Clinical Features and Outcomes of Patients with Pancreaticobiliary Malignancies in Los Angeles County and Their Association with CA 19-9 Levels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Variables and Primary Outcome
2.3. CA 19-9 Assays and Patient Stratification Based on CA 19-9 Level
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
3.2. Oncologic Profile of the Study Population
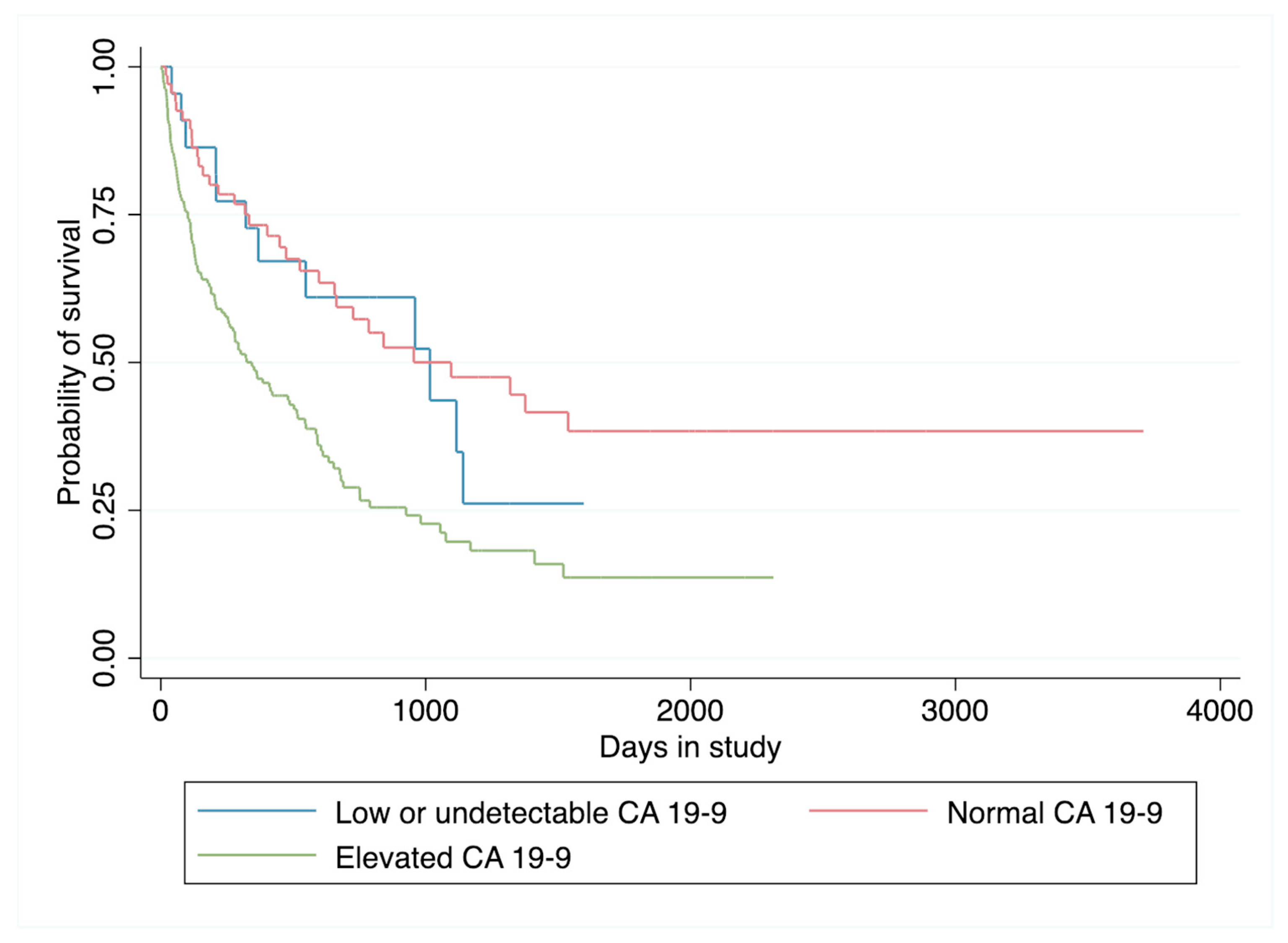
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer | Surveillance, Epidemiology, and End Results Program. 2021. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 26 December 2022).
- Cheng, H.; Luo, G.; Lu, Y.; Jin, K.; Guo, M.; Xu, J.; Long, J.; Liu, L.; Yu, X.; Liu, C. The combination of systemic inflammation-based marker nlr and circulating regulatory t cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology 2016, 16, 1080–1084. [Google Scholar] [CrossRef]
- Sohal, D.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Philip, P.A.; O’Reilly, E.M.; Uronis, H.E.; Ramanathan, R.K.; Crane, C.H.; Engebretson, A.; et al. Metastatic pancreatic cancer: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2016, 34, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Rosen, A.; Linder, S.; Harmenberg, U.; Pegert, S. Serum levels of CA 19–9 and CA50 in relation to Lewis blood cell status in patients with malignant and benign pancreatic disease. Pancreas 1993, 8, 160–165. [Google Scholar] [CrossRef]
- Molina, R.; Ojeda, B.; Filella, X.; Borras, G.; Jo, J.; Mas, E.; Lopez, J.J.; Ballesta, A. A prospective study of tumor markers CA 125 and CA 19.9 in patients with epithelial ovarian carcinomas. Tumour Biol. 1992, 13, 278–286. [Google Scholar] [CrossRef]
- Cherchi, P.L.; Dessole, S.; Ruiu, G.A.; Ambrosini, G.; Farina, M.; Capobianco, G.; Ambrosini, A. The value of serum CA 125 and association CA 125/CA 19–9 in endometrial carcinoma. Eur. J. Gynaecol. Oncol. 1999, 20, 315–317. [Google Scholar]
- Molina, R.; Auge, J.M.; Escudero, J.M.; Marrades, R.; Viñolas, N.; Carcereny, E.; Carcereny, E.; Ramirez, J.; Filella, X. Mucins CA 125, CA 19.9, CA 15.3 and TAG-72.3 as tumor markers in patients with lung cancer: Comparison with CYFRA 21–1, CEA, SCC and NSE. Tumour Biol. 2008, 29, 371–380. [Google Scholar] [CrossRef]
- Duffy, M.J.; Sturgeon, C.; Lamerz, R.; Haglund, C.; Holubec, V.L.; Klapdor, R.; Nicolini, A.; Topolcan, O.; Heinemann, V. Tumor markers in pancreatic cancer: A European Group on Tumor Markers (EGTM) status report. Ann. Oncol. 2010, 21, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based approach. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef]
- Furuya, N.; Kawa, S.; Hasebe, O.; Tokoo, M.; Mukawa, K.; Maejima, S.; Oguchi, H. Comparative study of CA 242 and CA19–9 in chronic pancreatitis. Br. J. Cancer 1996, 73, 372–376. [Google Scholar] [CrossRef]
- Barone, D.; Onetto, M.; Conio, M.; Paganuzzi, M.; Saccomanno, S.; Aste, H.; Pugliese, V. CA 19–9 assay in patients with extrahepatic cholestatic jaundice. Int. J. Biol. Markers 1988, 3, 95–100. [Google Scholar] [CrossRef]
- Peterli, R.; Meyer-Wyss, B.; Herzoq, U.; Tondelli, P. CA 19–9 has no value as a tumor marker in obstructive jaundice. Schweiz. Med. Wochenschr. 1999, 129, 77–79. [Google Scholar]
- Akdoğan, M.; Saşmaz, N.; Kayhan, B.; Biyikoğlu, I.; Dişibeyaz, S.; Sahin, B. Extraordinarily elevated CA 19–9 in benign conditions: A case report and review of the literature. Tumori J. 2001, 87, 337–339. [Google Scholar] [CrossRef]
- Vestergaard, E.M.; Hein, H.O.; Meyer, H.; Grunnet, N.; Jorgensen, J.; Wolf, H.; Orntoft, T.F. Reference values and biological variation for tumor marker CA 19–9 in serum for different Lewis and secretor genotypes and evaluation of secretor and Lewis genotyping in a Caucasian population. Clin Chem. 1999, 45, 54–61. [Google Scholar]
- Henrikson, N.B.; Bowles, E.J.A.; Blasi, P.R.; Morrison, C.C.; Nguyen, M.; Pillarisetty, V.G.; Lin, J.S. Screening for Pancreatic Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2019. [Google Scholar]
- Parra, -R.M.; Santos, V.M.; Canis, S.M.; Pla, X.F.; Fradera, J.M.A.; Porto, R.F. Relationship between CA 19.9 and the Lewis Phenotype: Options to Improve Diagnostic Efficiency. Anticancer Res. 2018, 38, 5883–5888. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The clinical utility of CA 19–9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, J.-C.; Yang, S.-H.; Tien, Y.-W.; Kuo, S.-H. Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer. J. Clin. Med. 2019, 8, 1115. [Google Scholar] [CrossRef]
- Motoi, F.; Murakami, Y.; Okada, K.I.; Matsumoto, I.; Uemura, K.; Satoi, S.; Sho, M.; Honda, G.; Fukumonto, T.; Yanagimoto, H.; et al. Sustained Elevation of Postoperative Serum Level of Carbohydrate Antigen 19–9 is High-Risk Stigmata for Primary Hepatic Recurrence in Patients with Curatively Resected Pancreatic Adenocarcinoma. World J. Surg. 2019, 43, 634–641. [Google Scholar] [CrossRef]
- Rieser, C.J.; Zenati, M.; Hamad, A.; Abbas, A.I.A.; Bahary, N.; Zureikat, A.H.; Zeh, H.J.; Hogg, M.E. CA19–9 on Postoperative Surveillance in Pancreatic Ductal Adenocarcinoma: Predicting Recurrence and Changing Prognosis over Time. Ann. Surg. Oncol. 2018, 25, 3483–3491. [Google Scholar] [CrossRef]
- George, B.; Kent, M.; Surinach, A.; Lamarre, N.; Cockrum, P. The Association of Real-World CA 19–9 Level Monitoring Patterns and Clinical Outcomes Among Patients With Metastatic Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2021, 11, 754687. [Google Scholar] [CrossRef]
- Shin, K.; Jung, E.K.; Park, S.J.; Jeong, S.; Kim, I.H.; Lee, M.A. Neutrophil-to-lymphocyte ratio and carbohydrate antigen 19–9 as prognostic markers for advanced pancreatic cancer patients receiving first-line chemotherapy. World J. Gastrointest. Oncol. 2021, 13, 915–928. [Google Scholar] [CrossRef]
- Tomishima, K.; Ishii, S.; Fujisawa, T.; Ikemura, M.; Ota, H.; Kabemura, D.; Ushio, M.; Fukuma, T.; Takahashi, S.; Yamagata, W.; et al. Duration of Reduced CA19–9 Levels Is a Better Prognostic Factor Than Its Rate of Reduction for Unresectable Locally Advanced Pancreatic Cancer. Cancers 2021, 13, 4224. [Google Scholar] [CrossRef]
- Duraker, N.; Hot, S.; Polat, Y.; Höbek, A.; Gençler, N.; Urhan, N. CEA, CA 19–9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J. Surg. Oncol. 2007, 95, 142–147. [Google Scholar] [CrossRef]
- Safi, F.; Roscher, R.; Bittner, R.; Schenkluhn, B.; Dopfer, H.P.; Beger, H.G. High sensitivity and specificity of CA 19–9 for pancreatic carcinoma in comparison to chronic pancreatitis. Serological and immunohistochemical findings. Pancreas 1987, 2, 398–403. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Lowenfels, A.B. Epidemiology of pancreatic cancer: An update. Dig. Dis. 2010, 28, 645–656. [Google Scholar] [CrossRef]
- Li, J.; Merl, M.Y.; Chabot, J.; Saif, M.W. Updates of adjuvant therapy in pancreatic cancer: Where are we and where are we going? JOP 2010, 11, 310–312. [Google Scholar] [PubMed]
- Koprowski, H.; Steplewsk, Z.; Mitchell, K.; Herlyn, M.; Herlyn, D.; Fuhrer, P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somat. Cell Genet. 1979, 5, 957–971. [Google Scholar] [CrossRef]
- Del Villano, B.C.; Brennan, S.; Brock, P.; Bucher, C.; Liu, V.; McClure, M.; Rake, B.; Space, S.; Westrick, B.; Schoemaker, H.; et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19–9. Clin. Chem. 1983, 29, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.R.; Finkelstein, D.M.; Thayer, S.P.; Muzikansky, A.; Fernandez-del Castillo, C.; Warshaw, A.L. Perioperative CA19–9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2006, 24, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.; Gocmen, E.; Tez, M.; Ertan, T.; Keskek, M.; Koc, M. Value of preoperative serum CA 19–9 levels in predicting resectability for pancreatic cancer. Can. J. Surg. 2006, 49, 241–244. [Google Scholar]
- Maithel, S.K.; Maloney, S.; Winston, C.; Gonen, M.; D’Angelica, M.I.; Dematteo, R.P.; Jarnagin, W.R.; Brennan, M.F.; Allen, P.J. Preoperative CA 19–9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann. Surg. Oncol. 2008, 15, 3512–3520. [Google Scholar] [CrossRef]
- Halm, U.; Schumann, T.; Schiefke, I.; Witzigmann, H.; Mossner, J.; Keim, V. Decrease of CA 19–9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br. J. Cancer 2000, 82, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Reni, M.; Cereda, S.; Balzano, G.; Passoni, P.; Rognone, A.; Fugazza, C.; Mazza, E.; Zerbi, A.; Carlo, V.D.; Villa, E. Carbohydrate antigen 19–9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer 2009, 115, 2630–2639. [Google Scholar] [CrossRef]
- Kondo, N.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Sudo, T.; Hashimoto, Y.; Nakashima, A.; Sakabe, R.; Shigemoto, N.; Kato, Y.; et al. Prognostic impact of perioperative serum CA 19–9 levels in patients with resectable pancreatic cancer. Ann. Surg. Oncol. 2010, 17, 2321–2329. [Google Scholar] [CrossRef]
- Nakao, A.; Oshima, K.; Nomoto, S.; Takeda, S.; Kaneko, T.; Ichihara, T.; Kurokawa, T.; Nonami, T.; Takagi, H. Clinical usefulness of CA-19–9 in pancreatic carcinoma. Semin. Surg Oncol. 1998, 15, 15–22. [Google Scholar] [CrossRef]
- Waraya, M.; Yamashita, K.; Katagiri, H.; Ishii, K.; Takahashi, Y.; Furuta, K.; Watanabe, M. Preoperative serum CA19–9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Ann. Surg. Oncol. 2009, 16, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.G.; Bois, J.P.; Sarr, M.G.; Wood, M.; Qin, R.; Thomsen, K.M.; Kendrick, M.L.; Farnell, M.B. Predictive and prognostic value of CA 19–9 in resected pancreatic adenocarcinoma. J. Gastrointest. Surg. 2009, 13, 2050–2058. [Google Scholar] [CrossRef]
- Berger, A.C.; Garcia, M., Jr.; Hoffman, J.P.; Regine, W.F.; Abrams, R.A.; Safran, H.; Konski, A.; Benson, A.B., 3rd; MacDonald, J.; Willet, C.G. Postresection CA 19–9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: A prospective validation by RTOG 9704. J. Clin. Oncol. 2008, 26, 5918–5922. [Google Scholar] [CrossRef]
- Hata, S.; Sakamoto, Y.; Yamamoto, Y.; Nara, S.; Esaki, M.; Shimada, K.; Kosuge, T. Prognostic impact of postoperative serum CA 19–9 levels in patients with resectable pancreatic cancer. Ann. Surg. Oncol. 2011, 19, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.H.; Myung, S.J.; Lim, B.C.; Park, E.T.; Yoo, K.S.; Seo, D.W.; Lee, S.K.; Min, Y.I. A new strategy for the application of CA19–9 in the differentiation of pancreaticobiliary cancer: Analysis using a receiver operating characteristic curve. Am. J. Gastroenterol. 1999, 94, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19–9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.C.; Meszoely, I.M.; Ross, E.A.; Watson, J.C.; Hoffman, J.P. Undetectable Preoperative Levels of Serum CA 19–9 Correlate with Improved Survival for Patients with Resectable Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2004, 11, 644–649. [Google Scholar] [CrossRef]
- Herremans, K.M.; Riner, A.N.; Winn, R.A.; Trevino, J.G. Diversity and Inclusion in Pancreatic Cancer Clinic Trials. Gastroenterology 2021, 161, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
| ICD-10 Code | Corresponding Disease Classification |
|---|---|
| C22.1 | Intrahepatic bile duct carcinoma |
| C23 | Malignant neoplasm of gallbladder |
| C25 | Malignant neoplasm of pancreas |
| C25.0 | Malignant neoplasm of head of pancreas |
| C25.1 | Malignant neoplasm of the body of the pancreas |
| C25.2 | Malignant neoplasm of tail of pancreas |
| C25.3 | Malignant neoplasm of the pancreatic duct |
| C25.4 | Malignant neoplasm of endocrine pancreas |
| C25.7 | Malignant neoplasm of other parts of pancreas |
| C25.8 | Malignant neoplasm of overlapping sites of pancreas |
| C25.9 | Malignant neoplasm of pancreas, unspecified |
| D01.5 | Carcinoma in situ of liver, gallbladder, and bile ducts |
| Patients with Low CA 19-9 (n = 23) | Patients with Normal CA 19-9 (n = 70) | Patients with Elevated CA 19-9 (n = 190) | |
|---|---|---|---|
| Age in years at diagnosis, median (IQR) | 60 (52–69) | 62 (53–70) | 61 (55–66) |
| Male, n (%) | 9 (39.1%) | 30 (42.9%) | 92 (48.4%) |
| BMI, median (IQR) | 26.0 (23.2–30.6) | 25.3 (22.4–28.7) | 25.4 (22.2–29.9) |
| Race, n (%) | |||
| Caucasian | 3 (13.0%) | 5 (7.1%) | 31 (16.4%) |
| Hispanic | 15 (65.2%) | 54 (77.1%) | 102 (54.0%) |
| Asian | 2 (8.7%) | 4 (5.7%) | 21 (11.1%) |
| African American | 2 (8.7%) | 4 (5.7%) | 15 (7.9%) |
| Middle Eastern/North African | 0 (0.0%) | 1 (1.4%) | 2 (1.1%) |
| Other | 1 (4.4%) | 2 (2.9%) | 18 (9.5%) |
| Comorbidities | |||
| None | 6 (26.1%) | 23 (32.9%) | 54 (28.4%) |
| Diabetes | 9 (39.1%) | 28 (40.0%) | 78 (41.1%) |
| Hypertension | 9 (39.1%) | 31 (44.3%) | 93 (49.0%) |
| Congestive heart failure | 0 (0.0%) | 3 (4.3%) | 8 (4.2%) |
| Coronary artery disease | 1 (4.4%) | 5 (7.1%) | 11 (5.8%) |
| COPD | 0 (0.0%) | 0 (0.0%) | 8 (4.2%) |
| Cerebrovascular accident | 0 (0.0%) | 1 (1.4%) | 8 (4.2%) |
| History of other cancer, n (%) | |||
| None | 21 (91.3%) | 61 (88.4%) | 175 (92.1%) |
| Non-GI | 2 (8.7%) | 5 (7.2%) | 12 (6.3%) |
| GI | 0 (0.0%) | 4 (5.8%) | 3 (1.6%) |
| Smoking history, n (%) | |||
| Never | 19 (82.6%) | 50 (72.5%) | 113 (60.1%) |
| Former | 1 (4.4%) | 14 (20.3%) | 47 (25.0%) |
| Current | 3 (13.0%) | 5 (7.3%) | 28 (14.9%) |
| Patients with Low CA 19-9 (n = 23) | Patients with Detectable but Normal CA 19-9 (n = 70) | Patients with Elevated CA 19-9 (n = 190) | p-Value | |
|---|---|---|---|---|
| CA 19-9 level at diagnosis, median (IQR) | 2.0 (2.0–3.0) | 16.5 (9.0–22.0) | 532.5 (147.0–3469.0) | 0.595 |
| CEA level at diagnosis, median (IQR) | 4.9 (2.8–6.6) | 2.0 (1.4–5.1) | 4.1 (1.9–17.8) | 0.508 |
| CA 125 level at diagnosis, median (IQR) | 30.6 (6.1–55.0) | 26.7 (10.4–48.9) | 61.3 (20.9–219.0) | 0.646 |
| Total bilirubin at diagnosis, median (IQR) | 0.6 (0.5–4.0) | 1.0 (0.6–2.1) | 1.7 (0.8–8.6) | 0.002 |
| Organ system with malignancy, n (%) | 0.054 | |||
| Cholangiocarcinoma | 5 (21.7%) | 18 (25.7%) | 46 (24.2%) | |
| Gallbladder adenocarcinoma | 4 (17.4%) | 13 (18.6%) | 13 (6.8%) | |
| Pancreatic adenocarcinoma | 14 (60.9%) | 39 (55.7%) | 131 (69.0%) | |
| Deceased by end of study period, n (%) | 12 (52.2%) | 32 (45.7%) | 120 (63.2%) | 0.035 |
| Survival time, median * | 1016 | 1096 | 344 | - |
| Follow-up time, median ** | 815 | 1243 | 662 | - |
| ECOG at time of diagnosis, n (%) | 0.236 | |||
| 0 | 9 (40.9%) | 45 (64.3%) | 90 (48.7%) | |
| 1 | 7 (31.8%) | 17 (24.3%) | 54 (29.2%) | |
| 2 | 4 (18.2%) | 5 (7.1%) | 17 (9.2%) | |
| 3 | 2 (9.1%) | 2 (2.9%) | 22 (11.9%) | |
| 4 | 0 (0.0%) | 1 (1.4%) | 2 (1.1%) | |
| Stage at time of diagnosis, n (%) | 0.017 | |||
| I | 4 (17.4%) | 19 (27.1%) | 21 (11.1%) | |
| II | 4 (17.4%) | 17 (24.3%) | 34 (17.9%) | |
| III | 3 (13.0%) | 6 (8.6%) | 39 (20.5%) | |
| IV | 12 (52.2%) | 28 (40.0%) | 96 (50.5%) | |
| Underwent curative treatment, n (%) | 8 (34.8%) | 38 (54.3%) | 44 (23.2%) | <0.001 |
| Type of treatment, n (%) | <0.001 | |||
| Chemotherapy | 12 (52.2%) | 20 (28.6%) | 92 (48.4%) | |
| Surgery | 3 (13.0%) | 16 (22.9%) | 14 (7.4%) | |
| Chemotherapy and surgery | 5 (21.7%) | 26 (37.1%) | 36 (19.0%) | |
| Hospice | 3 (13.0%) | 6 (8.6%) | 43 (22.6%) | |
| None or lost to follow-up | 0 (0.0%) | 2 (2.9%) | 5 (2.6%) |
| Predictor | Hazard Ratio | p-Value | 95% Confidence Interval |
|---|---|---|---|
| CA 19-9 level | |||
| Undetectable (reference) | - | - | - |
| Normal | 1.254 | 0.510 | 0.640–2.458 |
| Elevated | 1.993 | 0.025 * | 1.089–3.648 |
| Male | 0.973 | 0.866 | 0.709–1.336 |
| Age | 1.016 | 0.052 | 1.000–1.033 |
| BMI | 1.001 | 0.923 | 0.977–1.026 |
| Evidence of metastases at time of diagnosis | 1.815 | 0.002 * | 1.252–2.629 |
| Underwent curative treatment | 0.213 | <0.001 * | 0.127–0.356 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, J.; Trieu, H.; Kaleka, G.; Turkiewicz, J.; Palmer, S.; Lee, J.M.; Chen, K.T.; Tabibian, J.H. Clinical Features and Outcomes of Patients with Pancreaticobiliary Malignancies in Los Angeles County and Their Association with CA 19-9 Levels. Cancers 2023, 15, 1723. https://doi.org/10.3390/cancers15061723
Law J, Trieu H, Kaleka G, Turkiewicz J, Palmer S, Lee JM, Chen KT, Tabibian JH. Clinical Features and Outcomes of Patients with Pancreaticobiliary Malignancies in Los Angeles County and Their Association with CA 19-9 Levels. Cancers. 2023; 15(6):1723. https://doi.org/10.3390/cancers15061723
Chicago/Turabian StyleLaw, Jade, Harry Trieu, Guneet Kaleka, Joanna Turkiewicz, Samantha Palmer, Jennifer M. Lee, Kathryn T. Chen, and James H. Tabibian. 2023. "Clinical Features and Outcomes of Patients with Pancreaticobiliary Malignancies in Los Angeles County and Their Association with CA 19-9 Levels" Cancers 15, no. 6: 1723. https://doi.org/10.3390/cancers15061723
APA StyleLaw, J., Trieu, H., Kaleka, G., Turkiewicz, J., Palmer, S., Lee, J. M., Chen, K. T., & Tabibian, J. H. (2023). Clinical Features and Outcomes of Patients with Pancreaticobiliary Malignancies in Los Angeles County and Their Association with CA 19-9 Levels. Cancers, 15(6), 1723. https://doi.org/10.3390/cancers15061723







