Sodium New Houttuyfonate Induces Apoptosis of Breast Cancer Cells via ROS/PDK1/AKT/GSK3β Axis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis
2.2. Reagents and Antibodies
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Cell Migration Assay
2.6. Cell Invasion Assay
2.7. Apoptosis Assay
2.8. Intracellular ROS Assay
2.9. Transmission Electron Microscopy
2.10. Western Blot Analysis
2.11. Cellular Immunofluorescence Staining
2.12. In Vivo Experiment
2.13. Mouse Tissue Section Staining
2.14. Statistical Analysis
3. Results
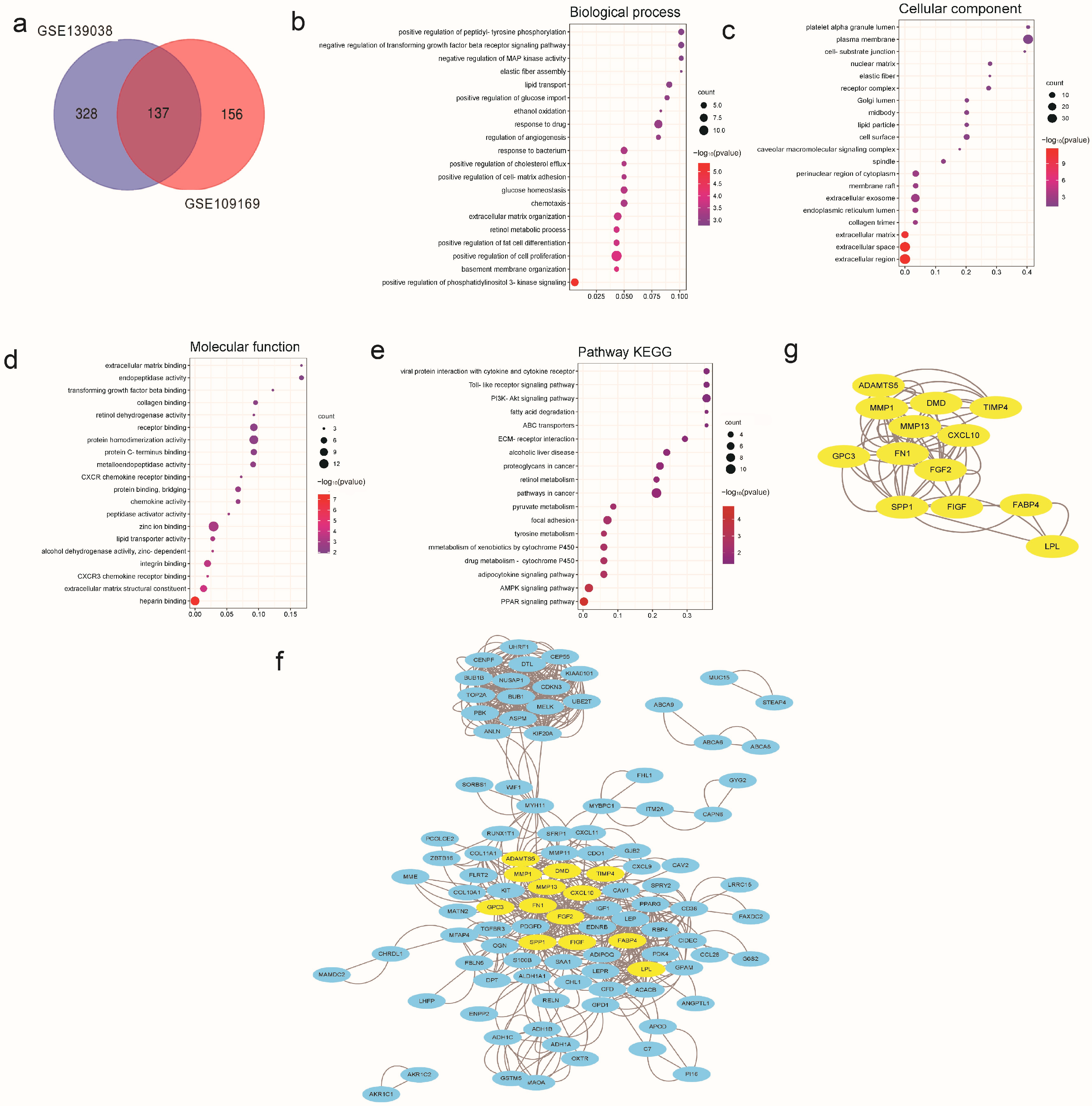
3.1. DEGs’ Analysis of Breast Cancer Based on the GEO Database
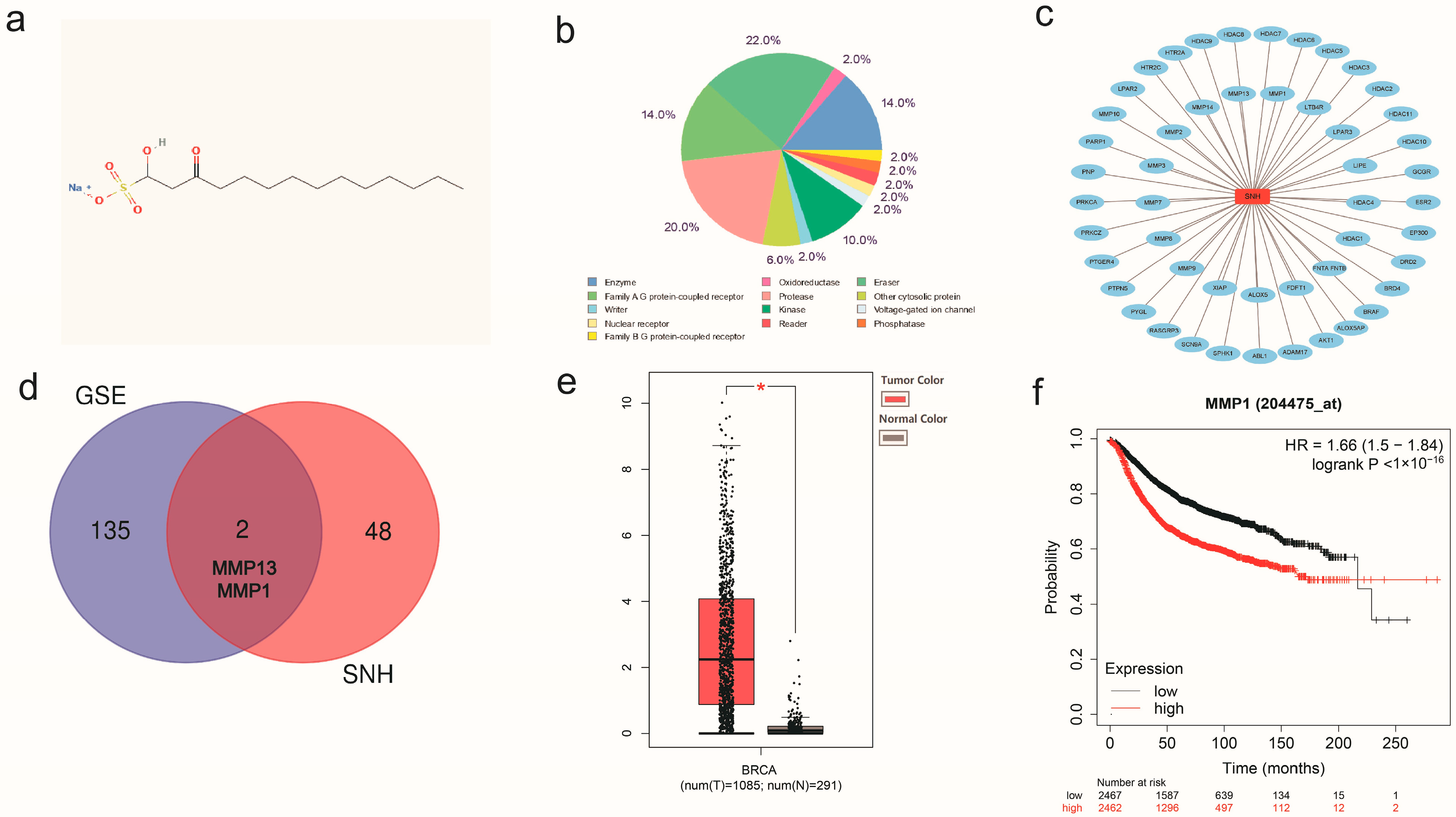
3.2. Network Pharmacological Analysis of SNH
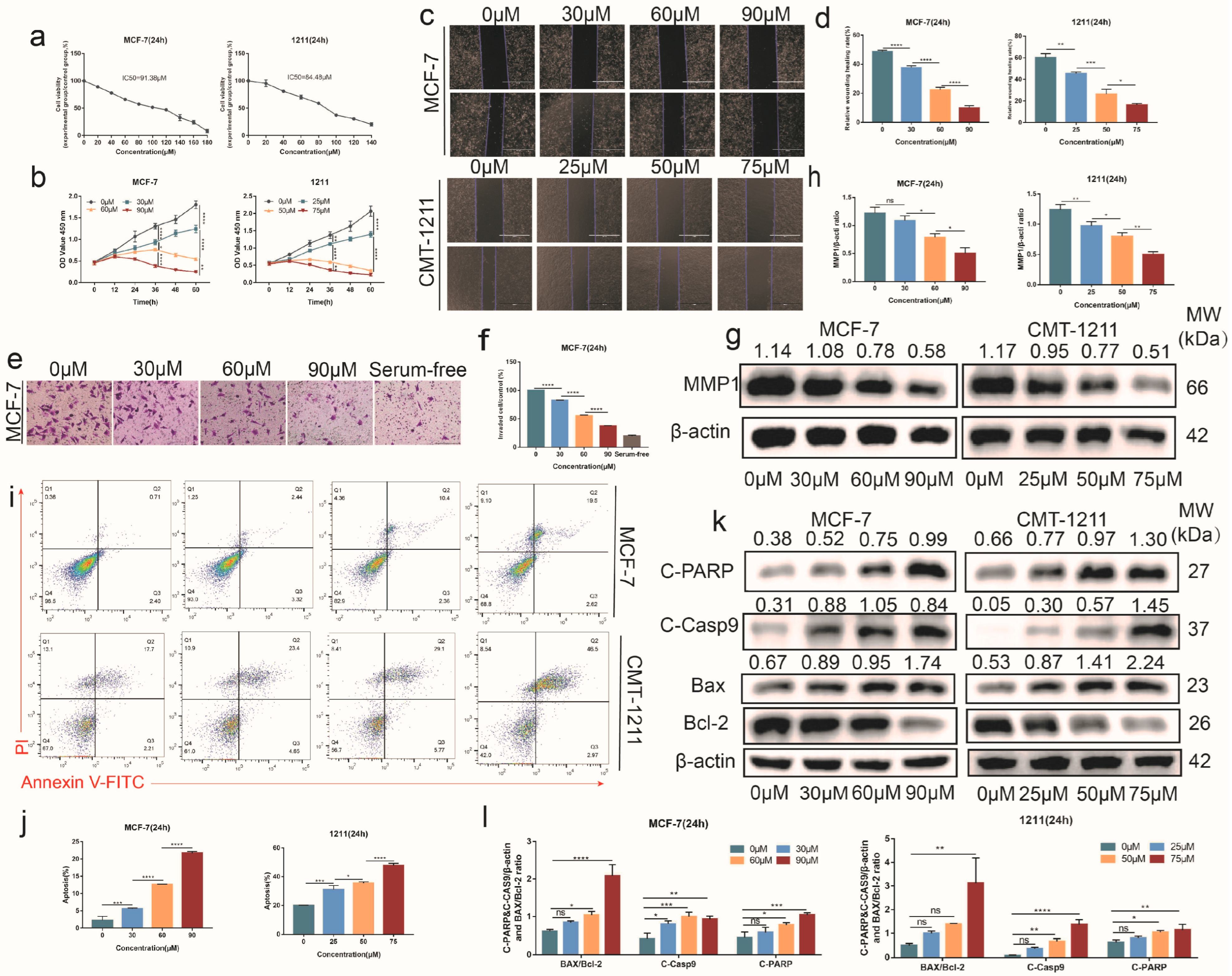
3.3. SNH Inhibited the Proliferation and Invasiveness of Breast Cancer Cells
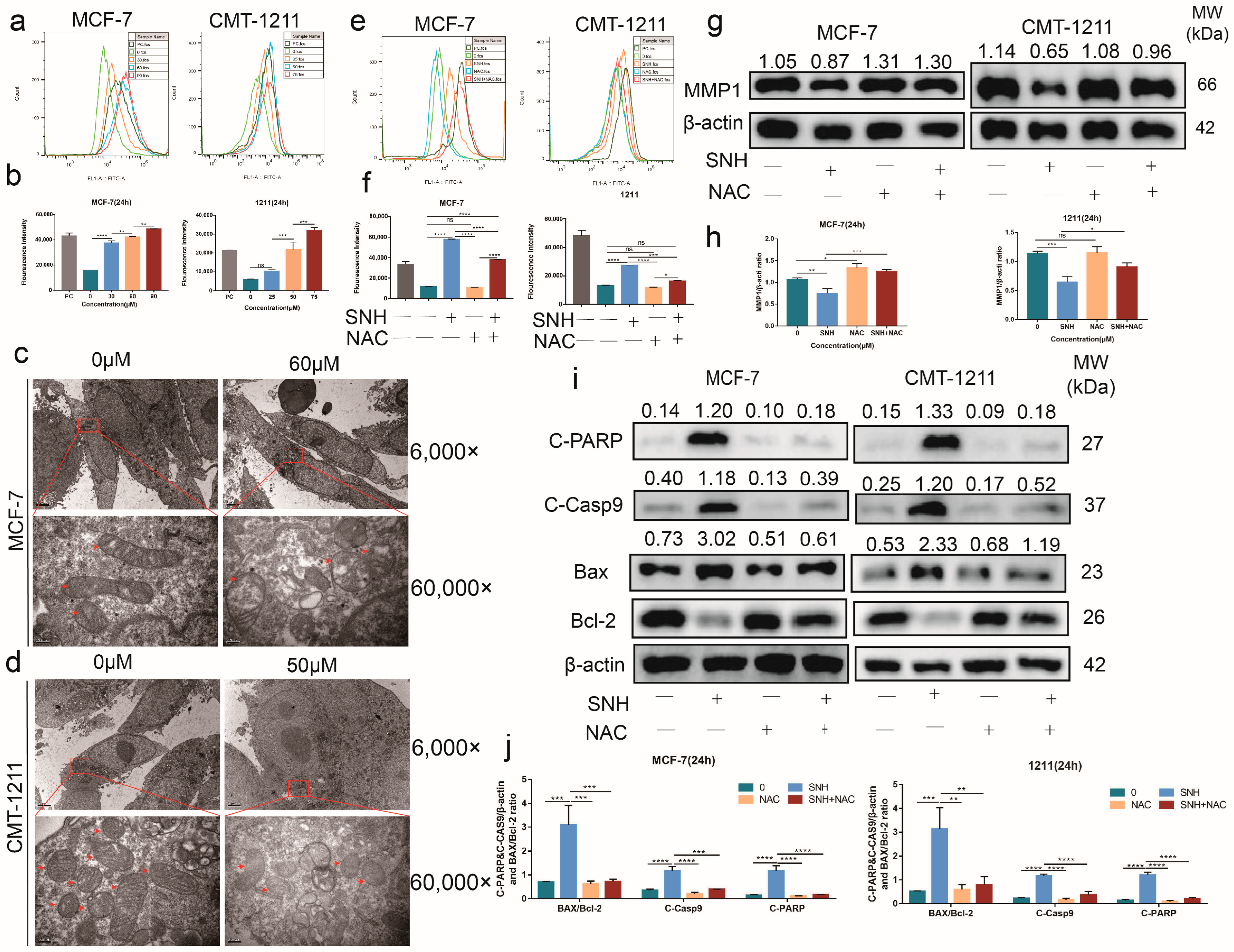
3.4. SNH Induced Cell Apoptosis by Increasing Intracellular ROS Level
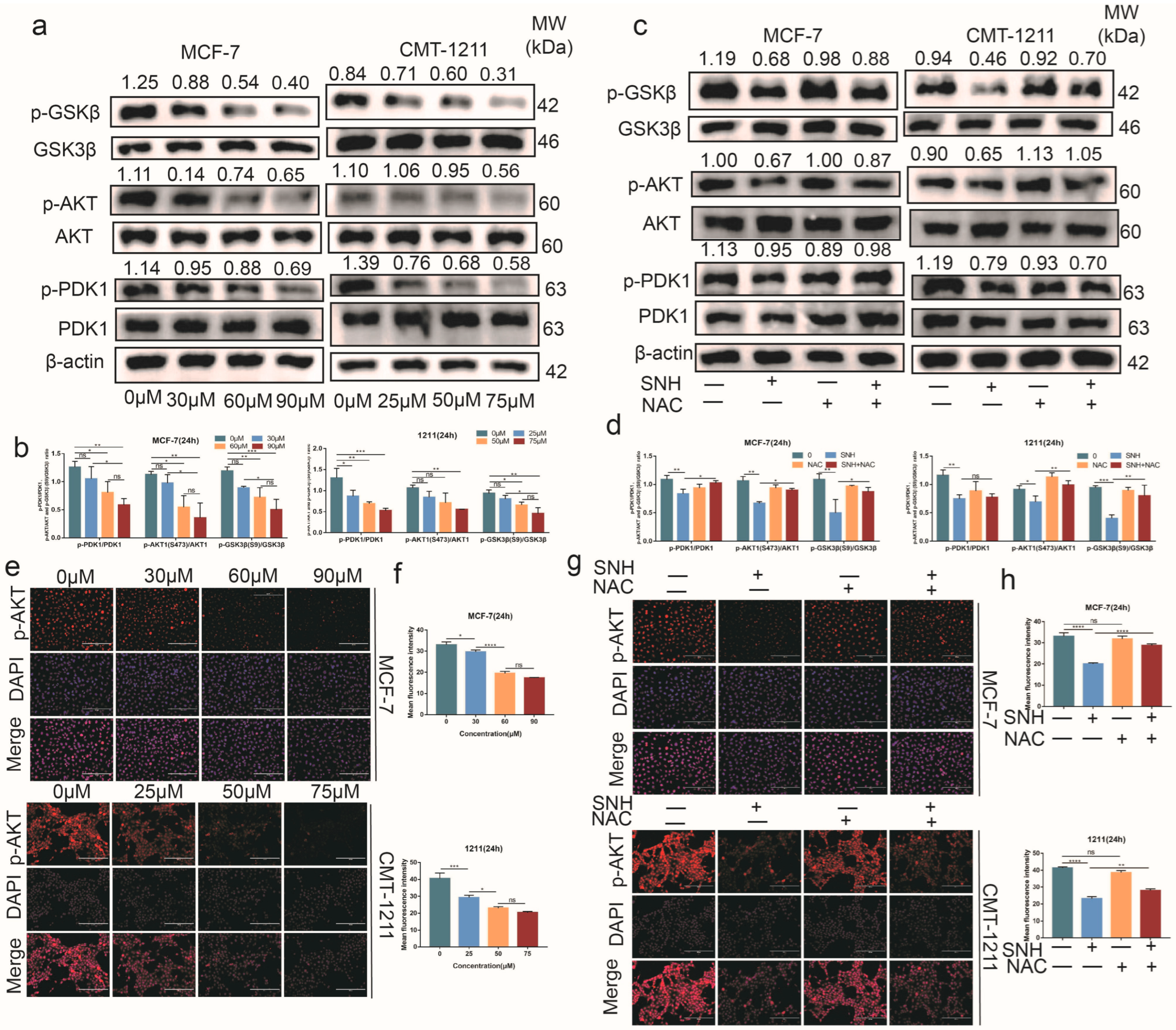
3.5. SNH Induced Apoptosis by Suppressing the Activation of the PDK1-AKT-GSK3β Pathway via ROS
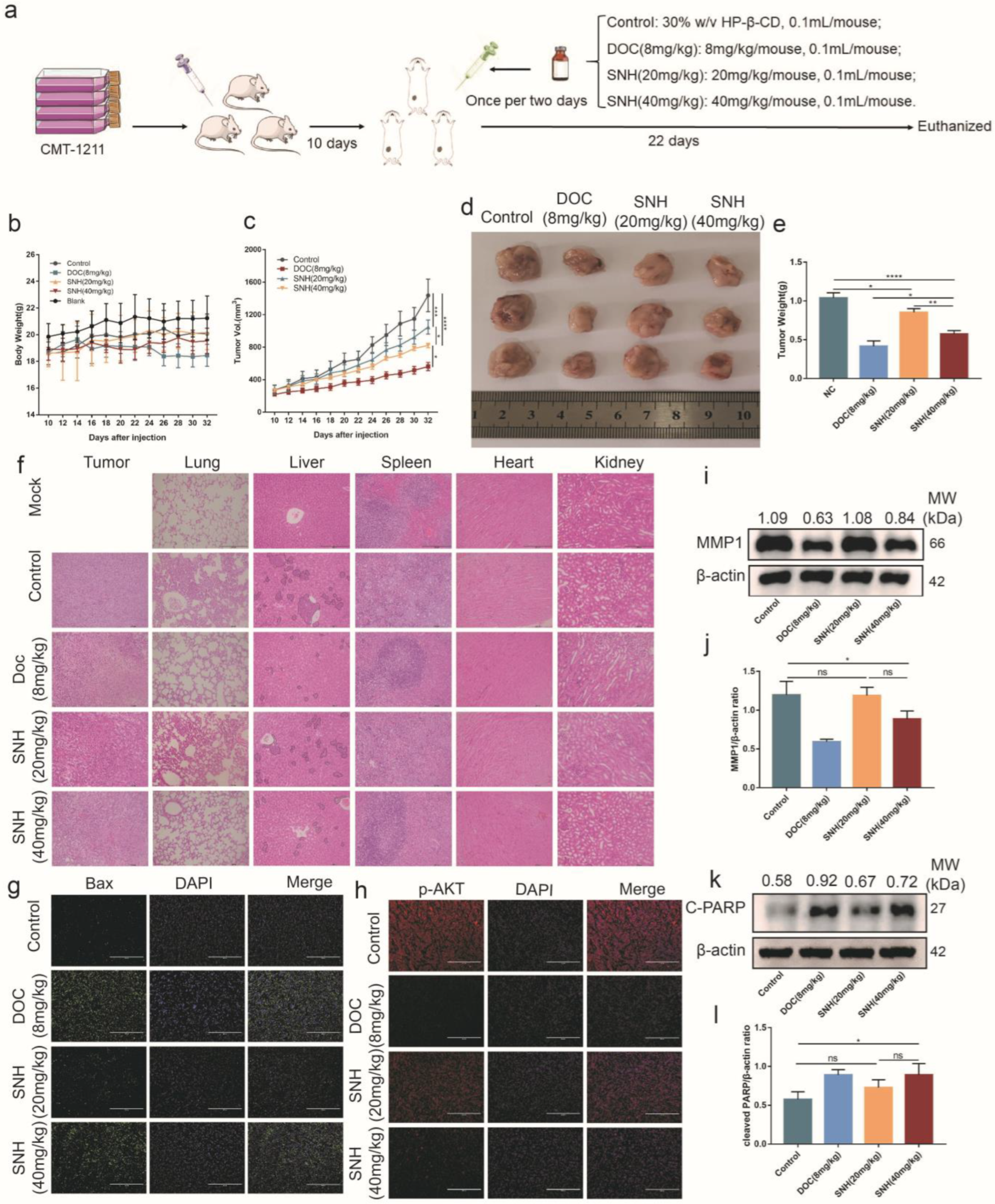
3.6. SNH Inhibited the Growth of Breast Tumor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| BAX | Bcl2-Associated X |
| Bcl-2 | B-cell lymphoma-2 |
| Casp9 | Caspase 9 |
| CCK-8 | Cell Counting Kit-8 |
| DCFH-DA | 2’,7’-Dichlorodihydrofluorescein diacetate |
| GO | Gene Ontology |
| GSK3β | Glycogensynthasekinase-3β |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NAC | N-Acetyl-L-cysteine |
| PARP | Poly ADP-ribose polymerase |
| PDK1 | 3-phosphoinositide-dependent protein kinase-1 |
| PI3K | Phosphatidylinositol3-kinase |
| PMSF | Phenylmethanesulfonyl fluoride |
| RIPA | Radio Immunoprecipitation Assay |
| ROS | Reactive oxygen species |
| SNH | Sodium new houttuyfonate |
References
- International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 15 December 2020).
- Nguyen, F.; Peña, L.; Ibisch, C.; Loussouarn, D.; Gama, A.; Rieder, N.; Belousov, A.; Campone, M.; Abadie, J. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: Natural history and prognostic factors. Breast Cancer Res. Treat. 2018, 167, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Yang, I.S.; Seung, B.J.; Lee, S.; Kim, D.; Ha, Y.J.; Seo, M.K.; Kim, K.K.; Kim, H.S.; Cheong, J.H.; et al. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat. Commun. 2020, 11, 3616. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lee, K.H.; Nam, A.R.; Cho, J.Y. Genome-Wide Methylation Profiling in Canine Mammary Tumor Reveals miRNA Candidates Associated with Human Breast Cancer. Cancers 2019, 11, 1466. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Deal, A.M.; Reeve, B.B.; Basch, E.; Chen, Y.T.; Park, J.H.; Shachar, S.S.; Carey, L.A.; Reeder-Hayes, K.E.; Dees, E.C.; et al. Congruence of patient- and clinician-reported toxicity in women receiving chemotherapy for early breast cancer. Cancer 2020, 126, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Shi, T.; Du, G.; Liu, W.; Yin, X.F.; Sun, X.; Pan, Y.; He, Q.Y. iTRAQ-Based Proteomics Revealed the Bactericidal Mechanism of Sodium New Houttuyfonate against Streptococcus pneumoniae. J. Agric. Food Chem. 2016, 64, 6375–6382. [Google Scholar] [CrossRef]
- Wu, J.; Wu, D.; Zhao, Y.; Si, Y.; Mei, L.; Shao, J.; Wang, T.; Yan, G.; Wang, C. Sodium New Houttuyfonate Inhibits Candida albicans Biofilm Formation by Inhibiting the Ras1-cAMP-Efg1 Pathway Revealed by RNA-seq. Front. Microbiol. 2020, 11, 2075. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, F.; Zeng, M.; Zhang, J.; Liu, Y.; Xin, C.; Mao, Y.; Song, Z. Antifungal Activity of Sodium New Houttuyfonate Against Aspergillus fumigatus in vitro and in vivo. Front. Microbiol. 2022, 13, 856272. [Google Scholar] [CrossRef]
- Dai, K.; Chen, L.; Liu, J.; Ding, Y.; Gu, C.; Lu, X. MiR-147a mediated by sodium new houttuyfonate could enhance radiosensitivity of non-small cell lung cancer cells via suppressing STAT3. Adv. Clin. Exp. Med. 2021, 30, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Hu, C.; Li, Q.; Cheng, Z.; Gu, L.; Li, H.; Guo, Y.; Li, Q.; Lu, Y.; Li, K.; et al. Sodium new houttuyfonate suppresses metastasis in NSCLC cells through the Linc00668/miR-147a/slug axis. J. Exp. Clin. Cancer Res. 2019, 38, 155. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside Induces Breast Cancer Cells Apoptosis via ROS-Mediated NF-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 21, 131. [Google Scholar] [CrossRef]
- Liu, J.B.; Li, Z.F.; Lu, L.; Wang, Z.Y.; Wang, L. Glyphosate damages blood-testis barrier via NOX1-triggered oxidative stress in rats: Long-term exposure as a potential risk for male reproductive health. Environ. Int. 2022, 159, 107038. [Google Scholar] [CrossRef]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020, 241, 117148. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.G.; Zhao, Y.; Wang, Z.Y.; Fan, R.F.; Liu, Z.P.; Wang, L. Epigenetic regulator BRD4 is involved in cadmium-induced acute kidney injury via contributing to lysosomal dysfunction, autophagy blockade and oxidative stress. J. Hazard Mater. 2022, 423, 127110. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, Y.; He, P.; Fan, Y.; Zhong, X.; Zheng, H.; Luo, T. PI3K/AKT/mTOR-Targeted Therapy for Breast Cancer. Cells 2022, 11, 2508. [Google Scholar] [CrossRef] [PubMed]
- Nagini, S. Breast Cancer: Current Molecular Therapeutic Targets and New Players. Anticancer Agents Med. Chem. 2017, 17, 152–163. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, S.; Zhu, X.; Qiu, J.; Deng, G.; Qiu, C. Alpinetin inhibits breast cancer growth by ROS/NF-κB/HIF-1α axis. J. Cell Mol. Med. 2020, 24, 8430–8440. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Q.; Zheng, Y.; Lu, J. Anti-breast cancer and toxicity studies of total secondary saponin from Anemone raddeana Rhizome on MCF-7 cells via ROS generation and PI3K/AKT/mTOR inactivation. J. Ethnopharmacol. 2020, 259, 112984. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Shyam, H.; Agarwal, S.; Sharma, R.; Nag, T.C.; Dwivedi, A.K.; Balapure, A.K. Genistein potentiates Centchroman induced antineoplasticity in breast cancer via PI3K/Akt deactivation and ROS dependent induction of apoptosis. Life Sci. 2019, 239, 117073. [Google Scholar] [CrossRef]
- Beurel, E.; Jope, R.S. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 2006, 79, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Liu, Q.; Wang, X.; He, L.; Zhang, T.; Zhou, H.; Zhu, X.; Zhou, T.; Deng, G.; Qiu, C. The combination of hydroxychloroquine and 2-deoxyglucose enhances apoptosis in breast cancer cells by blocking protective autophagy and sustaining endoplasmic reticulum stress. Cell Death Discov. 2022, 8, 286. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, 991–995. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array dataepository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Sheikh, A.; Hussain, S.A.; Ghori, Q.; Naeem, N.; Fazil, A.; Giri, S.; Sathian, B.; Mainali, P.; Al Tamimi, D.M. The spectrum of genetic mutations in breast cancer. Asian Pac. J. Cancer Prev. 2015, 16, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Gusterson, B.A.; Stein, T. Human breast development. Semin. Cell Dev. Biol. 2012, 23, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, J.; Li, Y.; Chen, Z.; Qi, D.; Zhang, Z. Immune Effect of Active Components of Traditional Chinese Medicine on Triple-Negative Breast Cancer. Front. Pharmacol. 2021, 12, 731741. [Google Scholar] [CrossRef] [PubMed]
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: Immunophenotypes and prognostic significance. Breast Cancer Res. Treat. 2018, 167, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, M.; Liu, Y.; Mu, W.; Deng, G.; Li, C.; Qiu, C. Selenium Induces an Anti-tumor Effect Via Inhibiting Intratumoral Angiogenesis in a Mouse Model of Transplanted Canine Mammary Tumor Cells. Biol. Trace Elem. Res. 2016, 171, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ji, W.; Zhong, H.; Wang, L.; Zhu, X.; Zhu, J. Anti-tumor effect of volatile oil from Houttuynia cordata Thunb. on HepG2 cells and HepG2 tumor-bearing mice. RSC Adv. 2019, 9, 31517–31526. [Google Scholar] [CrossRef]
- Maes, M.E.; Grosser, J.A.; Fehrman, R.L.; Schlamp, C.L.; Nickells, R.W. Completion of BAX recruitment correlates with mitochondrial fission during apoptosis. Sci. Rep. 2019, 9, 16565. [Google Scholar] [CrossRef] [PubMed]
- Grosser, J.A.; Fehrman, R.L.; Keefe, D.; Redmon, M.; Nickells, R.W. The effects of a mitochondrial targeted peptide (elamipretide/SS31) on BAX recruitment and activation during apoptosis. BMC Res. Notes 2021, 14, 198. [Google Scholar] [CrossRef]
- Renault, T.T.; Teijido, O.; Antonsson, B.; Dejean, L.M.; Manon, S. Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): Keep your friends close but your enemies closer. Int. J. Biochem. Cell Biol. 2013, 45, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Miao, Y.; Liu, S. Withaferin A induces apoptosis by ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2018, 503, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- DeHart, D.N.; Fang, D.; Heslop, K.; Li, L.; Lemasters, J.J.; Maldonado, E.N. Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells. Biochem. Pharmacol. 2018, 148, 155–162. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Zhang, X.; Ullah, R.; Tong, J.; Shen, Y. The role of the PI3K/AKT signalling pathway in the corneal epithelium: Recent updates. Cell Death Dis. 2022, 13, 513. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Dong, J.; Peng, J.; Zhang, H.; Mondesire, W.H.; Jian, W.; Mills, G.B.; Hung, M.C.; Meric-Bernstam, F. Role of glycogen synthase kinase 3beta in rapamycin-mediated cell cycle regulation and chemosensitivity. Cancer Res. 2005, 65, 1961–1972. [Google Scholar] [CrossRef]
- Farago, M.; Dominguez, I.; Landesman-Bollag, E.; Xu, X.; Rosner, A.; Cardiff, R.D.; Seldin, D.C. Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res. 2005, 65, 5792–5801. [Google Scholar] [CrossRef]
- Beurel, E.; Kornprobst, M.; Blivet-Van Eggelpoël, M.J.; Cadoret, A.; Capeau, J.; Desbois-Mouthon, C. GSK-3beta reactivation with LY294002 sensitizes hepatoma cells to chemotherapy-induced apoptosis. Int. J. Oncol. 2005, 27, 215–222. [Google Scholar]
- Armstrong, J.S. Mitochondrial membrane permeabilization: The sine qua non for cell death. Bioessays 2006, 28, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Bhat-Nakshatri, P.; Patel, N.M.; Constantinidou, D.; Ali, S.; Nakshatri, H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: A new model for anti-estrogen resistance. J. Biol. Chem. 2001, 276, 9817–9824. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44+/CD24-) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Zhang, E.; Shi, H.; Yang, L.; Wu, X.; Wang, Z. Ginsenoside Rd regulates the Akt/mTOR/p70S6K signaling cascade and suppresses angiogenesis and breast tumor growth. Oncol. Rep. 2017, 38, 359–367. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Feng, H.; Yin, B.; Li, W.; Wang, X.; Umar, T.; Gao, H.; Zhou, N.; Qiu, C. Sodium New Houttuyfonate Induces Apoptosis of Breast Cancer Cells via ROS/PDK1/AKT/GSK3β Axis. Cancers 2023, 15, 1614. https://doi.org/10.3390/cancers15051614
He L, Feng H, Yin B, Li W, Wang X, Umar T, Gao H, Zhou N, Qiu C. Sodium New Houttuyfonate Induces Apoptosis of Breast Cancer Cells via ROS/PDK1/AKT/GSK3β Axis. Cancers. 2023; 15(5):1614. https://doi.org/10.3390/cancers15051614
Chicago/Turabian StyleHe, Lixin, Huili Feng, Baoyi Yin, Wenxuan Li, Xiao Wang, Talha Umar, Hongbo Gao, Ning Zhou, and Changwei Qiu. 2023. "Sodium New Houttuyfonate Induces Apoptosis of Breast Cancer Cells via ROS/PDK1/AKT/GSK3β Axis" Cancers 15, no. 5: 1614. https://doi.org/10.3390/cancers15051614
APA StyleHe, L., Feng, H., Yin, B., Li, W., Wang, X., Umar, T., Gao, H., Zhou, N., & Qiu, C. (2023). Sodium New Houttuyfonate Induces Apoptosis of Breast Cancer Cells via ROS/PDK1/AKT/GSK3β Axis. Cancers, 15(5), 1614. https://doi.org/10.3390/cancers15051614




