Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
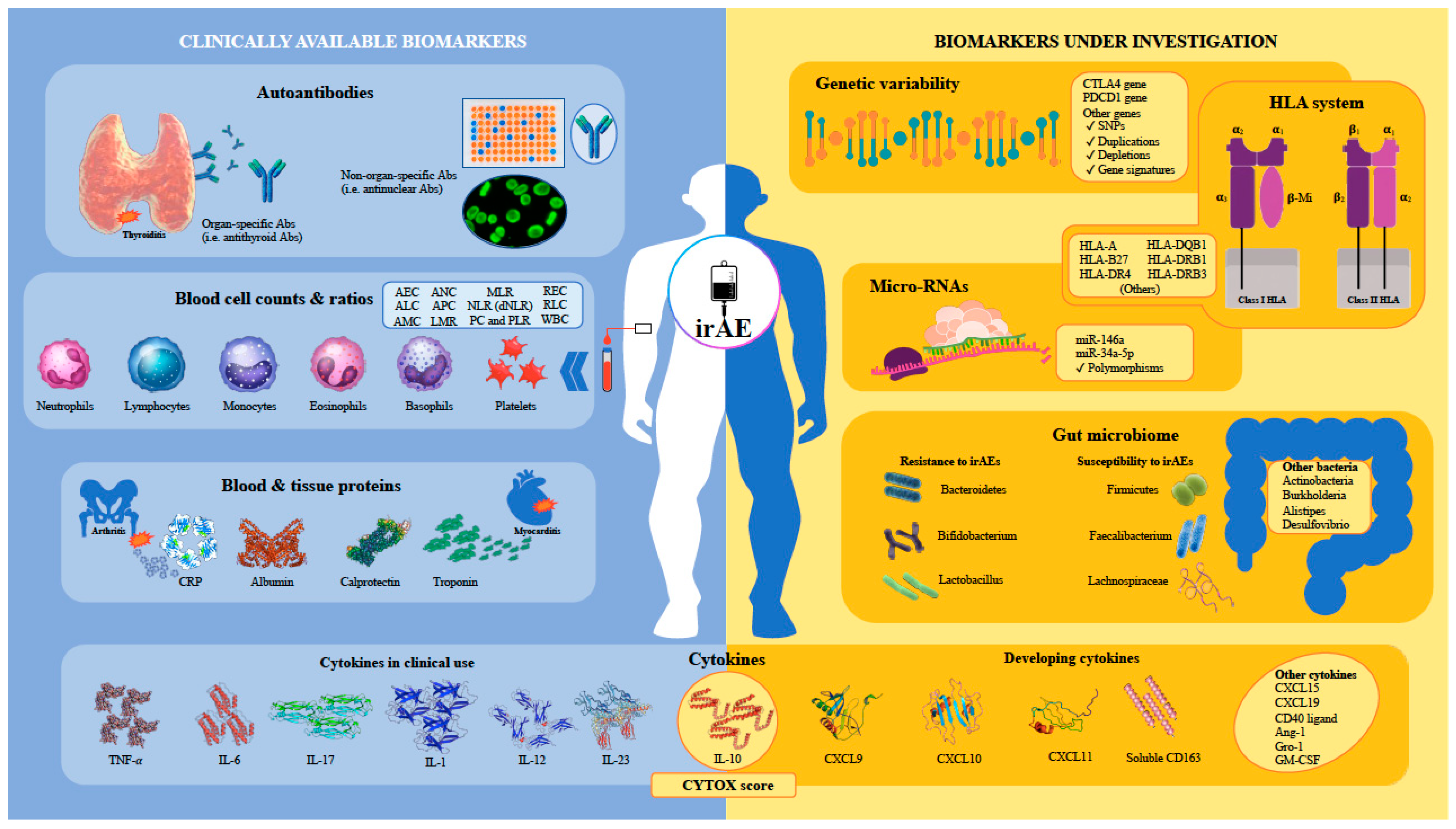
3.1. Biomarkers Available for Clinical Use
3.1.1. Autoantibodies
3.1.2. Blood Cell Counts and Ratios
3.1.3. Serum and Other Biological Fluid Proteins
3.1.4. Cytokine Profiles and Dynamics
3.2. Biomarkers under Investigation
3.2.1. Other Cytokines and Serum Proteins under Development
3.2.2. Genetic Variations and Gene Expression Profiling
3.2.3. Human Leucocyte Antigen Genotyping
3.2.4. Micro-RNAs
| Genetic Variants and Gene Expression Profiles | |||||
|---|---|---|---|---|---|
| Reference | Study Design (No. Patients) | Type of Tumor | Type of irAE | Associations | |
| Wölffer M. [131] | Prospective (n = 95) | Melanoma | All types | VARs on SMAD3 gene | Pancreatitis |
| CNVs on IL1RN and deletions on PRDM1 genes | Higher risk of irAEs | ||||
| Duplications on CD274 and CNVs on SLCO1B1 genes | Hepatitis | ||||
| CNVs on PRDM1 and CD274 genes | Encephalitis | ||||
| CNVs on PRDM1, CD274, TSHR and FAN1 genes | Myositis | ||||
| Abdel-Wahab N. [132] | Retrospective | Melanoma | All types | Several SNPs on GABRP, DSC2, BAZ2B, SEMA5A, OSBPL6, AGPS and LOC102724355, and near CFAP65 and LOC100129175 genes | Higher risk of irAEs |
| (n = 89) | Several SNPs on LOC105377125, RGMA, ANKRD42, PACRG, FAR2, LOC105374140, ROBO1, GLIS3, PVT1, PACRG and PREX2 genes | Lower risk of irAEs | |||
| Refae S. [133] | Retrospective (n = 94) | Pan-tumor | All types | Several SNPs on UNG, IFNW1, PD-L1, IFBL4 and CTLA-4 genes | Higher risk of irAEs |
| Jin Y. [134] | Retrospective (n = 46) | Gastric cancer | All types | Alterations in CEBPA, FGFR4, MET or KMT2B genes # | Higher risk of irAEs |
| Bins S. [135] | Retrospective (n = 322) | NSCLC | All types | Homozygous 804C > T (rs2227981) SNP on PDCD1 gene | Lower risk of any grade of irAEs |
| Kobayashi M. [136] | Retrospective (n = 106) | Renal cell cancer | All types | PD-1.6 SNP (G allele) on PDCD1 gene (rs10204525) | Higher risk of severe and multiple irAEs |
| Khan Z. [137] | Retrospective (n = 479) | Bladder cancer | Skin irAEs | Genetic variants related to vitiligo and psoriasis, assessed by a polygenic risk score | Higher risk of irAEs and better survival |
| Khan Z. [138] | Retrospective (n = 6075) | Pan-tumor | Thyroid dysfunction | Genetic variants related to autoimmune hypothyroidism, assessed by a polygenic risk score | Higher risk of irAEs and better survival |
| Friedlander P. [139] | Prospective (n = 150) | Melanoma | Diarrhea/colitis | Gene signature composed of 16 inflammation-related genes (CARD12, CCL3, CCR3, CXCL1, F5, FAM210B, GADD45A, IL18bp, IL2RA, IL5, IL8, MMP9, PTGS2, SOCS3, TLR9, UBE2C) | Differentiation between grade 0–1 and grade 2–4 diarrhea |
| Sahabi V. [140] | Prospective (n = 162) | Melanoma | Gastrointestinal irAEs | Increase in expression of CD177, CEACAM1 and immunoglobulin-related genes (IGHA1, IGHA2, IGHG1, and IGHV4–31) | Higher risk of gastrointestinal irAEs |
| Finke D. [141] | Retrospective (n = 19) | All types | Myocarditis | Upregulation of 3784 genes with overexpression of interferon-γ and inflammasome-regulating proteins (GBP5 and 6) | Higher risk of myocarditis |
| Adam BA. [142] | Retrospective (n = 75) * | All types | AIN | Overexpression of IFI27 gene (related to interferon-α) | Discrimination between AIN and TCMR |
| Zhang Y. [143] | Preclinical study (n not applicable) | Not applicable | Thyroid dysfunction | Overexpression of ALB, MAPK1, SPP1, PPARG and MIF genes | Hypothyroidism |
| Overexpression of ALB, FCGR2B, CD44, LCN2, and CD74 genes | Hyperthyroidism | ||||
| Jing Y. [144] | Retrospective (n = 18,706) | Pan-tumor (26 types) | General irAEs | Overexpression of LCP1 and ADPGK genes | Higher risk of irAEs |
| HLA Antigens | |||||
| Reference | Study Design (No. Patients) | Type of Tumor | Type of irAE | Associations | |
| Kobayashi T. [161] | Retrospective (n = 62) | All types | Endocrine irAEs | HLA-Cw12, HLA DR-15, HLA-DQ7 and HLA DPw9 | ACTH deficiency |
| HLA-Cw12 and HLA-DR15 | Hypophysitis | ||||
| HLA-DRB3*01:01 | Thrombocytopenia | ||||
| Jiang N. [163] | Retrospective (n = 530) | Pan-tumor | All types | HLA-DPB1*04:02 | Hypokalemia, hyponatremia, leukopenia and anemia |
| HLA-A*26:01 | Hyperbilirubinemia | ||||
| Capelli LC. [159] | Retrospective (n = 26) | Pan-tumor | Articular irAEs | HLA-DRB1*04:05 | Inflammatory arthritis |
| Correale P. [166] | Retrospective (n = 256, 29 with pneumonitis) | Pan-tumor | Lung irAEs | HLA-B*35 and HLA-DRB1*11 | Pneumonitis |
| Wölffer M. [131] | Prospective (n = 95) | Melanoma | All types | HLA class I homozygosity | Hepatitis |
| Stamatouli AM. [154] | Retrospective (n = 27) | Pan-tumor | Endocrine irAEs | HLA-DR4 | T1DM |
| Lo Preiato V. [155] | Retrospective ** (n =200) | Pan-tumor | All types | HLA-DR4 | T1DM |
| Inaba H. [167] | Retrospective (n = 25) | Pan-tumor | All types | HLA-B*46:01, HLA-C*14:02, HLA-DPA1*0103 and HLA-DPB1*02:01 | Higher risk of thyroid dysfunction |
| HLA-DPB1*05:01 | Lower risk of thyroid dysfunction | ||||
| Inaba H. [168] | Retrospective (n = 871, 7 with T1DM) | Pan-tumor | T1DM | HLA-DPA1*02:02, HLA-DPB1*05:01 and HLA-DRB1*04:05 | T1DM |
| Shi Y. [157] | Retrospective ** (n = 26) | Pan-tumor | APST2 | HLA-DR4 | APST2 |
| Chang H. [160] | Prospective (n = 290, 7 with encephalitis) | Breast and bladder cancer | Encephalitis | HLA-B*27:05 | Encephalitis |
| Yano S. [153] | Retrospective (n = 11) | Pan-tumor | Pituitary irAEs | HLA-DR15, HLA-B52 and HLA-Cw12 | Hypophysitis |
| Abed A. [164] | Retrospective (n = 179) | NSCLC | All types | HLA class I (but not class II) homozygosity | Lower risk of irAEs, especially pneumonitis |
| HLA-A03 | Higher risk of irAEs | ||||
| Hasan Ali O. [162] | Prospective (n = 102) | NSCLC Melanoma | All types | HLA-DRB1*11:01 | Pruritus |
| HLA-DQB1*03:01 | Colitis | ||||
| Kotwal A. [156] | Prospective (n = 10) | Pan-tumor | Endocrine irAEs | HLA-DR4-DR53 and HLA-DR15 | Thyroiditis |
| Purde MT. [165] | Prospective (n = 131, 11 hepatitis) | NSCLC Melanoma | Hepatitis | HLA- DRB1*04:01 and HLA- DRB1*15:01-DQB1*06:02 | Hepatitis |
| Clotman K. [152] | Retrospective ** (n = 42) | Pan-tumor | T1DM | HLA-DR3-DQ2, HLA-DRB1*04, HLA-DQB1*03:02, HLA-DR4, HLA-A2 and HLA-DR3DQ3, among others | T1DM |
| Magis Q. [169] | Retrospective (n = 163, 5 with T1DM) | Not available | T1DM | HLA-DRB01*03 or HLA-DRB01*04 | T1DM |
| Micro-RNAs | |||||
| Reference | Study Design (Sample Size) | Type of Tumor | Type of irAE | Associations | |
| Marschner D. [176] | Prospective (n = 179) | Pan-tumor | All types | Underexpression of miR-146a by SNP on MIR146A gene (rs2910164) | Higher risk of severe irAEs |
| Ivanova E. [177] | Prospective (n = 86) | ccRCC | All types | Underexpression of miR-146a by SNP on MIR146A gene (rs2910164) | Higher risk of severe irAEs |
| Xia W. [178] | Mouse model | Not applicable | Myocarditis | Overexpression of miR-34a-5p induced by PD-1 inhibitor-treated macrophages led to cardiac senescence | Higher risk of myocarditis |
3.2.5. Gastrointestinal Microbiome
3.2.6. Upcoming Biomarkers for irAE Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.M.; Evans, T.R.J.; Fraser, A.R.; Nibbs, R.J.B. Immune Checkpoint Inhibitors: New Strategies to Checkmate Cancer. Clin. Exp. Immunol. 2018, 191, 133–148. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Les, I.; Pérez-Francisco, I.; Cabero, M.; Sánchez, C.; Hidalgo, M.; Teijeira, L.; Arrazubi, V.; Domínguez, S.; Anaut, P.; Eguiluz, S.; et al. Prediction of Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors with a Panel of Autoantibodies: Protocol of a Multicenter, Prospective, Observational Cohort Study. Front. Pharmacol. 2022, 13, 894550. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Troiani, T.; Criscuolo, G.; Marone, G.; Ciardiello, F.; Tocchetti, C.G.; Varricchi, G. Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events. Front. Immunol. 2022, 13, 804597. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Grizzi, G.; Ghidini, M.; Ghidini, A.; Ratti, M.; Panni, S.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Parati, M.C.; et al. Immune-Related Adverse Events and Survival in Solid Tumors Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Immunother. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are Immune-Related Adverse Events Associated with the Efficacy of Immune Checkpoint Inhibitors in Patients with Cancer? A Systematic Review and Meta-Analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef]
- Park, R.; Lopes, L.; Saeed, A. Anti-PD-1/L1-Associated Immune-Related Adverse Events as Harbinger of Favorable Clinical Outcome: Systematic Review and Meta-Analysis. Clin. Transl. Oncol. 2021, 23, 100–109. [Google Scholar] [CrossRef]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]
- Food and Drug Administration; National Institutes of Health. BEST (Biomarkers, EndpointS and Other Tools) Resource; FDA-NIH Biomarker Working Group: Silver Spring, MD, USA, 2016.
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Tsunekawa, T.; Onoue, T.; Takagi, H.; Hagiwara, D.; Ito, Y.; Morishita, Y.; et al. Patients with Antithyroid Antibodies Are Prone to Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J. Endocr. Soc. 2018, 2, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Music, M.; Iafolla, M.; Soosaipillai, A.; Batruch, I.; Prassas, I.; Pintilie, M.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; et al. Predicting Response and Toxicity to PD-1 Inhibition Using Serum Autoantibodies Identified from Immuno-Mass Spectrometry. F1000Research 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- von Itzstein, M.S.; Khan, S.; Gerber, D.E. Investigational Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Event Prediction and Diagnosis. Clin. Chem. 2020, 66, 779–793. [Google Scholar] [CrossRef]
- Glehr, G.; Riquelme, P.; Yang Zhou, J.; Cordero, L.; Schilling, H.-L.; Kapinsky, M.; Schlitt, H.J.; Geissler, E.K.; Burkhardt, R.; Schmidt, B.; et al. External Validation of Biomarkers for Immune-Related Adverse Events after Immune Checkpoint Inhibition. Front. Immunol. 2022, 13, 1011040. [Google Scholar] [CrossRef]
- Ghosh, N.; Chan, K.K.; Jivanelli, B.; Bass, A.R. Autoantibodies in Patients with Immune-Related Adverse Events from Checkpoint Inhibitors: A Systematic Literature Review. JCR J. Clin. Rheumatol. 2022, 28, e498–e505. [Google Scholar] [CrossRef]
- Salim, A.; Tapia Rico, G.; Shaikh, A.; Brown, M. A Systematic Review of Immune Checkpoint Inhibitor-Related Neurological Adverse Events and Association with Anti-Neuronal Autoantibodies. Expert Opin. Biol. Ther. 2021, 21, 1237–1251. [Google Scholar] [CrossRef]
- Kostine, M.; Finckh, A.; Bingham, C.O.; Visser, K.; Leipe, J.; Schulze-Koops, H.; Choy, E.H.; Benesova, K.; Radstake, T.R.D.J.; Cope, A.P.; et al. EULAR Points to Consider for the Diagnosis and Management of Rheumatic Immune-Related Adverse Events due to Cancer Immunotherapy with Checkpoint Inhibitors. Ann. Rheum. Dis. 2021, 80, 36–48. [Google Scholar] [CrossRef]
- Mammen, A.L.; Rajan, A.; Pak, K.; Lehky, T.; Casciola-Rosen, L.; Donahue, R.N.; Lepone, L.M.; Zekeridou, A.; Pittock, S.J.; Hassan, R.; et al. Pre-Existing Antiacetylcholine Receptor Autoantibodies and B Cell Lymphopaenia Are Associated with the Development of Myositis in Patients with Thymoma Treated with Avelumab, an Immune Checkpoint Inhibitor Targeting Programmed Death-Ligand 1. Ann. Rheum. Dis. 2019, 78, 150–152. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Bomze, D.; Ring, S.S.; Berner, F.; Fässler, M.; Diem, S.; Abdou, M.-T.; Hammers, C.; Emtenani, S.; Braun, A.; et al. BP180-Specific IgG Is Associated with Skin Adverse Events, Therapy Response, and Overall Survival in Non-Small Cell Lung Cancer Patients Treated with Checkpoint Inhibitors. J. Am. Acad. Dermatol. 2020, 82, 854–861. [Google Scholar] [CrossRef]
- Seki, M.; Kitano, S.; Suzuki, S. Neurological Disorders Associated with Immune Checkpoint Inhibitors: An Association with Autoantibodies. Cancer Immunol. Immunother. 2022, 71, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-Mediated Thyroid Dysfunction during T-Cell Checkpoint Blockade in Patients with Non-Small-Cell Lung Cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Basak, E.A.; van der Meer, J.W.M.; Hurkmans, D.P.; Schreurs, M.W.J.; Oomen-de Hoop, E.; van der Veldt, A.A.M.; Bins, S.; Joosse, A.; Koolen, S.L.W.; Debets, R.; et al. Overt Thyroid Dysfunction and Anti-Thyroid Antibodies Predict Response to Anti-PD-1 Immunotherapy in Cancer Patients. Thyroid 2020, 30, 966–973. [Google Scholar] [CrossRef]
- Les, I.; Martínez, M.; Narro, A.; Pérez, I.; Sánchez, C.; Puntí, L.; Anaut, P.; Eguiluz, S.; Herrera, A.; Domínguez, S. Association of Immune-Related Adverse Events Induced by Nivolumab with a Battery of Autoantibodies. Ann. Med. 2021, 53, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Gogas, H.; Stavropoulou-Giokas, C.; Tsoutsos, D.; Papadopoulos, O.; Markopoulos, C.; Pectasides, D.; Kirkwood, J.M. Prognostic Significance of Autoimmunity during Treatment of Melanoma with Interferon. N. Engl. J. Med. 2006, 354, 709–718. [Google Scholar] [CrossRef]
- Toi, Y.; Sugawara, S.; Sugisaka, J.; Ono, H.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Aso, M.; Tsurumi, K.; et al. Profiling Preexisting Antibodies in Patients Treated with Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 376. [Google Scholar] [CrossRef]
- Yoneshima, Y.; Tanaka, K.; Shiraishi, Y.; Hata, K.; Watanabe, H.; Harada, T.; Otsubo, K.; Iwama, E.; Inoue, H.; Masuda, S.; et al. Safety and Efficacy of PD-1 Inhibitors in Non-Small Cell Lung Cancer Patients Positive for Antinuclear Antibodies. Lung Cancer 2019, 130, 5–9. [Google Scholar] [CrossRef]
- Sakakida, T.; Ishikawa, T.; Chihara, Y.; Harita, S.; Uchino, J.; Tabuchi, Y.; Komori, S.; Asai, J.; Narukawa, T.; Arai, A.; et al. Safety and Efficacy of PD-1/PD-L1 Blockade in Patients with Preexisting Antinuclear Antibodies. Clin. Transl. Oncol. 2020, 22, 919–927. [Google Scholar] [CrossRef]
- De Moel, E.C.; Rozeman, E.A.; Kapiteijn, E.H.; Verdegaal, E.M.E.; Grummels, A.; Bakker, J.A.; Huizinga, T.W.J.; Haanen, J.B.; Toes, R.E.M.; van der Woude, D. Autoantibody Development under Treatment with Immune-Checkpoint Inhibitors. Cancer Immunol. Res. 2019, 7, 6–11. [Google Scholar] [CrossRef]
- Giannicola, R.; D’Arrigo, G.; Botta, C.; Agostino, R.; Del Medico, P.; Falzea, A.; Barbieri, V.; Staropoli, N.; Del Giudice, T.; Pastina, P.; et al. Early Blood Rise in Auto-antibodies to Nuclear and Smooth Muscle Antigens Is Predictive of Prolonged Survival and Autoimmunity in Metastatic-non-small Cell Lung Cancer Patients Treated with PD-1 Immune-check Point Blockade by Nivolumab. Mol. Clin. Oncol. 2019, 11, 81–90. [Google Scholar] [CrossRef]
- Castel-Ajgal, Z.; Goulvestre, C.; Zaibet, S.; Arrondeau, J.; Bretagne, M.; Peyromaure, M.; Batteux, F.; Alexandre, J.; Goldwasser, F.; Huillard, O. Preexisting Autoantibodies and Immune Related Adverse Events in Metastatic Urothelial Carcinoma Patients Treated by Pembrolizumab. Clin. Genitourin. Cancer 2022, 20, e362–e368. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.A.; Stanzer, S.; Spiegelberg, J.; Bauernhofer, T.; Absenger, G.; Posch, F.; Lipp, R.; Halm, M.; Szkandera, J.; Balic, M.; et al. Evaluation of Autoantibodies as Predictors of Treatment Response and Immune-related Adverse Events during the Treatment with Immune Checkpoint Inhibitors: A Prospective Longitudinal Pan-cancer Study. Cancer Med. 2022, 11, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shi, Y.; Liu, X.; Liu, J.; Xu, Y.; Zhao, J.; Zhong, W.; Käsmann, L.; Hakozaki, T.; Provencio, M.; et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients with Preexisting Antinuclear Antibodies: A Retrospective Cohort Study. Transl. Lung Cancer Res. 2022, 11, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Geng, R.; Xu, X.; Wang, Y.; Zhou, J.; Zhang, S.; Zhao, L.; Guan, M.; Bai, C. Safety and Efficacy of PD-1/PD-L1 Inhibitors in Cancer Patients with Preexisting Autoantibodies. Front. Immunol. 2022, 13, 893179. [Google Scholar] [CrossRef] [PubMed]
- Izawa, N.; Shiokawa, H.; Onuki, R.; Hamaji, K.; Morikawa, K.; Saji, H.; Ohashi, H.; Kasugai, S.; Hayakawa, N.; Ohara, T.; et al. The Clinical Utility of Comprehensive Measurement of Autoimmune Disease-Related Antibodies in Patients with Advanced Solid Tumors Receiving Immune Checkpoint Inhibitors: A Retrospective Study. ESMO Open 2022, 7, 100415. [Google Scholar] [CrossRef] [PubMed]
- Mouri, A.; Kaira, K.; Yamaguchi, O.; Hashimoto, K.; Miura, Y.; Shiono, A.; Shinomiya, S.; Akagami, T.; Imai, H.; Kobayashi, K.; et al. Efficacy and Feasibility of Programmed Death-1/Programmed Death Ligand-1 Blockade Therapy in Non-Small Cell Lung Cancer Patients with High Antinuclear Antibody Titers. Front. Oncol. 2021, 11, 610952. [Google Scholar] [CrossRef]
- Alserawan, L.; Anguera, G.; Zamora Atenza, C.; Serra López, J.; Martínez-Martínez, L.; Riudavets Melià, M.; Sullivan, I.; Barba Joaquin, A.; Majem Tarruella, M.; Vidal, S. Association between Changes in the Patterns of Antinuclear Autoantibodies during Immune Checkpoint Inhibition Therapy and the Development of Severe Immune Related Adverse Events. Int. J. Mol. Sci. 2022, 23, 12641. [Google Scholar] [CrossRef]
- Ghosh, N.; Postow, M.; Zhu, C.; Jannat-Khah, D.; Li, Q.-Z.; Vitone, G.; Chan, K.K.; Bass, A.R. Lower Baseline Autoantibody Levels Are Associated with Immune-Related Adverse Events from Immune Checkpoint Inhibition. J. Immunother. Cancer 2022, 10, e004008. [Google Scholar] [CrossRef]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.M.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B Cell Changes Predict Autoimmunity Following Combination Immune Checkpoint Blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef]
- Diehl, A.; Yarchoan, M.; Hopkins, A.; Jaffee, E.; Grossman, S.A. Relationships between Lymphocyte Counts and Treatment-Related Toxicities and Clinical Responses in Patients with Solid Tumors Treated with PD-1 Checkpoint Inhibitors. Oncotarget 2017, 8, 114268–114280. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, R.; Maruyama, H.; Ishitsuka, Y.; Okiyama, N.; Watanabe, R.; Fujimoto, M.; Fujisawa, Y. Correlation between Blood Cell Count and Outcome of Melanoma Patients Treated with Anti-PD-1 Antibodies. Jpn. J. Clin. Oncol. 2019, 49, 431–437. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, X.; Wang, J.; Wu, S.; Wang, J.; Cao, B. Absolute Eosinophil Count May Be an Optimal Peripheral Blood Marker to Identify the Risk of Immune-Related Adverse Events in Advanced Malignant Tumors Treated with PD-1/PD-L1 Inhibitors: A Retrospective Analysis. World J. Surg. Oncol. 2022, 20, 242. [Google Scholar] [CrossRef] [PubMed]
- Ruste, V.; Goldschmidt, V.; Laparra, A.; Messayke, S.; Danlos, F.-X.; Romano-Martin, P.; Champiat, S.; Voisin, A.-L.; Baldini, C.; Massard, C.; et al. The Determinants of Very Severe Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors: A Prospective Study of the French REISAMIC Registry. Eur. J. Cancer 2021, 158, 217–224. [Google Scholar] [CrossRef]
- Pavan, A.; Calvetti, L.; Dal Maso, A.; Attili, I.; Del Bianco, P.; Pasello, G.; Guarneri, V.; Aprile, G.; Conte, P.; Bonanno, L. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist 2019, 24, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.; Kim, I.Y.; Sun, J.-M.; Lee, J.; Cha, H.-S.; Koh, E.-M.; Kim, H.; Lee, J. Risk Factors for Immune-Related Adverse Events Associated with Anti-PD-1 Pembrolizumab. Sci. Rep. 2019, 9, 14039. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Ma, F.; Sun, B.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Peripheral Blood Markers Associated with Immune-Related Adverse Effects in Patients Who Had Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors. Cancer Manag. Res. 2021, 13, 765–771. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Chen, X.; Li, L.; Song, W.; Li, W.; Zhao, Y.; Zhang, Y.; Han, F.; Lyu, Z.; et al. Analysis of Characteristics and Predictive Factors of Immune Checkpoint Inhibitor-Related Adverse Events. Cancer Biol. Med. 2021, 18, 1118–1133. [Google Scholar] [CrossRef]
- Fujimoto, A.; Toyokawa, G.; Koutake, Y.; Kimura, S.; Kawamata, Y.; Fukuishi, K.; Yamazaki, K.; Takeo, S. Association between Pretreatment Neutrophil-to-lymphocyte Ratio and Immune-related Adverse Events Due to Immune Checkpoint Inhibitors in Patients with Non-small Cell Lung Cancer. Thorac. Cancer 2021, 12, 2198–2204. [Google Scholar] [CrossRef]
- Michailidou, D.; Khaki, A.R.; Morelli, M.P.; Diamantopoulos, L.; Singh, N.; Grivas, P. Association of Blood Biomarkers and Autoimmunity with Immune Related Adverse Events in Patients with Cancer Treated with Immune Checkpoint Inhibitors. Sci. Rep. 2021, 11, 9029. [Google Scholar] [CrossRef]
- Suazo-Zepeda, E.; Bokern, M.; Vinke, P.C.; Hiltermann, T.J.N.; de Bock, G.H.; Sidorenkov, G. Risk Factors for Adverse Events Induced by Immune Checkpoint Inhibitors in Patients with Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cancer Immunol. Immunother. 2021, 70, 3069–3080. [Google Scholar] [CrossRef]
- Egami, S.; Kawazoe, H.; Hashimoto, H.; Uozumi, R.; Arami, T.; Sakiyama, N.; Ohe, Y.; Nakada, H.; Aomori, T.; Ikemura, S.; et al. Peripheral Blood Biomarkers Predict Immune-Related Adverse Events in Non-Small Cell Lung Cancer Patients Treated with Pembrolizumab: A Multicenter Retrospective Study. J. Cancer 2021, 12, 2105–2112. [Google Scholar] [CrossRef]
- Xu, H.; Feng, H.; Zhang, W.; Wei, F.; Zhou, L.; Liu, L.; Zhao, Y.; Lv, Y.; Shi, X.; Zhang, J.; et al. Prediction of Immune-Related Adverse Events in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors Based on Clinical and Hematological Markers: Real-World Evidence. Exp. Cell Res. 2022, 416, 113157. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Oen, K.Q.X.; Lim, G.R.S.; Hartono, J.L.; Muthiah, M.; Huang, D.Q.; Teo, F.S.W.; Li, A.Y.; Mak, A.; Chandran, N.S.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers 2021, 13, 1308. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Zhao, J.; Zhou, J.; Zhou, F.; Jiang, T.; Jiang, S.; Sun, X.; You, X.; Wu, F.; Ren, S.; et al. Association of Baseline Peripheral-Blood Eosinophil Count with Immune Checkpoint Inhibitor-Related Pneumonitis and Clinical Outcomes in Patients with Non-Small Cell Lung Cancer Receiving Immune Checkpoint Inhibitors. Lung Cancer 2020, 150, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Drobni, Z.D.; Zafar, A.; Zubiri, L.; Zlotoff, D.A.; Alvi, R.M.; Lee, C.; Hartmann, S.; Gilman, H.K.; Villani, A.; Nohria, A.; et al. Decreased Absolute Lymphocyte Count and Increased Neutrophil/Lymphocyte Ratio with Immune Checkpoint Inhibitor-Associated Myocarditis. J. Am. Heart Assoc. 2020, 9, e018306. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Murooka, H.; Tahatsu, K.; Yanase, M.; Umehara, K.; Hashishita, H.; Toru, H.; Satoru, M.; Sagawa, T.; Fujikawa, K.; et al. Identifying Early Predictive Markers for Immune-Related Adverse Events in Nivolumab-Treated Patients with Renal Cell Carcinoma and Gastric Cancer. Asian Pac. J. Cancer Prev. 2022, 23, 695–701. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Yoshino, K.; Otsuka, A.; Funakoshi, T.; Fujimura, T.; Yamamoto, Y.; Hata, H.; Gosho, M.; Tanaka, R.; Yamaguchi, K.; et al. Fluctuations in Routine Blood Count Might Signal Severe Immune-Related Adverse Events in Melanoma Patients Treated with Nivolumab. J. Dermatol. Sci. 2017, 88, 225–231. [Google Scholar] [CrossRef]
- Egami, S.; Kawazoe, H.; Hashimoto, H.; Uozumi, R.; Arami, T.; Sakiyama, N.; Ohe, Y.; Nakada, H.; Aomori, T.; Ikemura, S.; et al. Absolute Lymphocyte Count Predicts Immune-Related Adverse Events in Patients with Non-Small-Cell Lung Cancer Treated with Nivolumab Monotherapy: A Multicenter Retrospective Study. Front. Oncol. 2021, 11, 618570. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, T.; Qi, C.; Zhang, X.; Shen, L.; Peng, Z. Peripheral Blood Biomarkers Predictive of Efficacy Outcome and Immune-Related Adverse Events in Advanced Gastrointestinal Cancers Treated with Checkpoint Inhibitors. Cancers 2022, 14, 3736. [Google Scholar] [CrossRef]
- Subudhi, S.K.; Aparicio, A.; Gao, J.; Zurita, A.J.; Araujo, J.C.; Logothetis, C.J.; Tahir, S.A.; Korivi, B.R.; Slack, R.S.; Vence, L.; et al. Clonal Expansion of CD8 T Cells in the Systemic Circulation Precedes Development of Ipilimumab-Induced Toxicities. Proc. Natl. Acad. Sci. USA 2016, 113, 11919–11924. [Google Scholar] [CrossRef]
- Oh, D.Y.; Cham, J.; Zhang, L.; Fong, G.; Kwek, S.S.; Klinger, M.; Faham, M.; Fong, L. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-Cell Repertoire. Cancer Res. 2017, 77, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated with Ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Lalanne, C.; Quarneti, C.; Ferri, S.; Guidi, M.; Lenzi, M.; Muratori, P. Hepatocellular Carcinoma in Viral and Autoimmune Liver Diseases: Role of CD4+ CD25+ Foxp3+ Regulatory T Cells in the Immune Microenvironment. World J. Gastroenterol. 2021, 27, 2994–3009. [Google Scholar] [CrossRef] [PubMed]
- Arasanz, H.; Bocanegra, A.I.; Morilla, I.; Fernández-Irigoyen, J.; Martínez-Aguillo, M.; Teijeira, L.; Garnica, M.; Blanco, E.; Chocarro, L.; Ausin, K.; et al. Circulating Low Density Neutrophils Are Associated with Resistance to First Line Anti-PD1/PDL1 Immunotherapy in Non-Small Cell Lung Cancer. Cancers 2022, 14, 3846. [Google Scholar] [CrossRef] [PubMed]
- Muir, C.A.; Clifton-Bligh, R.J.; Long, G.V.; Scolyer, R.A.; Lo, S.N.; Carlino, M.S.; Tsang, V.H.M.; Menzies, A.M. Thyroid Immune-Related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e3704–e3713. [Google Scholar] [CrossRef]
- Yoon, J.H.; Hong, A.R.; Kim, H.K.; Kang, H.-C. Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors. Endocrinol. Metab. 2021, 36, 413–423. [Google Scholar] [CrossRef]
- Luongo, C.; Morra, R.; Gambale, C.; Porcelli, T.; Sessa, F.; Matano, E.; Damiano, V.; Klain, M.; Schlumberger, M.; Salvatore, D. Higher Baseline TSH Levels Predict Early Hypothyroidism during Cancer Immunotherapy. J. Endocrinol. Investig. 2021, 44, 1927–1933. [Google Scholar] [CrossRef]
- Isawa, T.; Toi, Y.; Sugawara, S.; Taguri, M.; Toyoda, S. Incidence, Clinical Characteristics and Predictors of Cardiovascular Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Oncologist 2022, 27, e410–e419. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, Y. Immune Checkpoint Inhibitor–Mediated Diarrhea and Colitis: A Clinical Review. JCO Oncol. Pract. 2020, 16, 453–461. [Google Scholar] [CrossRef]
- Zou, F.; Wang, X.; Glitza Oliva, I.C.; McQuade, J.L.; Wang, J.; Zhang, H.C.; Thompson, J.A.; Thomas, A.S.; Wang, Y. Fecal Calprotectin Concentration to Assess Endoscopic and Histologic Remission in Patients with Cancer with Immune-Mediated Diarrhea and Colitis. J. Immunother. Cancer 2021, 9, e002058. [Google Scholar] [CrossRef]
- Husain, B.; Kirchberger, M.C.; Erdmann, M.; Schüpferling, S.; Abolhassani, A.-R.; Fröhlich, W.; Berking, C.; Heinzerling, L. Inflammatory Markers in Autoimmunity Induced by Checkpoint Inhibitors. J. Cancer Res. Clin. Oncol. 2021, 147, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Shimozaki, K.; Sukawa, Y.; Sato, Y.; Horie, S.; Chida, A.; Tsugaru, K.; Togasaki, K.; Kawasaki, K.; Hirata, K.; Hayashi, H.; et al. Analysis of Risk Factors for Immune-Related Adverse Events in Various Solid Tumors Using Real-World Data. Future Oncol. 2021, 17, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Jiang, N.; Song, X.; Xu, J.; Zhu, X.; Chen, C.; Kong, C.; Wang, X.; Zong, D.; et al. Association of Blood Biochemical Indexes and Antibiotic Exposure with Severe Immune-Related Adverse Events in Patients with Advanced Cancers Receiving PD-1 Inhibitors. J. Immunother. 2022, 45, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Oyanagi, J.; Koh, Y.; Sato, K.; Mori, K.; Teraoka, S.; Akamatsu, H.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; et al. Predictive Value of Serum Protein Levels in Patients with Advanced Non-Small Cell Lung Cancer Treated with Nivolumab. Lung Cancer 2019, 132, 107–113. [Google Scholar] [CrossRef]
- Tas, F.; Yasasever, V.; Duranyildiz, D.; Camlica, H.; Ustuner, Z.; Aydiner, A.; Topuz, E. Clinical Value of Protein S100 and Melanoma-Inhibitory Activity (MIA) in Malignant Melanoma. Am. J. Clin. Oncol. 2004, 27, 225–228. [Google Scholar] [CrossRef]
- Kang, J.H.; Bluestone, J.A.; Young, A. Predicting and Preventing Immune Checkpoint Inhibitor Toxicity: Targeting Cytokines. Trends Immunol. 2021, 42, 293–311. [Google Scholar] [CrossRef]
- Tyan, K.; Baginska, J.; Brainard, M.; Giobbie-Hurder, A.; Severgnini, M.; Manos, M.; Haq, R.; Buchbinder, E.I.; Ott, P.A.; Hodi, F.S.; et al. Cytokine Changes during Immune-Related Adverse Events and Corticosteroid Treatment in Melanoma Patients Receiving Immune Checkpoint Inhibitors. Cancer Immunol. Immunother. 2021, 70, 2209–2221. [Google Scholar] [CrossRef]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFα Blockade Overcomes Resistance to Anti-PD-1 in Experimental Melanoma. Nat. Commun. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Perez-Ruiz, E.; Minute, L.; Otano, I.; Alvarez, M.; Ochoa, M.C.; Belsue, V.; de Andrea, C.; Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Marquez-Rodas, I.; et al. Prophylactic TNF Blockade Uncouples Efficacy and Toxicity in Dual CTLA-4 and PD-1 Immunotherapy. Nature 2019, 569, 428–432. [Google Scholar] [CrossRef]
- Yoshino, K.; Nakayama, T.; Ito, A.; Sato, E.; Kitano, S. Severe Colitis after PD-1 Blockade with Nivolumab in Advanced Melanoma Patients: Potential Role of Th1-Dominant Immune Response in Immune-Related Adverse Events: Two Case Reports. BMC Cancer 2019, 19, 1019. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.e22. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Okiyama, N.; Okune, M.; Ishitsuka, Y.; Watanabe, R.; Furuta, J.; Ohtsuka, M.; Otsuka, A.; Maruyama, H.; Fujisawa, Y.; et al. Serum Level of Interleukin-6 Is Increased in Nivolumab-Associated Psoriasiform Dermatitis and Tumor Necrosis Factor-α Is a Biomarker of Nivolumab Recativity. J. Dermatol. Sci. 2017, 86, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Oke, A.R.; Wheater, M.; Karydis, I.; Wallis, D. Successful Use of Adalimumab in Immune Checkpoint Inhibitor-Associated Inflammatory Arthritis. Rheumatol. Adv. Pract. 2018, 2, rky001. [Google Scholar] [CrossRef] [PubMed]
- Badran, Y.R.; Cohen, J.V.; Brastianos, P.K.; Parikh, A.R.; Hong, T.S.; Dougan, M. Concurrent Therapy with Immune Checkpoint Inhibitors and TNFα Blockade in Patients with Gastrointestinal Immune-Related Adverse Events. J. Immunother. Cancer 2019, 7, 226. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Wang, X.; Mallepally, N.; Chen, E.; Altan, M.; Bresalier, R.S.; Charabaty, A.; Dadu, R.; Jazaeri, A.; et al. Early Introduction of Selective Immunosuppressive Therapy Associated with Favorable Clinical Outcomes in Patients with Immune Checkpoint Inhibitor–Induced Colitis. J. Immunother. Cancer 2019, 7, 93. [Google Scholar] [CrossRef]
- Gao, J.; Miao, J.; Sun, H.; Fu, X.; Zhang, P.; Chen, Z.; Zhu, P. TNF-α Inhibitor Ameliorates Immune-Related Arthritis and Pneumonitis in Humanized Mice. Front. Immunol. 2022, 13, 955812. [Google Scholar] [CrossRef]
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Boers-Sonderen, M.J.; van der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1–Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020, 26, 2268–2274. [Google Scholar] [CrossRef]
- Chen, A.Y.; Wolchok, J.D.; Bass, A.R. TNF in the Era of Immune Checkpoint Inhibitors: Friend or Foe? Nat. Rev. Rheumatol. 2021, 17, 213–223. [Google Scholar] [CrossRef]
- Alvarez, M.; Otano, I.; Minute, L.; Ochoa, M.C.; Perez-Ruiz, E.; Melero, I.; Berraondo, P. Impact of Prophylactic TNF Blockade in the Dual PD-1 and CTLA-4 Immunotherapy Efficacy and Toxicity. Cell Stress 2019, 3, 236–239. [Google Scholar] [CrossRef]
- Montfort, A.; Filleron, T.; Virazels, M.; Dufau, C.; Milhès, J.; Pagès, C.; Olivier, P.; Ayyoub, M.; Mounier, M.; Lusque, A.; et al. Combining Nivolumab and Ipilimumab with Infliximab or Certolizumab in Patients with Advanced Melanoma: First Results of a Phase Ib Clinical Trial. Clin. Cancer Res. 2021, 27, 1037–1047. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. Erratum: Corrigendum: IL-6 as a Keystone Cytokine in Health and Disease. Nat. Immunol. 2017, 18, 1271. [Google Scholar] [CrossRef] [PubMed]
- Valpione, S.; Pasquali, S.; Campana, L.G.; Piccin, L.; Mocellin, S.; Pigozzo, J.; Chiarion-Sileni, V. Sex and Interleukin-6 Are Prognostic Factors for Autoimmune Toxicity Following Treatment with Anti-CTLA4 Blockade. J. Transl. Med. 2018, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, C.-K.; Chung, C.; Oh, I.-J.; Kim, Y.-C.; Park, D.; Kim, J.; Kwon, G.C.; Kwon, I.; Sun, P.; et al. Baseline Serum Interleukin-6 Levels Predict the Response of Patients with Advanced Non-Small Cell Lung Cancer to PD-1/PD-L1 Inhibitors. Immune Netw. 2020, 20, e27. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Hogan, S.; Menzies, A.M.; Dummer, R.; Long, G.V. Interleukin-6 Blockade for Prophylaxis and Management of Immune-Related Adverse Events in Cancer Immunotherapy. Eur. J. Cancer 2021, 157, 214–224. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Bentebibel, S.-E.; Daher, M.; Haymaker, C.; Wani, K.; Saberian, C.; Ogata, D.; et al. Interleukin-6 Blockade Abrogates Immunotherapy Toxicity and Promotes Tumor Immunity. Cancer Cell 2022, 40, 509–523.e6. [Google Scholar] [CrossRef]
- Campochiaro, C.; Farina, N.; Tomelleri, A.; Ferrara, R.; Lazzari, C.; De Luca, G.; Bulotta, A.; Signorelli, D.; Palmisano, A.; Vignale, D.; et al. Tocilizumab for the Treatment of Immune-Related Adverse Events: A Systematic Literature Review and a Multicentre Case Series. Eur. J. Intern. Med. 2021, 93, 87–94. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Wang, C.-H.; Wang, C.-W.; Chen, C.-J.; Huang, H.-Y.; Chung, F.-T.; Huang, Y.-C.; Lin, C.-W.; Lee, C.-S.; Lin, C.-Y.; et al. Increased Interleukin-17 and Glucocorticoid Receptor-β Expression in Interstitial Lung Diseases and Corticosteroid Insensitivity. Front. Immunol. 2022, 13, 905727. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline Circulating IL-17 Predicts Toxicity While TGF-Β1 and IL-10 Are Prognostic of Relapse in Ipilimumab Neoadjuvant Therapy of Melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef]
- Li, D.; Gál, I.; Vermes, C.; Alegre, M.-L.; Chong, A.S.F.; Chen, L.; Shao, Q.; Adarichev, V.; Xu, X.; Koreny, T.; et al. Cutting Edge: Cbl-b: One of the Key Molecules Tuning CD28- and CTLA-4-Mediated T Cell Costimulation. J. Immunol. 2004, 173, 7135–7139. [Google Scholar] [CrossRef]
- Bouguermouh, S.; Fortin, G.; Baba, N.; Rubio, M.; Sarfati, M. CD28 Co-Stimulation Down Regulates Th17 Development. PLoS ONE 2009, 4, e5087. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Patel, A.B.; Uemura, M.I.; Trinh, V.A.; Jackson, N.; Zobniw, C.M.; Tetzlaff, M.T.; Hwu, P.; Curry, J.L.; Diab, A. IL17A Blockade Successfully Treated Psoriasiform Dermatologic Toxicity from Immunotherapy. Cancer Immunol. Res. 2019, 7, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, L.; Giugliano, S.; D’Amico, P.; Belli, C.; Duso, B.A.; Rescigno, M.; Curigliano, G. Evidence for Interleukin 17 Involvement in Severe Immune-Related Neuroendocrine Toxicity. Eur. J. Cancer 2020, 141, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, Y.; Yang, X.; Gan, L.; Xue, J. Checkpoint Inhibitor Pneumonitis Induced by Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer: Occurrence and Mechanism. Front. Immunol. 2022, 13, 830631. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Miller, W.H. Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N. Engl. J. Med. 2017, 376, 1989–1991. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, C.; Inaba, H.; Ariyasu, H.; Iwakura, H.; Ueda, Y.; Uraki, S.; Takeshima, K.; Furukawa, Y.; Morita, S.; Yamamoto, Y.; et al. Predictive and Sensitive Biomarkers for Thyroid Dysfunctions during Treatment with Immune-checkpoint Inhibitors. Cancer Sci. 2020, 111, 1468–1477. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, J.; Li, Y.; Jiao, X.; Wang, Y.; Zhuo, N.; Gao, M.; Gong, J.; Li, J.; Zhang, X.; et al. Serological Biomarkers Predict Immune-Related Adverse Events and Clinical Benefit in Patients with Advanced Gastrointestinal Cancers. Front. Immunol. 2022, 13, 987568. [Google Scholar] [CrossRef]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.-S.; Derosa, L.; Khan, M.A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut Microbiota Signatures Are Associated with Toxicity to Combined CTLA-4 and PD-1 Blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Jackson, M.A.; Korsunsky, I.; Bullers, S.J.; Rue-Albrecht, K.; Christoforidou, Z.; Sathananthan, D.; Thomas, T.; Ravindran, R.; et al. IL-1-Driven Stromal–Neutrophil Interactions Define a Subset of Patients with Inflammatory Bowel Disease That Does Not Respond to Therapies. Nat. Med. 2021, 27, 1970–1981. [Google Scholar] [CrossRef]
- Schindler, H.; Lusky, F.; Daniello, L.; Elshiaty, M.; Gaissmaier, L.; Benesova, K.; Souto-Carneiro, M.; Angeles, A.K.; Janke, F.; Eichhorn, F.; et al. Serum Cytokines Predict Efficacy and Toxicity, but Are Not Useful for Disease Monitoring in Lung Cancer Treated with PD-(L)1 Inhibitors. Front. Oncol. 2022, 12, 1010660. [Google Scholar] [CrossRef]
- Thomas, A.S.; Ma, W.; Wang, Y. Ustekinumab for Refractory Colitis Associated with Immune Checkpoint Inhibitors. N. Engl. J. Med. 2021, 384, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Ngiow, S.F.; Young, A.; Blake, S.J.; Hill, G.R.; Yagita, H.; Teng, M.W.L.; Korman, A.J.; Smyth, M.J. Agonistic CD40 MAb-Driven IL12 Reverses Resistance to Anti-PD1 in a T-Cell–Rich Tumor. Cancer Res. 2016, 76, 6266–6277. [Google Scholar] [CrossRef]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161.e7. [Google Scholar] [CrossRef]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 Promotes Tumour Incidence and Growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, F.; Zhao, C.; Cheng, L.; Zhou, C.; Qiao, M.; Li, X.; Chen, X. Interleukin-10 Is a Promising Marker for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving Immunotherapy. Front. Immunol. 2022, 13, 840313. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, J.; Gu, Q.; Huang, M.; Zhang, W.; Guo, J.; Zhou, X. Reciprocal Expression of IL-35 and IL-10 Defines Two Distinct Effector Treg Subsets That Are Required for Maintenance of Immune Tolerance. Cell Rep. 2017, 21, 1853–1869. [Google Scholar] [CrossRef]
- Takai, R.; Funakoshi, Y.; Suto, H.; Nagatani, Y.; Imamura, Y.; Toyoda, M.; Yakushijin, K.; Kiyota, N.; Harada, K.-I.; Yamashita, K.; et al. Serum Soluble Interleukin-2 Receptor as a Potential Biomarker for Immune-Related Adverse Events. Anticancer Res. 2021, 41, 1021–1026. [Google Scholar] [CrossRef]
- Hirashima, T.; Kanai, T.; Suzuki, H.; Yoshida, H.; Matsushita, A.; Kawasumi, H.; Samejima, Y.; Noda, Y.; Nasu, S.; Tanaka, A.; et al. The Levels of Interferon-Gamma Release as a Biomarker for Non-Small-Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Anticancer Res. 2019, 39, 6231–6240. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Sato, Y.; Tanita, K.; Kambayashi, Y.; Otsuka, A.; Fujisawa, Y.; Yoshino, K.; Matsushita, S.; Funakoshi, T.; Hata, H.; et al. Serum Levels of Soluble CD163 and CXCL5 May Be Predictive Markers for Immune-Related Adverse Events in Patients with Advanced Melanoma Treated with Nivolumab: A Pilot Study. Oncotarget 2018, 9, 15542–15551. [Google Scholar] [CrossRef]
- Khan, S.; Khan, S.A.; Luo, X.; Fattah, F.J.; Saltarski, J.; Gloria-McCutchen, Y.; Lu, R.; Xie, Y.; Li, Q.; Wakeland, E.; et al. Immune Dysregulation in Cancer Patients Developing Immune-Related Adverse Events. Br. J. Cancer 2019, 120, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yi, Y.; Cao, W.; Fu, X.; Mei, N.; Li, C. Serum Cytokine Levels for Predicting Immune-Related Adverse Events and the Clinical Response in Lung Cancer Treated with Immunotherapy. Front. Oncol. 2022, 12, 923531. [Google Scholar] [CrossRef] [PubMed]
- Ciecko, A.E.; Foda, B.; Barr, J.Y.; Ramanathan, S.; Atkinson, M.A.; Serreze, D.V.; Geurts, A.M.; Lieberman, S.M.; Chen, Y.-G. Interleukin-27 Is Essential for Type 1 Diabetes Development and Sjögren Syndrome-like Inflammation. Cell Rep. 2019, 29, 3073–3086.e5. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Lee, J.H.; Gide, T.N.; Menzies, A.M.; Guminski, A.; Carlino, M.S.; Breen, E.J.; Yang, J.Y.H.; Ghazanfar, S.; Kefford, R.F.; et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin. Cancer Res. 2019, 25, 1557–1563. [Google Scholar] [CrossRef]
- Schett, G.; McInnes, I.B.; Neurath, M.F. Reframing Immune-Mediated Inflammatory Diseases through Signature Cytokine Hubs. N. Engl. J. Med. 2021, 385, 628–639. [Google Scholar] [CrossRef]
- McGonagle, D.; McDermott, M.F. A Proposed Classification of the Immunological Diseases. PLoS Med. 2006, 3, e297. [Google Scholar] [CrossRef]
- Omarjee, O.; Picard, C.; Frachette, C.; Moreews, M.; Rieux-Laucat, F.; Soulas-Sprauel, P.; Viel, S.; Lega, J.-C.; Bader-Meunier, B.; Walzer, T.; et al. Monogenic Lupus: Dissecting Heterogeneity. Autoimmun. Rev. 2019, 18, 102361. [Google Scholar] [CrossRef]
- Yin, X.; Kim, K.; Suetsugu, H.; Bang, S.-Y.; Wen, L.; Koido, M.; Ha, E.; Liu, L.; Sakamoto, Y.; Jo, S.; et al. Meta-Analysis of 208370 East Asians Identifies 113 Susceptibility Loci for Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2021, 80, 632–640. [Google Scholar] [CrossRef]
- Chye, A.; Allen, I.; Barnet, M.; Burnett, D.L. Insights into the Host Contribution of Endocrine Associated Immune-Related Adverse Events to Immune Checkpoint Inhibition Therapy. Front. Oncol. 2022, 12, 894015. [Google Scholar] [CrossRef]
- Wölffer, M.; Battke, F.; Schulze, M.; Feldhahn, M.; Flatz, L.; Martus, P.; Forschner, A. Biomarkers Associated with Immune-Related Adverse Events under Checkpoint Inhibitors in Metastatic Melanoma. Cancers 2022, 14, 302. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Diab, A.; Yu, R.K.; Futreal, A.; Criswell, L.A.; Tayar, J.H.; Dadu, R.; Shannon, V.; Shete, S.S.; Suarez-Almazor, M.E. Genetic Determinants of Immune-Related Adverse Events in Patients with Melanoma Receiving Immune Checkpoint Inhibitors. Cancer Immunol. Immunother. 2021, 70, 1939–1949. [Google Scholar] [CrossRef]
- Refae, S.; Gal, J.; Ebran, N.; Otto, J.; Borchiellini, D.; Peyrade, F.; Chamorey, E.; Brest, P.; Milano, G.; Saada-Bouzid, E. Germinal Immunogenetics Predict Treatment Outcome for PD-1/PD-L1 Checkpoint Inhibitors. Invest. New Drugs 2020, 38, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, D.-L.; Wang, F.; Yang, C.; Chen, X.-X.; You, J.; Huang, J.-S.; Shao, Y.; Zhu, D.-Q.; Ouyang, Y.-M.; et al. The Predicting Role of Circulating Tumor DNA Landscape in Gastric Cancer Patients Treated with Immune Checkpoint Inhibitors. Mol. Cancer 2020, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Bins, S.; Basak, E.A.; el Bouazzaoui, S.; Koolen, S.L.W.; de Hoop, E.O.; van der Leest, C.H.; van der Veldt, A.A.M.; Sleijfer, S.; Debets, R.; van Schaik, R.H.N.; et al. Association between Single-Nucleotide Polymorphisms and Adverse Events in Nivolumab-Treated Non-Small Cell Lung Cancer Patients. Br. J. Cancer 2018, 118, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Numakura, K.; Hatakeyama, S.; Muto, Y.; Sekine, Y.; Sasagawa, H.; Kashima, S.; Yamamoto, R.; Koizumi, A.; Nara, T.; et al. Severe Immune-Related Adverse Events in Patients Treated with Nivolumab for Metastatic Renal Cell Carcinoma Are Associated with PDCD1 Polymorphism. Genes 2022, 13, 1204. [Google Scholar] [CrossRef]
- Khan, Z.; Di Nucci, F.; Kwan, A.; Hammer, C.; Mariathasan, S.; Rouilly, V.; Carroll, J.; Fontes, M.; Ley Acosta, S.; Guardino, E.; et al. Polygenic Risk for Skin Autoimmunity Impacts Immune Checkpoint Blockade in Bladder Cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 12288–12294. [Google Scholar] [CrossRef]
- Khan, Z.; Hammer, C.; Carroll, J.; Di Nucci, F.; Acosta, S.L.; Maiya, V.; Bhangale, T.; Hunkapiller, J.; Mellman, I.; Albert, M.L.; et al. Genetic Variation Associated with Thyroid Autoimmunity Shapes the Systemic Immune Response to PD-1 Checkpoint Blockade. Nat. Commun. 2021, 12, 3355. [Google Scholar] [CrossRef]
- Friedlander, P.; Wood, K.; Wassmann, K.; Christenfeld, A.M.; Bhardwaj, N.; Oh, W.K. A Whole-Blood RNA Transcript-Based Gene Signature Is Associated with the Development of CTLA-4 Blockade-Related Diarrhea in Patients with Advanced Melanoma Treated with the Checkpoint Inhibitor Tremelimumab. J. Immunother. Cancer 2018, 6, 90. [Google Scholar] [CrossRef]
- Shahabi, V.; Berman, D.; Chasalow, S.D.; Wang, L.; Tsuchihashi, Z.; Hu, B.; Panting, L.; Jure-Kunkel, M.; Ji, R.-R. Gene Expression Profiling of Whole Blood in Ipilimumab-Treated Patients for Identification of Potential Biomarkers of Immune-Related Gastrointestinal Adverse Events. J. Transl. Med. 2013, 11, 75. [Google Scholar] [CrossRef]
- Finke, D.; Heckmann, M.; Salatzki, J.; Riffel, J.; Herpel, E.; Heinzerling, L.; Meder, B.; Völkers, M.; Müller, O.; Frey, N.; et al. Comparative Transcriptomics of Immune Checkpoint Inhibitor Myocarditis Identifies Guanylate Binding Protein 5 and 6 Dysregulation. Cancers 2021, 13, 2498. [Google Scholar] [CrossRef]
- Adam, B.A.; Murakami, N.; Reid, G.; Du, K.; Jasim, R.; Boils, C.L.; Bu, L.; Hill, P.D.; Murray, A.G.; Renaudin, K.; et al. Gene Expression Profiling in Kidney Transplants with Immune Checkpoint Inhibitor–Associated Adverse Events. Clin. J. Am. Soc. Nephrol. 2021, 16, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Garofano, F.; Wu, X.; Schmid, M.; Krawitz, P.; Essler, M.; Schmidt-Wolf, I.G.H. Integrative Analysis of Key Candidate Genes and Signaling Pathways in Autoimmune Thyroid Dysfunction Related to Anti-CTLA-4 Therapy by Bioinformatics. Investig. New Drugs 2020, 38, 1717–1729. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, J.; Ye, Y.; Pan, L.; Deng, H.; Wang, Y.; Yang, Y.; Diao, L.; Lin, S.H.; Mills, G.B.; et al. Multi-Omics Prediction of Immune-Related Adverse Events during Checkpoint Immunotherapy. Nat. Commun. 2020, 11, 4946. [Google Scholar] [CrossRef]
- Queirolo, P.; Dozin, B.; Morabito, A.; Banelli, B.; Carosio, R.; Fontana, V.; Ferrucci, P.F.; Martinoli, C.; Cocorocchio, E.; Ascierto, P.A.; et al. CTLA-4 Gene Variant -1661A>G May Predict the Onset of Endocrine Adverse Events in Metastatic Melanoma Patients Treated with Ipilimumab. Eur. J. Cancer 2018, 97, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.L.; Reuben, A.; Szczepaniak-Sloane, R.; Ning, J.; Milton, D.R.; Lee, C.H.; Hudgens, C.; George, S.; Torres-Cabala, C.; Johnson, D.; et al. Gene Expression Profiling of Lichenoid Dermatitis Immune-related Adverse Event from Immune Checkpoint Inhibitors Reveals Increased CD14+ and CD16+ Monocytes Driving an Innate Immune Response. J. Cutan. Pathol. 2019, 46, 627–636. [Google Scholar] [CrossRef]
- He, X.; Yu, J.; Shi, H. Pan-Cancer Analysis Reveals Alternative Splicing Characteristics Associated with Immune-Related Adverse Events Elicited by Checkpoint Immunotherapy. Front. Pharmacol. 2021, 12, 797852. [Google Scholar] [CrossRef]
- Martín-Villa, J.M.; Vaquero-Yuste, C.; Molina-Alejandre, M.; Juarez, I.; Suárez-Trujillo, F.; López-Nares, A.; Palacio-Gruber, J.; Barrera-Gutiérrez, L.; Fernández-Cruz, E.; Rodríguez-Sainz, C.; et al. HLA-G: Too Much or Too Little? Role in Cancer and Autoimmune Disease. Front. Immunol. 2022, 13, 796054. [Google Scholar] [CrossRef]
- Bodis, G.; Toth, V.; Schwarting, A. Role of Human Leukocyte Antigens (HLA) in Autoimmune Diseases. Rheumatol. Ther. 2018, 5, 5–20. [Google Scholar] [CrossRef]
- Brown, N.K.; Guandalini, S.; Semrad, C.; Kupfer, S.S. A Clinician’s Guide to Celiac Disease HLA Genetics. Am. J. Gastroenterol. 2019, 114, 1587–1592. [Google Scholar] [CrossRef]
- Diaconu, A.-D.; Ceasovschih, A.; Șorodoc, V.; Pomîrleanu, C.; Lionte, C.; Șorodoc, L.; Ancuța, C. Practical Significance of Biomarkers in Axial Spondyloarthritis: Updates on Diagnosis, Disease Activity, and Prognosis. Int. J. Mol. Sci. 2022, 23, 11561. [Google Scholar] [CrossRef]
- Clotman, K.; Janssens, K.; Specenier, P.; Weets, I.; De Block, C.E.M. Programmed Cell Death-1 Inhibitor–Induced Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2018, 103, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Ashida, K.; Sakamoto, R.; Sakaguchi, C.; Ogata, M.; Maruyama, K.; Sakamoto, S.; Ikeda, M.; Ohe, K.; Akasu, S.; et al. Human Leucocyte Antigen DR15, a Possible Predictive Marker for Immune Checkpoint Inhibitor–Induced Secondary Adrenal Insufficiency. Eur. J. Cancer 2020, 130, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced with Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Lo Preiato, V.; Salvagni, S.; Ricci, C.; Ardizzoni, A.; Pagotto, U.; Pelusi, C. Diabetes Mellitus Induced by Immune Checkpoint Inhibitors: Type 1 Diabetes Variant or New Clinical Entity? Review of the Literature. Rev. Endocr. Metab. Disord. 2021, 22, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, A.; Gustafson, M.P.; Bornschlegl, S.; Kottschade, L.; Delivanis, D.A.; Dietz, A.B.; Gandhi, M.; Ryder, M. Immune Checkpoint Inhibitor-Induced Thyroiditis Is Associated with Increased Intrathyroidal T Lymphocyte Subpopulations. Thyroid 2020, 30, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shen, M.; Zheng, X.; Chen, Y.; Zhao, R.; Gu, Y.; Yang, T. ICPis-Induced Autoimmune Polyendocrine Syndrome Type 2: A Review of the Literature and a Protocol for Optimal Management. J. Clin. Endocrinol. Metab. 2020, 105, e4208–e4218. [Google Scholar] [CrossRef]
- Delivanis, D.A.; Gustafson, M.P.; Bornschlegl, S.; Merten, M.M.; Kottschade, L.; Withers, S.; Dietz, A.B.; Ryder, M. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights into Underlying Involved Mechanisms. J. Clin. Endocrinol. Metab. 2017, 102, 2770–2780. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Dorak, M.T.; Bettinotti, M.P.; Bingham, C.O.; Shah, A.A. Association of HLA-DRB1 Shared Epitope Alleles and Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis. Rheumatology 2019, 58, 476–480. [Google Scholar] [CrossRef]
- Chang, H.; Shin, Y.; Keam, B.; Kim, M.; Im, S.; Lee, S. HLA-B27 Association of Autoimmune Encephalitis Induced by PD-L1 Inhibitor. Ann. Clin. Transl. Neurol. 2020, 7, 2243–2250. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwama, S.; Sugiyama, D.; Yasuda, Y.; Okuji, T.; Ito, M.; Ito, S.; Sugiyama, M.; Onoue, T.; Takagi, H.; et al. Anti-Pituitary Antibodies and Susceptible Human Leukocyte Antigen Alleles as Predictive Biomarkers for Pituitary Dysfunction Induced by Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e002493. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Berner, F.; Bomze, D.; Fässler, M.; Diem, S.; Cozzio, A.; Jörger, M.; Früh, M.; Driessen, C.; Lenz, T.L.; et al. Human Leukocyte Antigen Variation Is Associated with Adverse Events of Checkpoint Inhibitors. Eur. J. Cancer 2019, 107, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yu, Y.; Zhang, M.; Tang, Y.; Wu, D.; Wang, S.; Fang, Y.; Zhang, Y.; Meng, L.; Li, Y.; et al. Association between Germ-Line HLA and Immune-Related Adverse Events. Front. Immunol. 2022, 13, 952099. [Google Scholar] [CrossRef]
- Abed, A.; Law, N.; Calapre, L.; Lo, J.; Bhat, V.; Bowyer, S.; Millward, M.; Gray, E.S. Human Leucocyte Antigen Genotype Association with the Development of Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Treated with Single Agent Immunotherapy. Eur. J. Cancer 2022, 172, 98–106. [Google Scholar] [CrossRef]
- Purde, M.-T.; Niederer, R.; Wagner, N.B.; Diem, S.; Berner, F.; Hasan Ali, O.; Hillmann, D.; Bergamin, I.; Joerger, M.; Risch, M.; et al. Presence of Autoantibodies in Serum Does Not Impact the Occurrence of Immune Checkpoint Inhibitor-Induced Hepatitis in a Prospective Cohort of Cancer Patients. J. Cancer Res. Clin. Oncol. 2022, 148, 647–656. [Google Scholar] [CrossRef]
- Correale, P.; Saladino, R.E.; Giannarelli, D.; Sergi, A.; Mazzei, M.A.; Bianco, G.; Giannicola, R.; Iuliano, E.; Forte, I.M.; Calandruccio, N.D.; et al. HLA Expression Correlates to the Risk of Immune Checkpoint Inhibitor-Induced Pneumonitis. Cells 2020, 9, 1964. [Google Scholar] [CrossRef]
- Inaba, H.; Kaido, Y.; Ito, S.; Hirobata, T.; Inoue, G.; Sugita, T.; Yamamoto, Y.; Jinnin, M.; Kimura, H.; Kobayashi, T.; et al. Human Leukocyte Antigens and Biomarkers in Type 1 Diabetes Mellitus Induced by Immune-Checkpoint Inhibitors. Endocrinol. Metab. 2022, 37, 84–95. [Google Scholar] [CrossRef]
- Inaba, H.; Ariyasu, H.; Iwakura, H.; Kurimoto, C.; Takeshima, K.; Morita, S.; Furuta, H.; Hotomi, M.; Akamizu, T. Distinct Clinical Features and Prognosis between Persistent and Temporary Thyroid Dysfunctions by Immune-Checkpoint Inhibitors. Endocr. J. 2021, 68, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Magis, Q.; Gaudy-Marqueste, C.; Basire, A.; Loundou, A.; Malissen, N.; Troin, L.; Monestier, S.; Mallet, S.; Hesse, S.; Richard, M.-A.; et al. Diabetes and Blood Glucose Disorders Under Anti-PD1. J. Immunother. 2018, 41, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Iafolla, M.A.J.; Yang, C.; Chandran, V.; Pintilie, M.; Li, Q.; Bedard, P.L.; Hansen, A.; Lheureux, S.; Spreafico, A.; Razak, A.A.; et al. Predicting Toxicity and Response to Pembrolizumab Through Germline Genomic HLA Class 1 Analysis. JNCI Cancer Spectr. 2021, 5, pkaa115. [Google Scholar] [CrossRef]
- Berman, D.; Parker, S.M.; Siegel, J.; Chasalow, S.D.; Weber, J.; Galbraith, S.; Targan, S.R.; Wang, H.L. Ipilimumab efficacy and safety in patients with advanced melanoma: A retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010, 10, 6. [Google Scholar]
- Rusca, N.; Monticelli, S. MiR-146a in Immunity and Disease. Mol. Biol. Int. 2011, 2011, 437301. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, T.; Horos, R.; Jehn, J.; Schenz, J.; Muley, T.; Pelea, O.; Hofmann, S.; Kittner, P.; Kahraman, M.; Heuvelman, M.; et al. A Blood-Based MiRNA Signature with Prognostic Value for Overall Survival in Advanced Stage Non-Small Cell Lung Cancer Treated with Immunotherapy. NPJ Precis. Oncol. 2022, 6, 19. [Google Scholar] [CrossRef]
- Mastroianni, J.; Stickel, N.; Andrlova, H.; Hanke, K.; Melchinger, W.; Duquesne, S.; Schmidt, D.; Falk, M.; Andrieux, G.; Pfeifer, D.; et al. MiR-146a Controls Immune Response in the Melanoma Microenvironment. Cancer Res. 2019, 79, 183–195. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Marschner, D.; Falk, M.; Javorniczky, N.R.; Hanke-Müller, K.; Rawluk, J.; Schmitt-Graeff, A.; Simonetta, F.; Haring, E.; Dicks, S.; Ku, M.; et al. MicroRNA-146a Regulates Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors. JCI Insight 2020, 5, e132334. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Asadullina, D.; Rakhimov, R.; Izmailov, A.; Izmailov, A.; Gilyazova, G.; Galimov, S.; Pavlov, V.; Khusnutdinova, E.; Gilyazova, I. Exosomal MiRNA-146a Is Downregulated in Clear Cell Renal Cell Carcinoma Patients with Severe Immune-Related Adverse Events. Non-Coding RNA Res. 2022, 7, 159–163. [Google Scholar] [CrossRef]
- Xia, W.; Chen, H.; Chen, D.; Ye, Y.; Xie, C.; Hou, M. PD-1 Inhibitor Inducing Exosomal MiR-34a-5p Expression Mediates the Cross Talk between Cardiomyocyte and Macrophage in Immune Checkpoint Inhibitor–Related Cardiac Dysfunction. J. Immunother. Cancer 2020, 8, e001293. [Google Scholar] [CrossRef]
- Runtsch, M.C.; Hu, R.; Alexander, M.; Wallace, J.; Kagele, D.; Petersen, C.; Valentine, J.F.; Welker, N.C.; Bronner, M.P.; Chen, X.; et al. MicroRNA-146a Constrains Multiple Parameters of Intestinal Immunity and Increases Susceptibility to DSS Colitis. Oncotarget 2015, 6, 28556–28572. [Google Scholar] [CrossRef]
- Bogunia-Kubik, K.; Wysoczańska, B.; Piątek, D.; Iwaszko, M.; Ciechomska, M.; Świerkot, J. Significance of Polymorphism and Expression of MiR-146a and NFkB1 Genetic Variants in Patients with Rheumatoid Arthritis. Arch. Immunol. Ther. Exp. 2016, 64, 131–136. [Google Scholar] [CrossRef]
- Srivastava, A.; Nikamo, P.; Lohcharoenkal, W.; Li, D.; Meisgen, F.; Xu Landén, N.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. MicroRNA-146a Suppresses IL-17–Mediated Skin Inflammation and Is Genetically Associated with Psoriasis. J. Allergy Clin. Immunol. 2017, 139, 550–561. [Google Scholar] [CrossRef]
- Pantazi, D.; Tselepis, A.D. Cardiovascular Toxic Effects of Antitumor Agents: Pathogenetic Mechanisms. Thromb. Res. 2022, 213, S95–S102. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Nickl, L.; Walch-Rueckheim, B.; Krammes, L.; Rheinheimer, S.; Diener, C.; Taenzer, T.; Kehl, T.; Sester, M.; Lenhof, H.-P.; et al. Wrinkle in the Plan: MiR-34a-5p Impacts Chemokine Signaling by Modulating CXCL10/CXCL11/CXCR3-Axis in CD4+, CD8+ T Cells and M1 Macrophages. J. Immunother. Cancer 2020, 8, e001617. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.; Chaudhari, D.; Dhotre, D.; Shouche, Y.; Saroj, S. Restoration of Dysbiotic Human Gut Microbiome for Homeostasis. Life Sci. 2021, 278, 119622. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Darfeuille–Michaud, A. The Commensal Microbiota and Enteropathogens in the Pathogenesis of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1720–1728.e3. [Google Scholar] [CrossRef]
- Sakai, K.; Sakurai, T.; De Velasco, M.A.; Nagai, T.; Chikugo, T.; Ueshima, K.; Kura, Y.; Takahama, T.; Hayashi, H.; Nakagawa, K.; et al. Intestinal Microbiota and Gene Expression Reveal Similarity and Dissimilarity Between Immune-Mediated Colitis and Ulcerative Colitis. Front. Oncol. 2021, 11, 763468. [Google Scholar] [CrossRef]
- Bellaguarda, E.; Hanauer, S. Checkpoint Inhibitor-Induced Colitis. Am. J. Gastroenterol. 2020, 115, 202–210. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti–PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Sakurai, T.; De Velasco, M.A.; Sakai, K.; Nagai, T.; Nishiyama, H.; Hashimoto, K.; Uemura, H.; Kawakami, H.; Nakagawa, K.; Ogata, H.; et al. Integrative Analysis of Gut Microbiome and Host Transcriptomes Reveals Associations between Treatment Outcomes and Immunotherapy-induced Colitis. Mol. Oncol. 2022, 16, 1493–1507. [Google Scholar] [CrossRef]
- Mao, J.; Wang, D.; Long, J.; Yang, X.; Lin, J.; Song, Y.; Xie, F.; Xun, Z.; Wang, Y.; Wang, Y.; et al. Gut Microbiome Is Associated with the Clinical Response to Anti-PD-1 Based Immunotherapy in Hepatobiliary Cancers. J. Immunother. Cancer 2021, 9, e003334. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint-Blockade-Induced Colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R.; et al. Intestinal Microbiota Signatures of Clinical Response and Immune-Related Adverse Events in Melanoma Patients Treated with Anti-PD-1. Nat. Med. 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Chau, J.; Yadav, M.; Liu, B.; Furqan, M.; Dai, Q.; Shahi, S.; Gupta, A.; Nace Mercer, K.; Eastman, E.; Abu Hejleh, T.; et al. Prospective Correlation between the Patient Microbiome with Response to and Development of Immune-Mediated Adverse Effects to Immunotherapy in Lung Cancer. J. Clin. Oncol. 2021, 39, e21024. [Google Scholar] [CrossRef]
- Liu, T.; Xiong, Q.; Li, L.; Hu, Y. Intestinal Microbiota Predicts Lung Cancer Patients at Risk of Immune-Related Diarrhea. Immunotherapy 2019, 11, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, F.; Sun, B.; Liu, Y.; Tang, H.; Luo, J.; Chen, H.; Luo, Z. Intestinal Microbiome Associated with Immune-Related Adverse Events for Patients Treated with Anti-PD-1 Inhibitors, a Real-World Study. Front. Immunol. 2021, 12, 756872. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Liu, Y.; Tang, H.; Chen, D.; Xu, Y.; Chen, M.; Li, Y.; Wang, M.; Qian, J. Gut Microbiota Shed New Light on the Management of Immune-Related Adverse Events. Thorac. Cancer 2022, 13, 2681–2691. [Google Scholar] [CrossRef]
- Usyk, M.; Pandey, A.; Hayes, R.B.; Moran, U.; Pavlick, A.; Osman, I.; Weber, J.S.; Ahn, J. Bacteroides Vulgatus and Bacteroides Dorei Predict Immune-Related Adverse Events in Immune Checkpoint Blockade Treatment of Metastatic Melanoma. Genome Med. 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Gil-Cruz, C.; Perez-Shibayama, C.; De Martin, A.; Ronchi, F.; van der Borght, K.; Niederer, R.; Onder, L.; Lütge, M.; Novkovic, M.; Nindl, V.; et al. Microbiota-Derived Peptide Mimics Drive Lethal Inflammatory Cardiomyopathy. Science 2019, 366, 881–886. [Google Scholar] [CrossRef]
- Wang, F.; Yin, Q.; Chen, L.; Davis, M.M. Bifidobacterium Can Mitigate Intestinal Immunopathology in the Context of CTLA-4 Blockade. Proc. Natl. Acad. Sci. USA 2018, 115, 157–161. [Google Scholar] [CrossRef]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium Alters the Gut Microbiota and Modulates the Functional Metabolism of T Regulatory Cells in the Context of Immune Checkpoint Blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 27509–27515. [Google Scholar] [CrossRef]
- Hakozaki, T.; Richard, C.; Elkrief, A.; Hosomi, Y.; Benlaïfaoui, M.; Mimpen, I.; Terrisse, S.; Derosa, L.; Zitvogel, L.; Routy, B.; et al. The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 1243–1250. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurised Bacterium Blunts Colitis Associated Tumourigenesis by Modulation of CD8+ T Cells in Mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Hu, Z.I.; Link, V.M.; Lima-Junior, D.S.; Delaleu, J.; Bouladoux, N.; Han, S.-J.; Collins, N.; Belkaid, Y. Immune Checkpoint Inhibitors Unleash Pathogenic Immune Responses against the Microbiota. Proc. Natl. Acad. Sci. USA 2022, 119, e2200348119. [Google Scholar] [CrossRef]
- Pham, F.; Moinard-Butot, F.; Coutzac, C.; Chaput, N. Cancer and Immunotherapy: A Role for Microbiota Composition. Eur. J. Cancer 2021, 155, 145–154. [Google Scholar] [CrossRef]
- Wang, Q.; McLoughlin, R.M.; Cobb, B.A.; Charrel-Dennis, M.; Zaleski, K.J.; Golenbock, D.; Tzianabos, A.O.; Kasper, D.L. A Bacterial Carbohydrate Links Innate and Adaptive Responses through Toll-like Receptor 2. J. Exp. Med. 2006, 203, 2853–2863. [Google Scholar] [CrossRef]
- Park, R.; Umar, S.; Kasi, A. Immunotherapy in Colorectal Cancer: Potential of Fecal Transplant and Microbiota-Augmented Clinical Trials. Curr. Colorectal Cancer Rep. 2020, 16, 81–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Li, W.; Du, Y.; Hu, W.; Zhao, J. Biomarkers and Risk Factors for the Early Prediction of Immune-Related Adverse Events: A Review. Hum. Vaccines Immunother. 2022, 18, 2018894. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Zhang, Y.; Zhang, C.; Liu, X.; Shi, C. Intestinal Microbiota: A Potential Target for Enhancing the Antitumor Efficacy and Reducing the Toxicity of Immune Checkpoint Inhibitors. Cancer Lett. 2021, 509, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Fu, J.; Peng, X.; Tang, D.; Song, J. Intestinal Microbiota: The Driving Force behind Advances in Cancer Immunotherapy. Cancers 2022, 14, 4796. [Google Scholar] [CrossRef] [PubMed]
- Holmstroem, R.B.; Nielsen, O.H.; Jacobsen, S.; Riis, L.B.; Theile, S.; Bjerrum, J.T.; Vilmann, P.; Johansen, J.S.; Boisen, M.K.; Eefsen, R.H.L.; et al. COLAR: Open-Label Clinical Study of IL-6 Blockade with Tocilizumab for the Treatment of Immune Checkpoint Inhibitor-Induced Colitis and Arthritis. J. Immunother. Cancer 2022, 10, e005111. [Google Scholar] [CrossRef]
- Zamora, A.E.; Crawford, J.C.; Thomas, P.G. Hitting the Target: How T Cells Detect and Eliminate Tumors. J. Immunol. 2018, 200, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Bethune, M.T.; Joglekar, A.V. Personalized T Cell-Mediated Cancer Immunotherapy: Progress and Challenges. Curr. Opin. Biotechnol. 2017, 48, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Berner, F.; Bomze, D.; Lichtensteiger, C.; Walter, V.; Niederer, R.; Ali, O.H.; Wyss, N.; Bauer, J.; Freudenmann, L.K.; Marcu, A.; et al. Autoreactive Napsin A-Specific T Cells Are Enriched in Lung Tumors and Inflammatory Lung Lesions during Immune Checkpoint Blockade. Sci. Immunol. 2022, 7, eabn9644. [Google Scholar] [CrossRef]
- Tahir, S.A.; Gao, J.; Miura, Y.; Blando, J.; Tidwell, R.S.S.; Zhao, H.; Subudhi, S.K.; Tawbi, H.; Keung, E.; Wargo, J.; et al. Autoimmune Antibodies Correlate with Immune Checkpoint Therapy-Induced Toxicities. Proc. Natl. Acad. Sci. USA 2019, 116, 22246–22251. [Google Scholar] [CrossRef] [PubMed]
- Gowen, M.F.; Giles, K.M.; Simpson, D.; Tchack, J.; Zhou, H.; Moran, U.; Dawood, Z.; Pavlick, A.C.; Hu, S.; Wilson, M.A.; et al. Baseline Antibody Profiles Predict Toxicity in Melanoma Patients Treated with Immune Checkpoint Inhibitors. J. Transl. Med. 2018, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Johannet, P.; Liu, W.; Fenyo, D.; Wind-Rotolo, M.; Krogsgaard, M.; Mehnert, J.M.; Weber, J.S.; Zhong, J.; Osman, I. Baseline Serum Autoantibody Signatures Predict Recurrence and Toxicity in Melanoma Patients Receiving Adjuvant Immune Checkpoint Blockade. Clin. Cancer Res. 2022, 28, 4121–4130. [Google Scholar] [CrossRef]
- Colen, R.R.; Fujii, T.; Bilen, M.A.; Kotrotsou, A.; Abrol, S.; Hess, K.R.; Hajjar, J.; Suarez-Almazor, M.E.; Alshawa, A.; Hong, D.S.; et al. Radiomics to Predict Immunotherapy-Induced Pneumonitis: Proof of Concept. Investig. New Drugs 2018, 36, 601–607. [Google Scholar] [CrossRef]
- Jing, Y.; Yang, J.; Johnson, D.B.; Moslehi, J.J.; Han, L. Harnessing Big Data to Characterize Immune-Related Adverse Events. Nat. Rev. Clin. Oncol. 2022, 19, 269–280. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Primer 2020, 6, 38. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing Toxicities Associated with Immune Checkpoint Inhibitors: Consensus Recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immune Checkpoint Inhibitor-Related Adverse Events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of Response, Resistance, and Toxicity to Immune Checkpoint Blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-G.; Wong, A.H.-H.; Wang, H.; Tan, F.; Chen, X.; Jin, S.-H.; He, S.-S.; Shen, G.; Wang, Y.-J.; Frey, B.; et al. Elucidation of the Application of Blood Test Biomarkers to Predict Immune-Related Adverse Events in Atezolizumab-Treated NSCLC Patients Using Machine Learning Methods. Front. Immunol. 2022, 13, 862752. [Google Scholar] [CrossRef]
- Iivanainen, S.; Ekstrom, J.; Virtanen, H.; Kataja, V.V.; Koivunen, J.P. Electronic Patient-Reported Outcomes and Machine Learning in Predicting Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapies. BMC Med. Inform. Decis. Mak. 2021, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Booth, A. A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies: A Typology of Reviews. Maria J. Grant Andrew Booth. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Type of Parameter | Design (No. Patients) | Type of Tumor | Type of irAE | Main Findings | Reference |
|---|---|---|---|---|---|
| ANA, RF and ATA (detected before ICI initiation) | Retrospective (n = 137) | NSCLC | All types | Autoantibodies were associated with a higher risk of irAEs (OR 3.25, p = 0.001) | Toi Y. JAMA Oncol 2019 [27] |
| ANA (detected before ICI initiation) | Retrospective (n = 83) | NSCLC | All types | ANA were not associated with irAEs, though the risk of irAEs tended to be higher with higher titers of ANAs | Yoneshima Y. Lung Cancer 2019 [28] |
| ANA (detected before ICI initiation) | Retrospective (n = 191) | Pan-tumor | All types | ANA were not associated with irAEs, except for colitis (22% vs. 1.6%, p = 0.002) | Sakakida T. Clin Transl Oncol 2020 [29] |
| ANA, anti-dsDNA antibody, ENA *, RF, ACPA, ASMA, AMA, anti-LKM antibody and ATA (developing after ICI initiation) | Retrospective (n = 133) | Melanoma | All types | The association between irAEs and seroconversions was nonsignificant considering all irAEs and any autoantibody (OR 2.92, p = 0.12), but became significant when focusing on irAEs related to the autoantibodies tested (OR 3.64, p = 0.04) | de Moel EC. Cancer Immunol Res 2019 [30] |
| ANA, ENA and ASMA (developing after ICI initiation, within 30 days) | Retrospective (n = 92) | NSCLC | All types | Early detection of autoantibodies was associated with a higher risk of irAEs (HR not available, p = 0.002) | Giannicola R. Mol Clin Oncol 2019 [31] |
| ATA (titer increase from baseline) | Prospective (n = 78) | Pan-tumor | All types | Increases in anti-Tg and anti-TPO titers ≥ 1.5 from baseline were associated with irAE occurrence (OR 17.4, p = 0.015; OR 6.1, p = 0.035; respectively) | Music M. F1000Res 2020 [14] |
| ANA, RF, ATA and ANCA (before and after ICI initiation) | Retrospective (n = 69) | Pan-tumor | All types | Positivity for any autoantibody was associated with a higher risk of irAEs (OR 46.61, p = 0.010) | Les I. Ann Med 2021 [25] |
| ANA (detected before ICI initiation) | Retrospective (n = 68) | Urothelial carcinoma | All types | Patients with ANA positivity at a titer >1:160 developed irAEs more frequently (p = 0.029) and earlier (p = 0.052) | Castel-Ajgal Z. Clin Genitourin Cancer 2022 [32] |
| ANA, ENA **, RF, ACPA, autoimmune hepatopathy profile # and myopathy profile † (detected before ICI initiation) | Prospective (n = 44) | Pan-cancer | All types | The frequency of irAEs did not differ as a function of positivity for any autoantibody (OR 0.62, p = 0.480) or ANA titers (OR 0.79, p = 0.529) | Barth DA. Cancer Med 2022 [33] |
| ANA and ATA (detected before ICI initiation) | Retrospective (n = 159) | NSCLC | All types | ANA titer ≥ 1:320 was related to irAEs (OR 4.9, p = 0.01), especially to skin subtypes (9.7% in patients with ANA <1:320 vs. 32% in patients with ANA ≥ 1:320, p = 0.003) | Zhang D. Transl Lung Cancer Res 2022 [34] |
| ANA, anti-Ro52 and ATA (detected before ICI initiation) | Retrospective (n = 177) | Pan-tumor | All types | ANA and anti-Ro52 positivity was not associated with a higher risk of irAEs. ATA positivity was more common in patients with than without thyroiditis (75% vs. 13.8%, p < 0.001) | Tang H. Front Immunol 2022 [35] |
| ANA, ATA, AGAD, AChR and PA-IgG (detected before ICI initiation) | Retrospective (n = 275) | Pan-tumor | All types | There were no associations between autoantibodies and irAEs, except between ATA and thyroiditis (39.5% in anti-Tg-positive vs. 12.5% in anti-Tg-negative patients, p < 0.01) | Izawa N. ESMO 2022 [36] |
| ANA (detected before ICI initiation) | Retrospective (n = 266) | NSCLC | All types | There were no significant differences in the frequency of irAEs between positive and negative ANA patients and between high and low ANA titers | Mouri A. Front Oncol 2021 [37] |
| ANA (before and after ICI initiation) | Prospective (n = 152) | Pan-tumor | All types | There was no association between irAEs and ANA at baseline or developing. Patients who became ANA-positive during follow-up were more likely to have severe irAEs than those who were ANA-positive at baseline and ANA-negative patients (42.8% vs. 26.1% vs. 9.1%, p = 0.05) | Alserawan L. Int J Mol Sci 2022 [38] |
| ANA, RF and ACPA (before and 6 weeks after ICI initiation) | Prospective (n = 60) | Melanoma | All types | There was no association between baseline seropositivity for ANA/RF/ACPA and time to first irAE (p = 0.39). ANA/RF/ACPA-negative patients experienced more thyroid irAEs than ANA/RF/ACPA-positive patients (p = 0.006) | Gosh N. J Immunother Cancer 2022 [39] |
| At Baseline (Before Immune-Checkpoint Inhibitor Initiation) | |||||
|---|---|---|---|---|---|
| Type of Parameter | Study Design (No. Patients) | Type of Tumor | Type of irAE | Main Findings | Reference |
| ALC | Retrospective (n = 167) | Pan-tumor | All types | Grade ≥ 2 irAEs were associated with ALC > 2000/μL (OR 1.996, p < 0.05) | Diehl A. Oncotarget 2017 [41] |
| AEC | Retrospective (n = 45) | Melanoma | Endocrine irAEs | irAEs were associated with AEC > 240/μL (OR 1.601, p = 0.045) | Nakamura Y. Jpn J Clin Oncol 2019 [42] |
| AEC | Retrospective (n = 95) | Pan-tumor | All types | AEC > 0.045 × 109/L was predictive of irAEs (OR 4.114, p = 0.014) | Ma Y. World J Surg Oncol 2022 [43] |
| NLR | Prospective (n = 1187) | Pan-tumor (blood and solid organ cancers) | All types | NLR > 4.78 was predictive of grade 4 and 5 irAEs (OR not available, p = 0.0137) * | Ruste V. Eur J Cancer 2021 [44] |
| NLR and PLR | Retrospective (n = 184) | NSCLC | All types | PLR < 180 was the only independent predictor of irAEs (OR 2.3, p = 0.017) | Pavan A. Oncologist 2019 [45] |
| dNLR | Retrospective (n = 391) | Pan-tumor | All types | dNLR ≥ 3 was protective against irAEs (OR 0.37, p = 0.012) | Eun Y. Sci Rep 2019 [46] |
| ANC, PC, NLR and PLR | Retrospective (n = 150) | NSCLC | All types | Grade 3–4 irAEs were associated with ANC (p = 0.009), PC (p = 0.023), NLR (p= 0.023) and PLR (p = 0.0016) * (cut-off values and ORs not provided) | Liu W. Cancer Manag Res 2021 [47] |
| RLC and AEC | Retrospective (n = 105) | Pan-tumor | All types | irAEs were associated with RLC < 28.5% (OR 3.60, p = 0.027) and AEC > 0.175 × 109/L (OR 3.44, p = 0.020) | Bai R. Cancer Biol Med 2021 [48] |
| NLR | Retrospective (n = 115) | NSCLC | All types | irAEs were associated with NLR < 2.86 (OR 2.69, p = 0.016) | Fujimoto A. Thorac Cancer 2021 [49] |
| ALC, AMC, APC, NLR, MLR and PLR | Retrospective (n = 470) | Pan-tumor | All types | irAEs were associated with ALC > 2.6 K/μL (aOR 4.3, p = 0.002), AMC > 0.29 K/μL (aOR 2.34, p = 0.03), PC > 145 K/μL (aOR 2.23, p = 0.03), NLR ≤ 5.3 (aOR 2.07, p = 0.01), MLR ≤ 0.76 (aOR 2.96, p = 0.01) and PLR ≤ 534 (aOR 5.05, p = 0.04) ** | Michailidou D. Sci Rep 2021 [50] |
| NLR | Metanalysis # (n = 6696) | NSCLC | All types | irAEs were associated with NLR ≥ 5 (OR = 1.046, p = 0.026) | Suazo-Zepeda E. Cancer Immunol Immunother 2021 [51] |
| ALC, LMR, NLR and PLR | Retrospective (n = 92) | NSCLC | All types | ALC > 1450/mm3 (aOR 0.24, p = 0.003) and LMR > 1.6 (OR 0.12, p = 0.004) were associated with a lower risk of irAEs. NLR > 2.3 (aOR 5.99, p = 0.005) and PLR > 165 (OR = 2.87, p = 0.022) were associated with a higher risk of irAEs † | Egami S. J Cancer 2021 [52] |
| ALC | Retrospective (n = 667) | NSCLC | All types | ALC was positively associated with irAE risk (OR 2.556, p = 0.001; ALC cut-off value not provided) | Xu H. Exp Cell Res 2022 [53] |
| NLR | Retrospective (n = 147) | Pan-tumor | All types | NLR < 3 was associated with a higher rate of irAE (aOR 2.27, p = 0.034) | Lee PY. Cancers (Basel) 2021 [54] |
| AEC | Retrospective (n = 300) | NSCLC | Pneumonitis | Pneumonitis was associated with AEC ≥ 0.125 × 109/L (HR 2.825, p < 0.001) | Chu X. Lung Cancer 2020 [55] |
| ALC | Retrospective (n = 110) | Pan-tumor | Myocarditis | ALC 1.6 K/μL in myocarditis group vs. 1.3 K/μL in non-myocarditis group (p = 0.02) * | Drobni ZD. J Am Heart Assoc 2020 [56] |
| NLR | Retrospective (n = 73) | Gastric and renal cancers | Grade 3 and 4 irAEs | NLR < 4.3 was associated with lower risk of grade 3–4 irAEs (OR 0.024, p = 0.014) | Takada S. Asian Pac J Cancer Prev 2022 [57] |
| During Follow-Up (After Immune-Checkpoint Inhibitor Initiation) | |||||
| Type of Parameter | Study Design | Type of Tumor | Type of irAE | Main Findings | Reference |
| WBC RLC (on the day of irAE detection) | Retrospective (n =101) | Melanoma | Lung and gastrointestinal irAEs | 59.1% increase in WBC (OR = 6.04, p = 0.014) and 32.3% decrease in RLC (OR = 5.01, p = 0.012) were predictive of irAEs | Fujisawa Y. J Dermatol Sci 2017 [58] |
| ALC at 1 month | Retrospective (n = 167) | Pan-tumor | All types | Grade ≥ 2 irAEs were associated with ALC > 2000/μL (OR = 1.813, p < 0.05) | Diehl A. Oncotarget 2017 [41] |
| REC at 1 month WBC at 1 month | Retrospective (n = 45) | Melanoma | Endocrine irAEs and vitiligo | REC > 3.2% was predictive of irAEs (OR = 5.111, p = 0.025) *. A summative increase in WBC by 100 was protective against vitiligo (OR = 0.823, p = 0.0023). | Nakamura Y. Jpn J Clin Oncol 2019 [42] |
| ANC NLR PLR (treatment cycle before onset of the irAE) | Retrospective (n = 150) | NSCLC | All types | Multiple univariate associations were described, namely, between *: 1.91 × 109/L decrease in ANC and grade 1–2 irAEs (p = 0.013) 1.11 × 109/L decrease in ANC and grade 3–4 irAEs (p = 0.003) 0.62 decrease in NLR and grade 1–2 irAEs (p = 0.013) 0.76 decrease in NLR and grade 3–4 irAEs (p = 0.011) 89.26 decrease in PLR and grade 1–2 irAEs (p = 0.011) (comparative data between baseline and pre-irAE cycle) | Liu W. Cancer Manag Res 2021 [47] |
| ALC at 2 weeks | Retrospective (n = 171) | NSCLC | All types | Early onset of irAEs was associated with ALC > 820/mm3 (aOR = 3.58, p = 0.07) † | Egami S. Front Oncol 2021 [59] |
| NLR at second course (2 to 3 weeks after the first dose) | Retrospective (n = 243) | Esophageal, gastric and colon cancer | All types | irAEs (any grade) were associated with NLR < 3 (OR = 0.894, p= 0.044) | Zhang Z. Cancers (Basel) 2022 [60] |
| ALC NLR (from baseline to last ICI dose; and from baseline to myocarditis onset) | Retrospective (n = 110) | Pan-tumor | Myocarditis | irAEs were associated with a decrease in ALC (1.6 K/μL to 1.4 K/μL to 1.1 K/μL, p < 0.001) and an increase in NLR (3.5 to 4.1 to 6.6, p < 0.001) * | Drobni ZD. J Am Heart Assoc 2020 [56] |
| NLR (at the onset of the irAE) | Retrospective (n = 73) | Gastric and renal cancers | Grade 3 and 4 irAEs | ∆NLR >120% was associated with increased risk of irAEs (OR = 10.48, p = 0.033) | Takada S. Asian Pac J Cancer Prev 2022 [57] |
| Type of Taxonomic Category or Microorganism | Type of Effect | Type of irAE Assessed | Related References |
|---|---|---|---|
| Bacteroidetes phylum * | Protective factor | Colitis | Dubin K. Nat Commun 2016 [191] Chaput N. Ann Oncol 2019 [63] Liu T. Immunotherapy 2019 [194] Sakai K. Front Oncol 2021 [186] Liu W. Front Immunol 2021 [195] |
| Bacteroidetes phylum | Protective factor | Pancreatic irAEs | Tan B. Thorac Cancer 2021 [196] |
| Bacteroides dorei Bacteroides vulgatus | Risk factor Protective factor | General irAEs | Usyk M. Genome Med 2021 [197] |
| Bacteroides fragilis Bacteroides thetaiotaomicron | Protective factor | Colitis (in mice) | Vétizou M. Science 2015 [198] |
| Bacteroides thetaiotaomicron Bacteroides faecis | Risk factor | Myocarditis | Gil-Cruz C. Science 2019 [199] |
| Bacteroides intestinalis | Risk factor | General irAEs (grade ≥ 3) | Andrews MC. Nat Med 2021 [109] |
| Prevotellamassilia timonensis (from Bacteroidetes phylum) | Risk factor | Severe colitis | Mao J. J Immunother Cancer 2021 [190] |
| Firmicutes phylum ** | Risk factor | Colitis | Dubin K. Nat Commun 2016 [191] Chaput N. Ann Oncol 2019 [63] Gopalakrishnan V. Science 2018 [188] Liu T. Immunotherapy 2019 [194] |
| Firmicutes phylum | Risk factor | Pancreatic irAEs | Tan B. Thorac Cancer 2021 [196] |
| Phascolarctobacterium genus (from Firmicutes phylum) | Protective factor | Colitis | Liu T. Immunotherapy 2019 [194] |
| Faecalibacterium genus (from Firmicutes phylum) | Protective factor | Absent or grade 0–2 colitis | Liu W. Front Immunol 2021 [195] |
| Bifidobacterium Bifidobacterium breve # | Protective factor | Colitis (in mice) | Wang F. Proc Natl Acad Sci USA 2018 [200] Sun S. Proc Natl Acad Sci USA 2020 [201] |
| Bifidobacterium | Protective factor | General irAEs | Chau J. J Clin Oncol 2021 [193] |
| Lactobacillus rhamnosum | Protective factor | Colitis (in mice) | Sun S. Proc Natl Acad Sci USA 2020 [201] |
| Lactobacillaceae family Raoultella genus Akkermansia species Agathobacter genus | Protective factor Protective factor Protective factor Risk factor | Low-grade irAEs Low-grade irAEs Low-grade irAEs High-grade irAEs | Hakozaki T. Cancer Immunol Res 2020 [202] |
| Enterobacteriaceae family † | Protective factor (remission of colitis) | Colitis | Sakurai T. Mol Oncol 2022 [189] |
| Intestinibacter barlettii Anaerotignum lactatifermentans Dorea formicigenerans | Risk factor Protective factor Protective factor | General irAEs (grade ≥ 3) | Andrews MC. Nat Med 2021 [109] |
| Streptococcus genus Paecalibacterium genus Stenotrophomonas genus | Risk factor | General irAEs (grade ≥ 3) | Liu W. Front Immunol 2021 [195] |
| Lachnospiraceae species Streptococcaceae species | Risk factor | General irAEs | McCulloch JA. Nat Med 2022 [192] |
| Akkermansia muciniphila | Protective factor | Colitis | Wang L. Gut 2020 [203] |
| Alispides genus | Protective factor | Pancreatic irAEs | Tan B. Thorac Cancer 2021 [196] |
| Lachnospiraceae genus | Risk factor | Pancreatic irAEs | Tan B. Thorac Cancer 2021 [196] |
| Burkholderia cepacia | Protective factor | Colitis in mice | Vétizou M. Science 2015 [198] |
| Proteobacteria phylum | |||
| Desulfovibrio | Protective factor | General irAEs | Chau J. J Clin Oncol 2021 [193] |
| Veillonela | Risk factor | Colitis | Liu T. Immunotherapy 2019 [194] |
| Staphylococcus epidermidis | Risk factor | Dermatitis (in mice) | Hu ZI. Proc Natl Acad Sci USA 2022 [204] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Les, I.; Martínez, M.; Pérez-Francisco, I.; Cabero, M.; Teijeira, L.; Arrazubi, V.; Torrego, N.; Campillo-Calatayud, A.; Elejalde, I.; Kochan, G.; et al. Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events. Cancers 2023, 15, 1629. https://doi.org/10.3390/cancers15051629
Les I, Martínez M, Pérez-Francisco I, Cabero M, Teijeira L, Arrazubi V, Torrego N, Campillo-Calatayud A, Elejalde I, Kochan G, et al. Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events. Cancers. 2023; 15(5):1629. https://doi.org/10.3390/cancers15051629
Chicago/Turabian StyleLes, Iñigo, Mireia Martínez, Inés Pérez-Francisco, María Cabero, Lucía Teijeira, Virginia Arrazubi, Nuria Torrego, Ana Campillo-Calatayud, Iñaki Elejalde, Grazyna Kochan, and et al. 2023. "Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events" Cancers 15, no. 5: 1629. https://doi.org/10.3390/cancers15051629
APA StyleLes, I., Martínez, M., Pérez-Francisco, I., Cabero, M., Teijeira, L., Arrazubi, V., Torrego, N., Campillo-Calatayud, A., Elejalde, I., Kochan, G., & Escors, D. (2023). Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events. Cancers, 15(5), 1629. https://doi.org/10.3390/cancers15051629





