3–10.4) | −0.2 (−0.8 to 0.1) | −7.1 (−34.3 to 1.2) | <0.0001 |
| FV90% | 3.0 (0.6–9.9) | 2.8 (0.6–9.5) | −0.2 (−0.8 to 0.2) | −5.7 (−22.3 to 4.4) | <0.0001 |
| Anatomical | 2.7 (0.8–7.5) | 2.5 (0.7–7.2) | −0.1 (−0.5 to 0.3) | −4.7 (−15.8 to 8.5) | <0.0001 |
| Anatomical Plan | Functional Plan | Absolute Difference | Relative Difference | Difference (p-Value) | ||
|---|---|---|---|---|---|---|
| Median (Range) | Median (Range) | Median (Range) | Median % (Range) | |||
| FV50% | V5Gy | 16.1% (0.1% to 48.2%) | 14.0% (0% to 46.8%) | −1.4% (−8.4% to 4.5%) | −11.4% (−100% to 19.2%) | <0.0001 |
| V10Gy | 8.6% (0% to 41.2%) | 7.7% (0% to 40.0%) | −0.8% (−5.1%to 0.9%) | −12.5% (−99.9% to 6.5%) | <0.0001 | |
| V15Gy | 5.5% (0.0% to 30.5%) | 4.7% (0.0% to 29.5%) | −0.5% (−5.3% to 0.3%) | −10.6% (−90.4% to 7.5%) | <0.0001 | |
| V20Gy | 3.6% (0.0% to 22.6%) | 3.3% (0.0% to 21.8%) | −0.3% (−2.9% to 0.3%) | −9.2% (−100% to 9.2%) | <0.0001 | |
| FV70% | V5Gy | 15.2% (0.3% to 9.9%) | 13.5% (0.1% to 47.9%) | −1.3% (−6.6% to 3.6%) | −10.4% (−76.5% to 16.1%) | <0.0001 |
| V10Gy | 8.7%% (0% to 34.9%) | 7.5% (0% to 32.1%) | −0.7% (−4.1%to 0.5%) | −11.3% (−99.9% to 12.7%) | <0.0001 | |
| V15Gy | 5.7% (0.0% to 22.8%) | 4.9% (0.0% to 22.0%) | −0.4% (−4.4%to 0.1%) | −10.0% (−93.3% to 13.9%) | <0.0001 | |
| V20Gy | 3.6% (0.0% to 16.7%) | 3.3% (0.0% to 16.2%) | −0.2% (−2.1% to 0.3%) | −7.2% (−100% to 22.2%) | <0.0001 | |
| FV90% | V5Gy | 14.0% (1.2% to 49.2%) | 13.0% (1.0% to 46.7%) | −1.0 (−5.3% to 3.4%) | −7.9% (−42.8% to 16.6%) | <0.0001 |
| V10Gy | 8.5% (0.5% to 32.0%) | 7.5% (0.3% to 29.7%) | −0.6% (−3.7%to 0.5%) | −8.0% (−37.8%to 5.9%) | <0.0001 | |
| V15Gy | 5.7% (0.2% to 20.9%) | 4.9% (0.2% to 19.8%) | −0.4% (−3.2%to 0.3%) | −7.7% (−41.8% to 11.5%) | <0.0001 | |
| V20Gy | 3.5% (0.2% to 15.0%) | 3.2% (0.1% to 14.4%) | −0.1% (−1.5% to 0.2%) | −5.3% (−38.8% to 13.3%) | <0.0001 | |
| AV | V5Gy | 13.6% (3.5% to 43.0%) | 12.2% (2.8% to 40.7%) | −0.7 (−4.7% to 3.4%) | −6.0% (−23.9% to 18.0%) | <0.0001 |
| V10Gy | 7.6% (1.9% to 27.1%) | 7.0% (1.9% to 25.5%) | −0.5% (−3.0% to 0.7%) | −6.4% (−31.0% to 6.6%) | <0.0001 | |
| V15Gy | 4.7% (1% to 17.5%) | 4.3% (1.0% to 16.8%) | −0.3% (−2.2% to 0.4%) | −5.1% (−41.2% to 11.2%) | <0.0001 | |
| V20Gy | 3.1% (0.7% to 12.1%) | 3.0% (0.6% to 11.7%) | −0.1% (−1.3% to 0.5%) | −2.7% (−25.5% to 13.3%) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucia, F.; Hamya, M.; Pinot, F.; Goasduff, G.; Blanc-Béguin, F.; Bourhis, D.; Pradier, O.; Lucia, A.-S.; Hennebicq, S.; Mauguen, M.; et al. A Feasibility Study of Functional Lung Volume Preservation during Stereotactic Body Radiotherapy Guided by Gallium-68 Perfusion PET/CT. Cancers 2023, 15, 1726. https://doi.org/10.3390/cancers15061726
Lucia F, Hamya M, Pinot F, Goasduff G, Blanc-Béguin F, Bourhis D, Pradier O, Lucia A-S, Hennebicq S, Mauguen M, et al. A Feasibility Study of Functional Lung Volume Preservation during Stereotactic Body Radiotherapy Guided by Gallium-68 Perfusion PET/CT. Cancers. 2023; 15(6):1726. https://doi.org/10.3390/cancers15061726
Chicago/Turabian StyleLucia, François, Mohamed Hamya, Fanny Pinot, Gaëlle Goasduff, Frédérique Blanc-Béguin, David Bourhis, Olivier Pradier, Anne-Sophie Lucia, Simon Hennebicq, Maëlle Mauguen, and et al. 2023. "A Feasibility Study of Functional Lung Volume Preservation during Stereotactic Body Radiotherapy Guided by Gallium-68 Perfusion PET/CT" Cancers 15, no. 6: 1726. https://doi.org/10.3390/cancers15061726
APA StyleLucia, F., Hamya, M., Pinot, F., Goasduff, G., Blanc-Béguin, F., Bourhis, D., Pradier, O., Lucia, A.-S., Hennebicq, S., Mauguen, M., Floch, R., Schick, U., Bourbonne, V., Salaün, P.-Y., & Le Roux, P.-Y. (2023). A Feasibility Study of Functional Lung Volume Preservation during Stereotactic Body Radiotherapy Guided by Gallium-68 Perfusion PET/CT. Cancers, 15(6), 1726. https://doi.org/10.3390/cancers15061726





