A Dual Biomarker TK1 Protein and CA125 or HE4-Based Algorithm as a Better Diagnostic Tool than ROMA Index in Early Detection of Ovarian Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Patient Characteristics
2.3. Measurement of CA125 and HE4
2.4. The Risk of Ovarian Malignancy Algorithm (ROMA Index)
2.5. Serum TK Activity (STK1a) and TK1 Protein (STK1p) Determinations
2.6. Statistical Analysis
3. Results
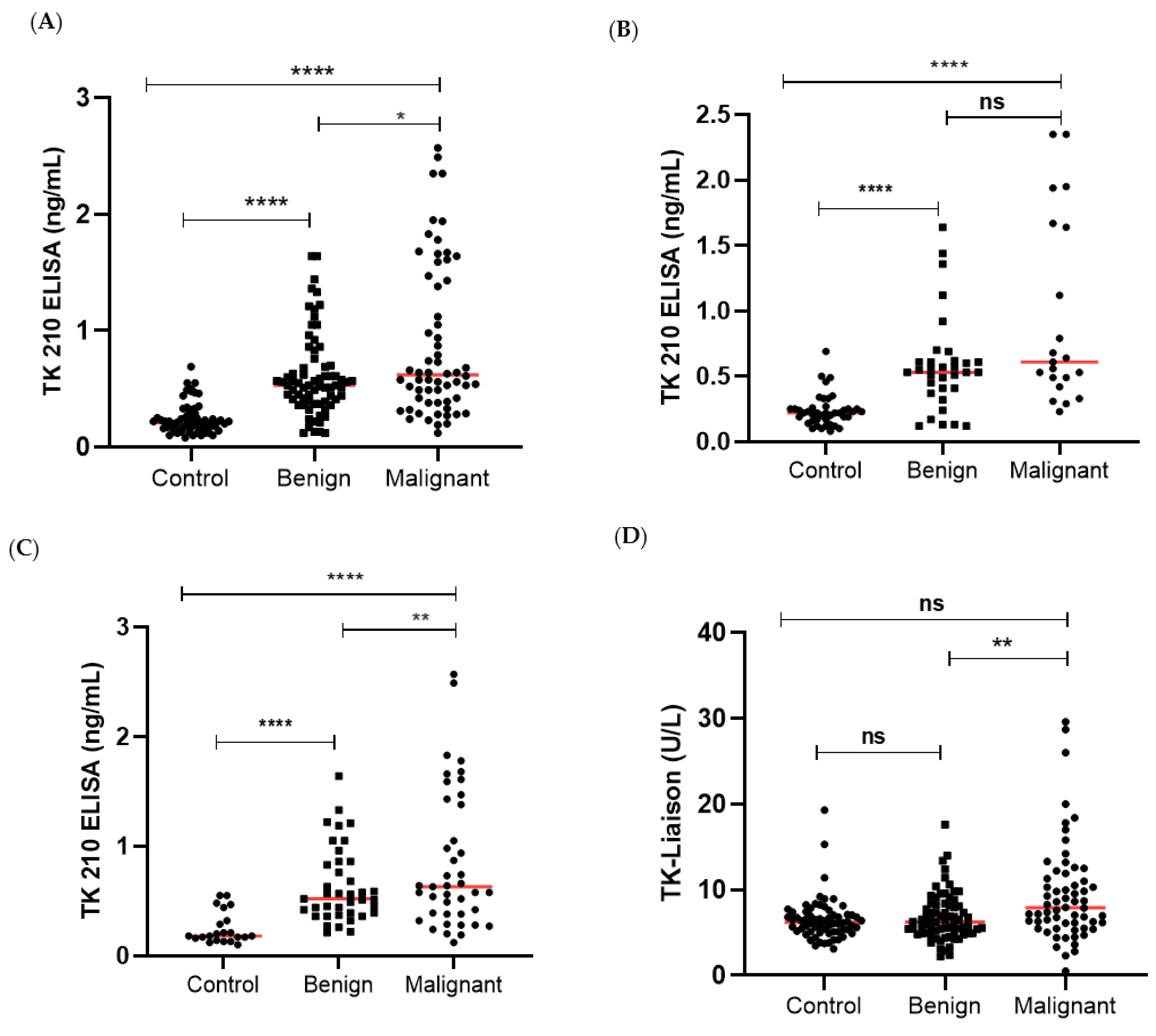
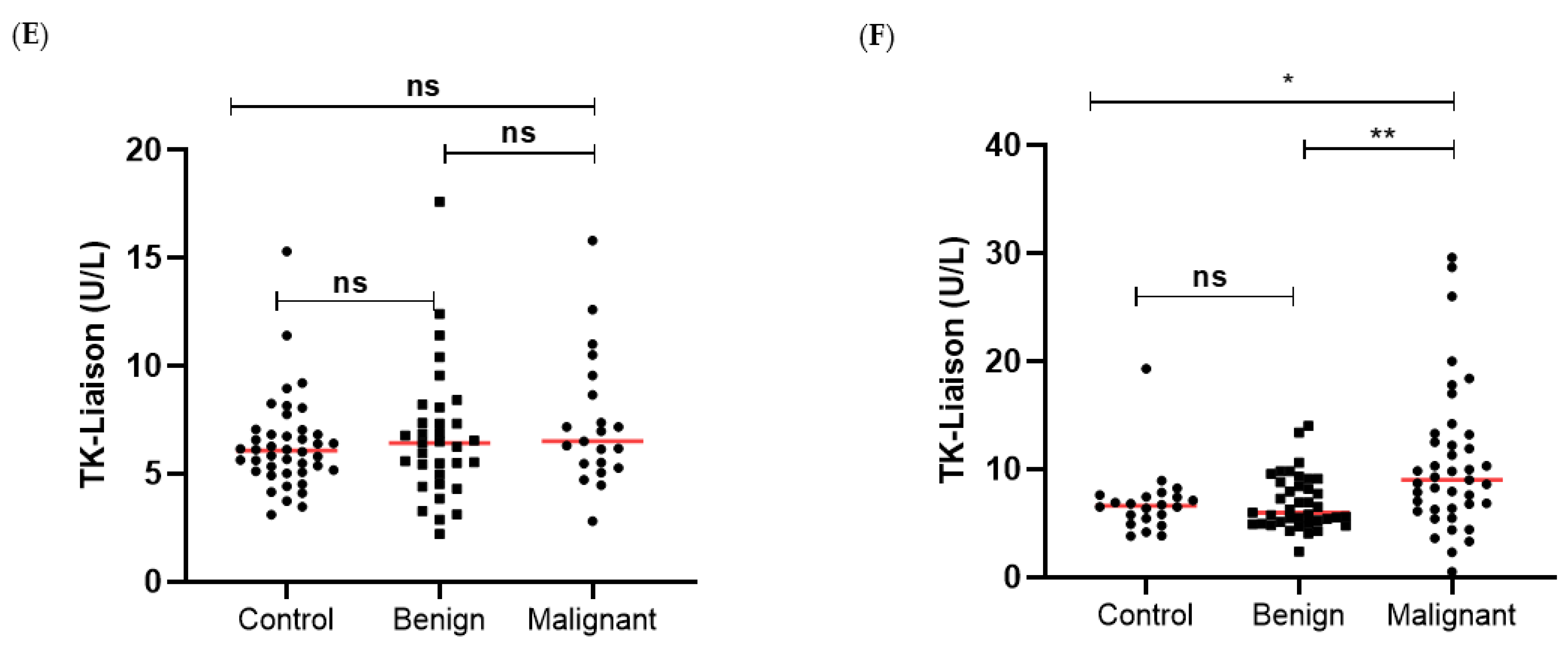
3.1. TK1 Levels in the Different Patient Groups
3.2. All Ovarian Cancers Versus all Healthy Controls
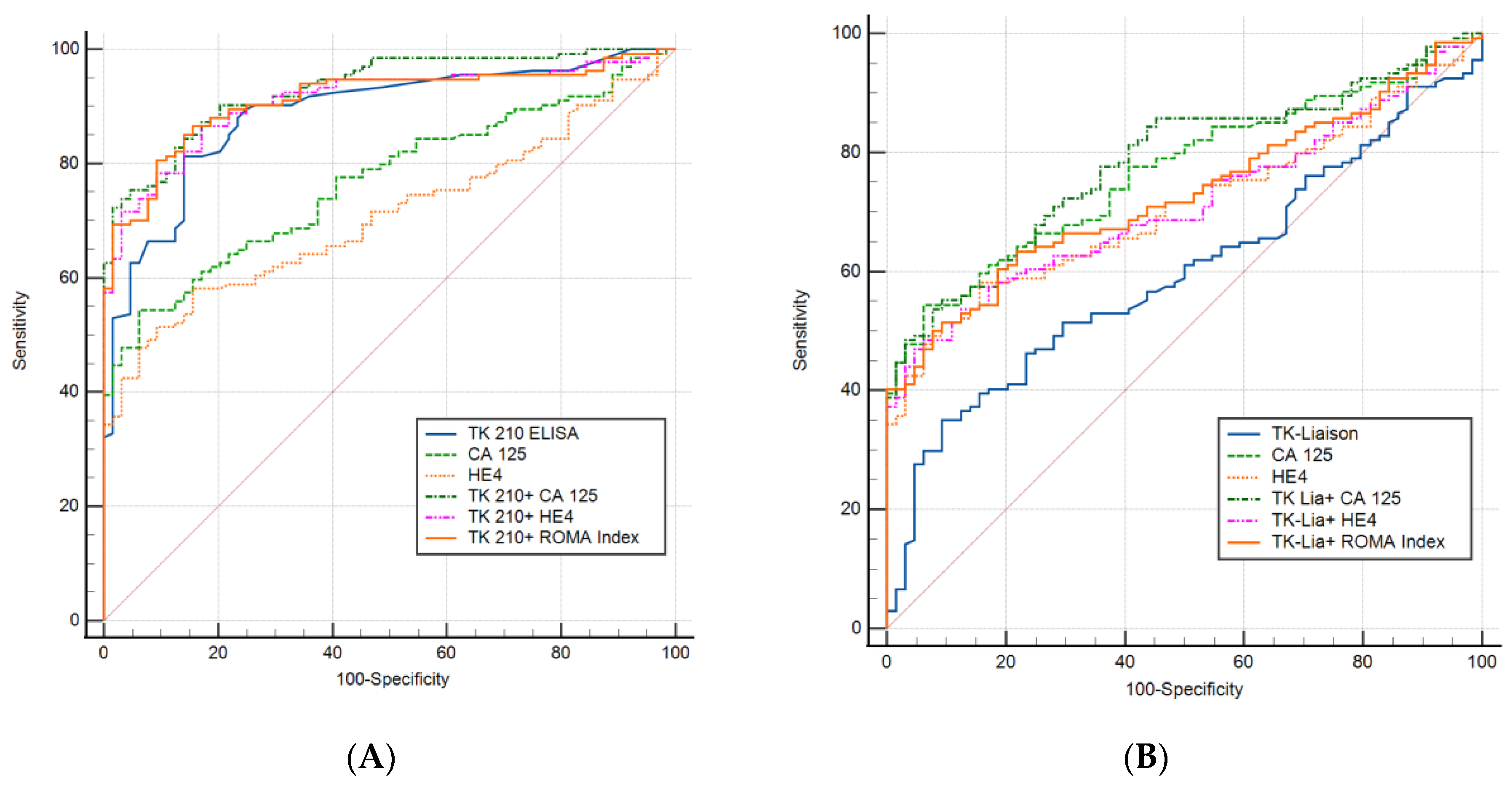
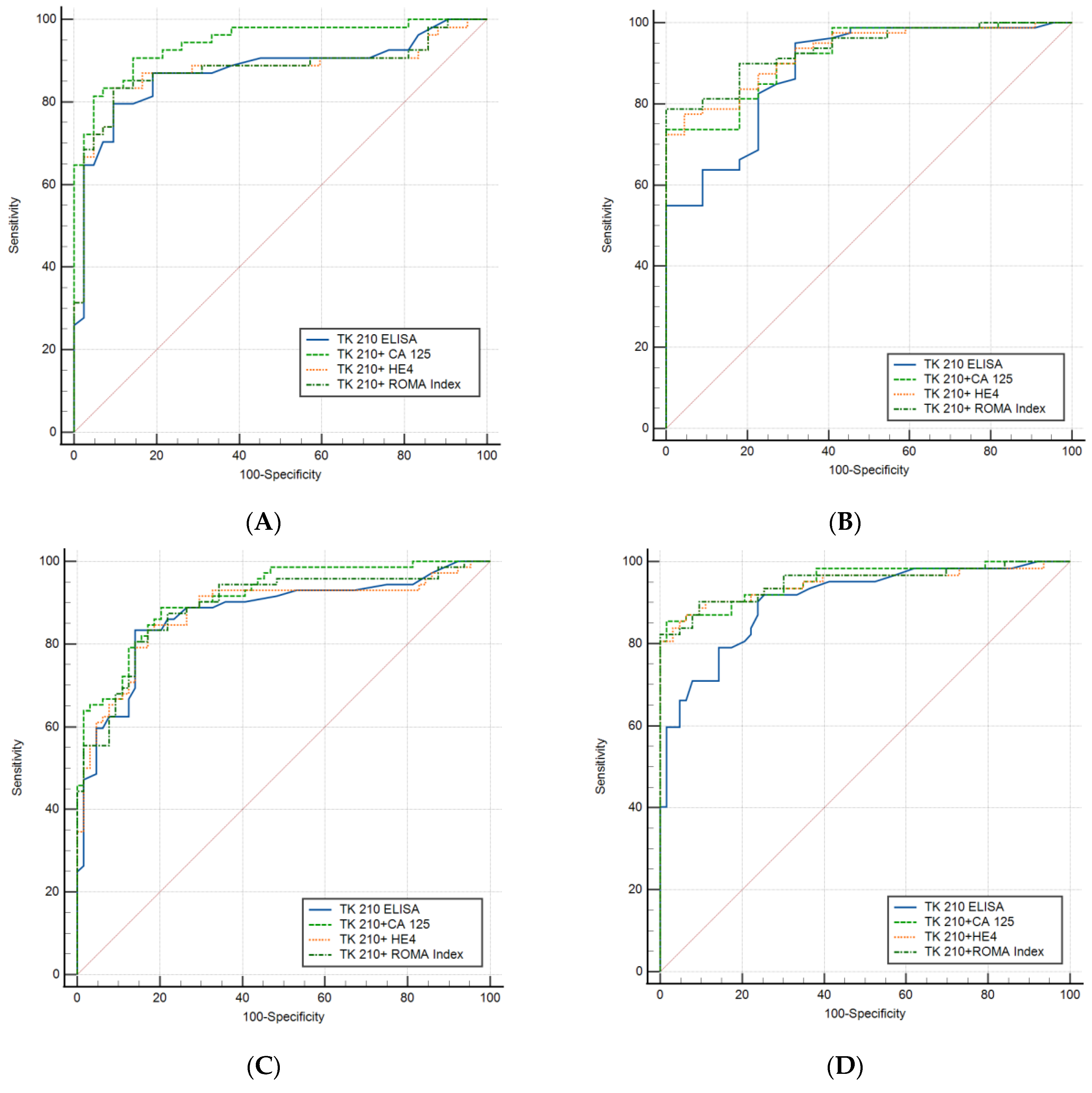
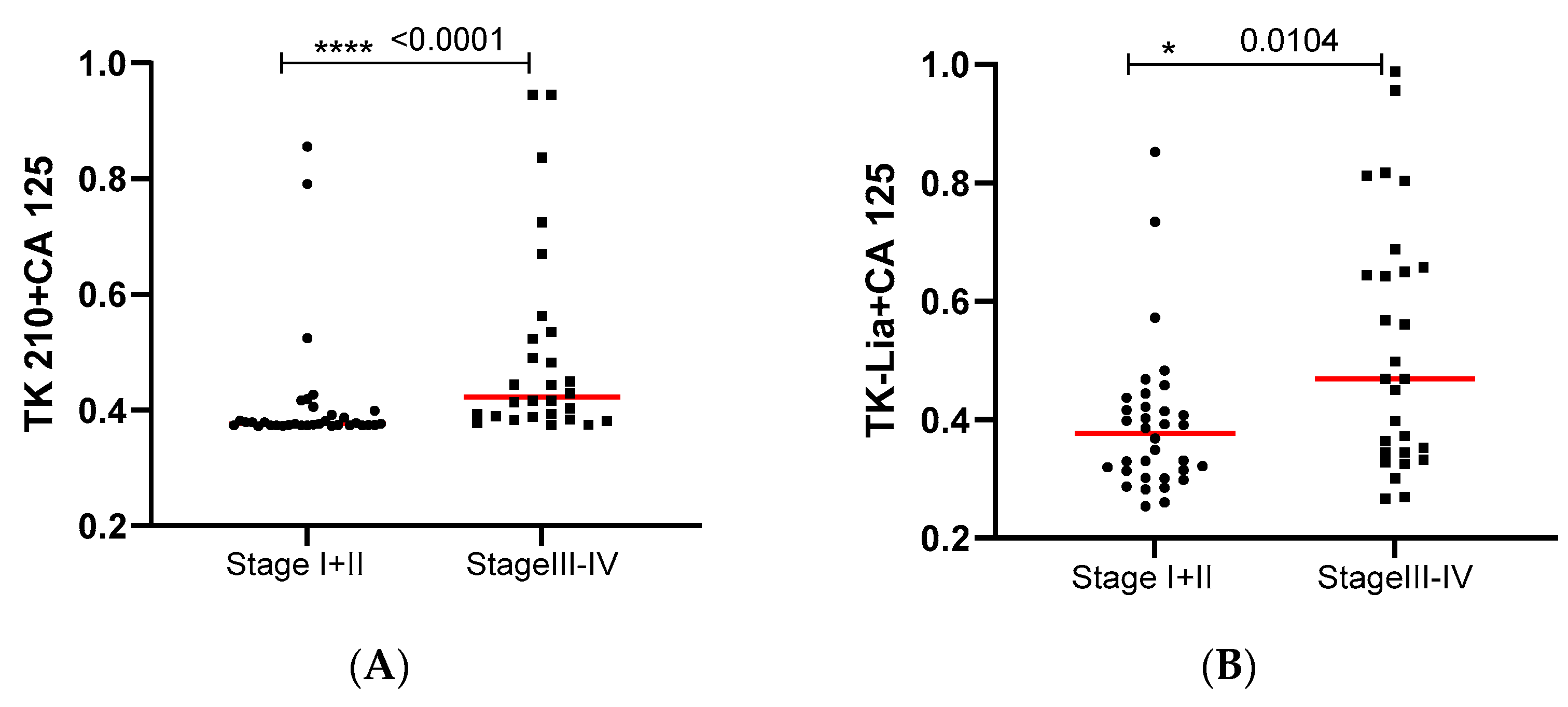
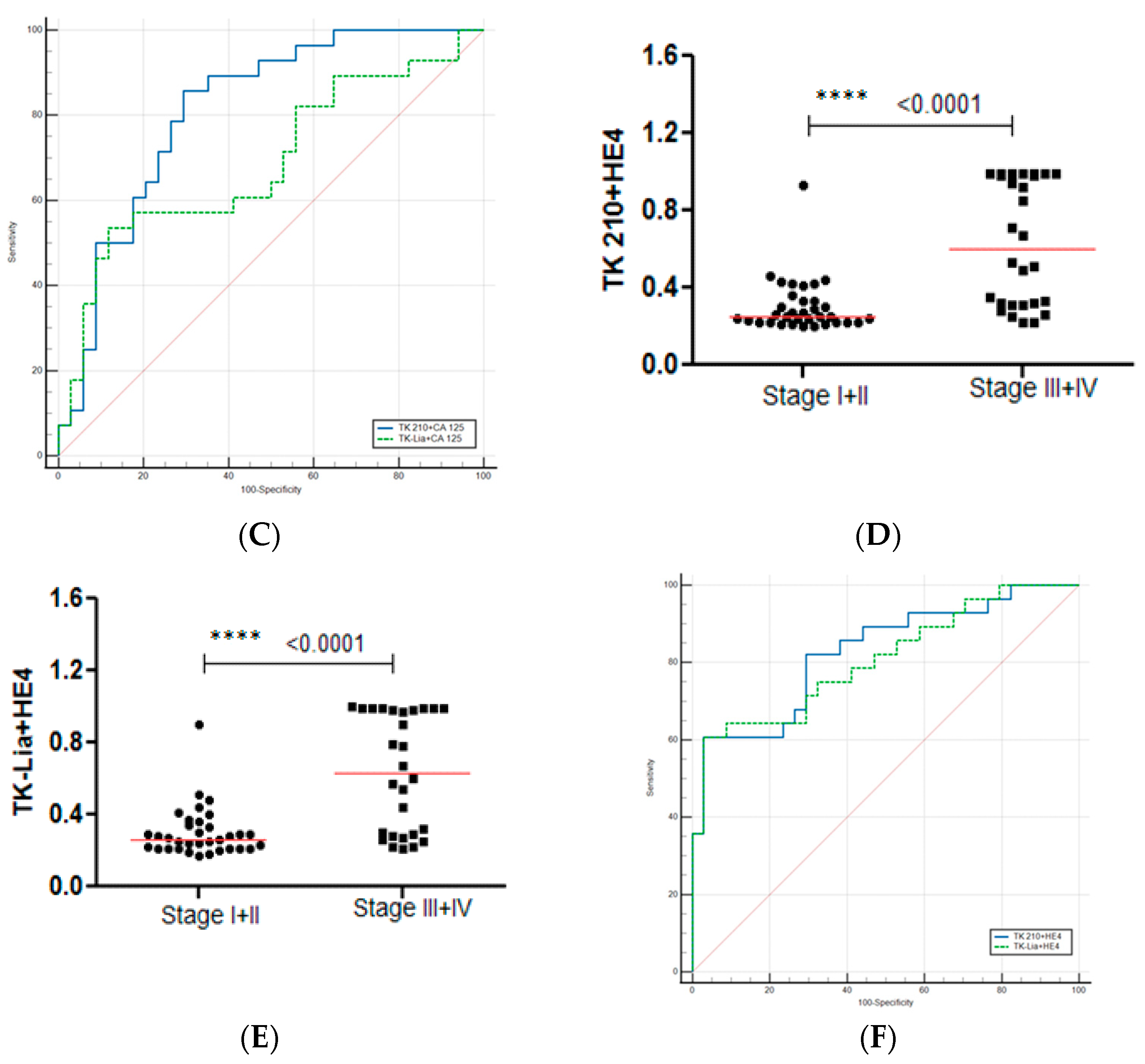
3.3. Performance Evaluation of Dual Biomarkers in Sub-Groups of Ovarian Cancer Patients
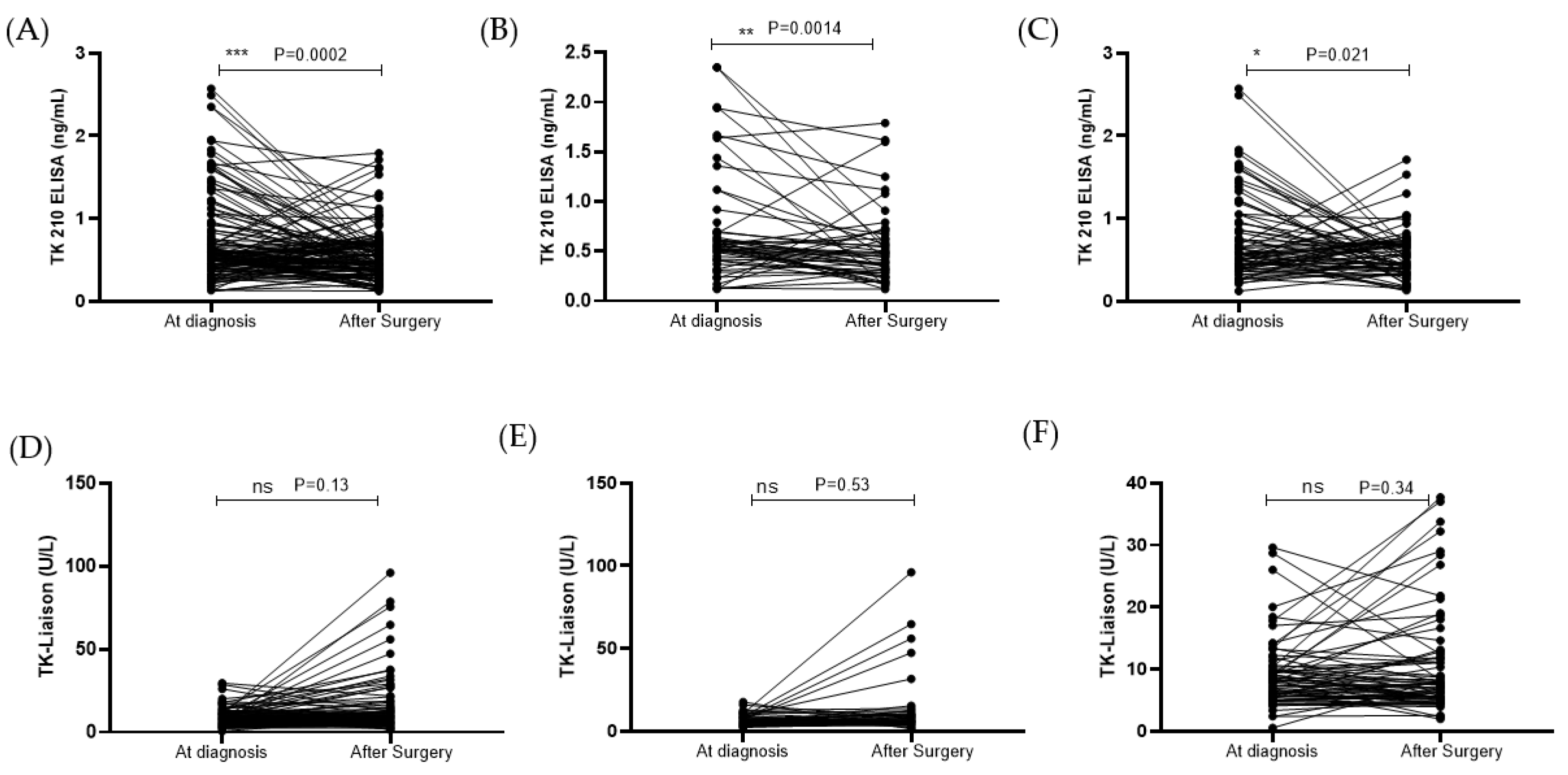
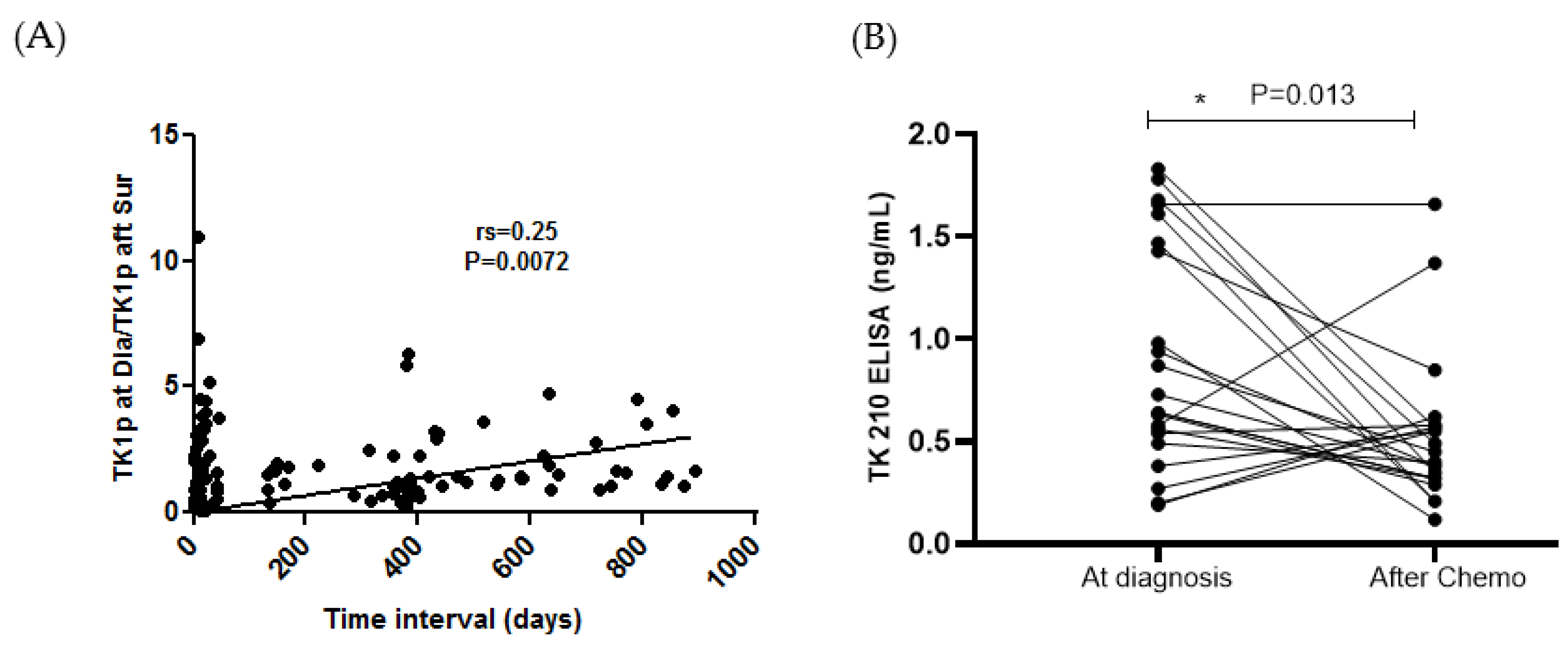
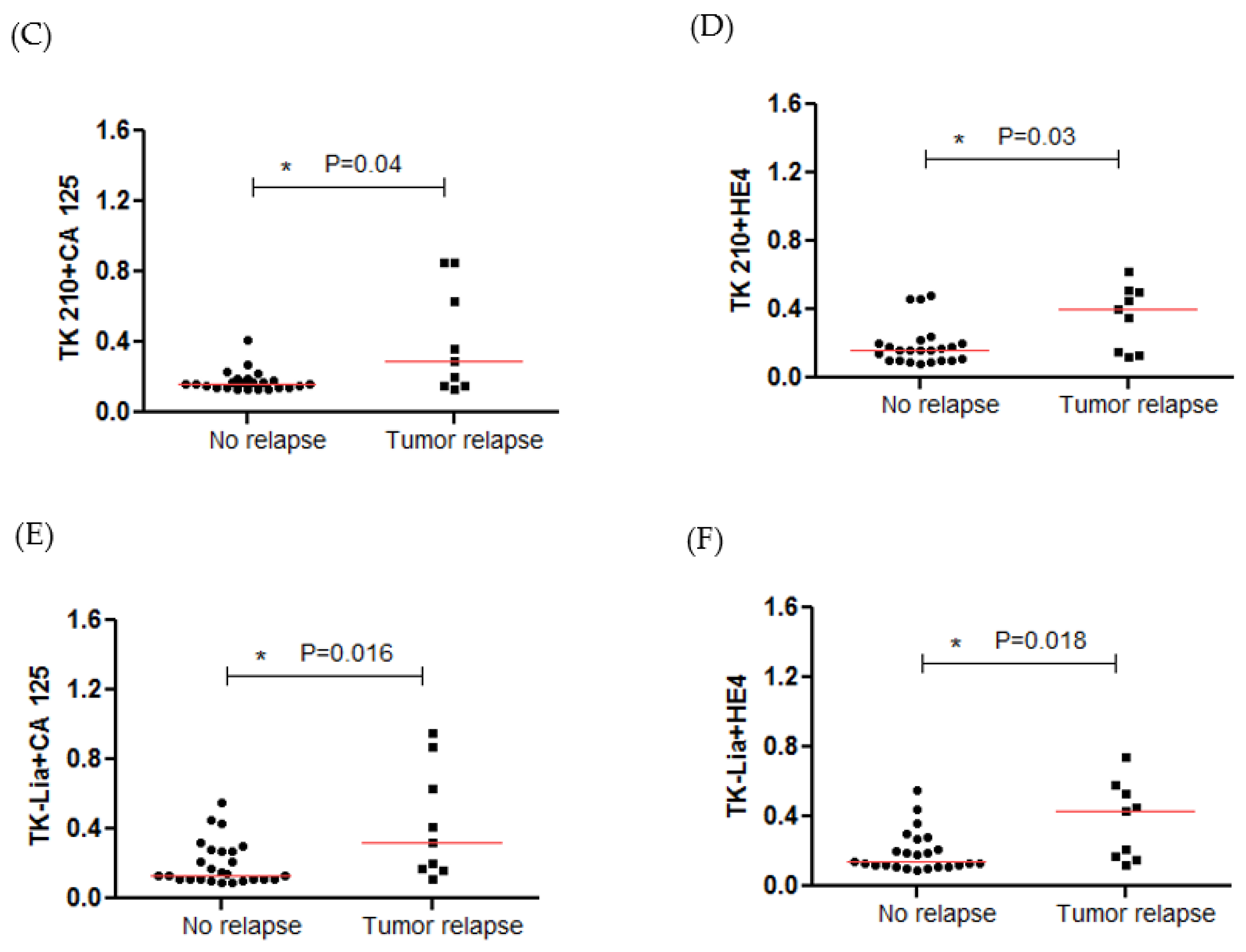
3.4. STK1p and STK1a Levels before and after Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Bellone, S.; Siegel, E.R.; Altwerger, G.; Menderes, G.; Bonazzoli, E.; Takata, T.; Pettinella, F.; Bianchi, A.; Riccio, F.; et al. A novel multiple biomarker panel for the early detection of high-grade serous ovarian carcinoma. Gynecol Oncol. 2018, 149, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Cancer in Slovenia 2019; Institute of Oncology Ljubljana, Epidemiology and Cancer Registry, Slovenian Cancer Registry: Ljubljana, Slovenia, 2022.
- Holschneider, H.C.; Berek, S.J. Ovarian Cancer: Epidemiology, Biology, and Prognostic Factors. Semin. Surg. Oncol. 2000, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, A.A.J.; Claussen, N.; Kapur, K.A.; Connor, P.J.; Patankar, S.M. The detection, treatment, and biology of epithelial ovarian cancer. J. Ovarian Res. 2010, 3, 8. [Google Scholar] [CrossRef]
- Urban, N.; McIntosh, M.W.; Andersen, M.R.; Karlan, B.Y. Ovarian cancer screening. Hematol./Oncol. Clin. 2003, 17, 989–1005. [Google Scholar] [CrossRef] [PubMed]
- Buamah, P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265. [Google Scholar] [CrossRef]
- Hellstrom, I.; Raycraft, J.; Hayden-Ledbetter, M.; Ledbetter, J.A.; Schummer, M.; McIntosh, M.; Drescher, C.; Urban, N.; Hellstrom, K.E. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003, 63, 3695–3700. [Google Scholar]
- Drapkin, R.; von Horsten, H.H.; Lin, Y.; Mok, S.C.; Crum, C.P.; Welch, W.R.; Hecht, J.L. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005, 65, 2162–2169. [Google Scholar] [CrossRef]
- Granato, T.; Porpora, M.; Longo, F.; Angeloni, A.; Manganaro, L.; Anastasi, E. HE4 in the differential diagnosis of ovarian masses. Clin. Chim. Acta 2015, 446, 147–155. [Google Scholar] [CrossRef]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Skates, S.; Allard, W.J.; Verch, T.; Steinhoff, M.; Messerlian, G.; DiSilvestro, P.; Granai, C.O.; et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol. Oncol. 2008, 108, 402–408. [Google Scholar] [CrossRef]
- Sandri, M.T.; Bottari, F.; Franchi, D.; Boveri, S.; Candiani, M.; Ronzoni, S.; Peiretti, M.; Radice, D.; Passerini, R.; Sideri, M. Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: Correlation with pathological outcome. Gynecol. Oncol. 2013, 128, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tie, R.; Chang, K.; Wang, F.; Deng, S.; Lu, W.; Yu, L.; Chen, M. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and CA125 in predicting epithelial ovarian cancer: A meta-analysis. BMC Cancer 2012, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Hada, A.; Han, L.P.; Chen, Y.; Hu, Q.H.; Yuan, Y.; Liu, L. Comparison of the predictive performance of risk of malignancy indexes 1–4, HE4 and risk of malignancy algorithm in the triage of adnexal masses. J. Ovarian Res. 2020, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Escudero, M.J.; Auge, M.J.; Filella, X.; Foj, L.; Torne, A.; Lejarcegui, J.; Pahisa, J. HE4 a novel tumor marker for ovarian cancer: Comparison with CA 125 and ROMA algorithm in patients with gynecological diseases. Tumor Biol. 2011, 32, 1087–1095. [Google Scholar] [CrossRef]
- Jacob, F.; Meier, M.; Caduff, R.; Goldstein, D.; Pochechueva, T.; Hacker, N.; Fink, D.; Heinzelmann-Schwarz, V. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol. Oncol. 2011, 121, 487–491. [Google Scholar] [CrossRef]
- Jagarlamudi, K.K.; Shaw, M. Thymidine kinase 1 as a tumor biomarker: Technical advances offer new potential to an old biomarker. Biomark. Med. 2018, 12, 1035–1048. [Google Scholar] [CrossRef]
- Bitter, E.E.; Townsend, H.M.; Erickson, R.; Allen, C.; O’Neill, L.K. Thymidine kinase 1 through the ages: A comprehensive review. Cell Biosci. 2020, 10, 138. [Google Scholar] [CrossRef]
- Sherley, J.L.; Kelly, T.J. Regulation of human thymidine kinase during the cell cycle. J. Biol. Chem. 1988, 263, 8350–8358. [Google Scholar] [CrossRef]
- He, Q.; Zhang, P.; Zou, L.; Li, H.; Wang, X.; Zhou, S.; Fornander, T.; Skog, S. Concentration of thymidine kinase 1 in serum (s-tk1) is a more sensitive proliferation marker in human solid tumors than its activity. Oncol. Rep. 2005, 14, 1013–1019. [Google Scholar] [CrossRef]
- Jagarlamudi, K.K.; Swinkels, L.; Zupan, M.; Osredkar, J.; Venge, P.; Eriksson, S. Analytical and clinical characterization of an optimized dual monoclonal sandwich ELISA for the quantification of thymidine kinase 1 (TK1) protein in human blood samples. PLoS ONE. 2022, 17, e0275444. [Google Scholar] [CrossRef]
- Jagarlamudi, K.K.; Zupan, M.; Kumer, K.; Fabjan, T.; Hlebič, G.; Eriksson, S.; Osredkar, J.; Smrkolj, T. The combination of AroCell TK 210 ELISA with Prostate Health Index or prostate-specific antigen density can improve the ability to differentiate prostate cancer from noncancerous conditions. Prostate 2019, 79, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.K.; Aronsson, A.C.; Pilko, G.; Zupan, M.; Kumer, K.; Fabjan, T.; Osredkar, J.; Eriksson, S. A clinical evaluation of the TK 210 ELISA in sera from breast cancer patients demonstrates high sensitivity and specificity in all stages of disease. Tumour Biol. 2016, 37, 11937–11945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, N.; Zhong, A.; Xiao, K.; Lu, R.; Guo, L. A combined strategy of TK1, HE4 and CA125 shows better diagnostic performance than risk of ovarian malignancy algorithm (ROMA) in ovarian carcinoma. Clin. Chim. Acta 2022, 524, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ohrvik, A.; Lindh, M.; Einarsson, R.; Grassi Eriksson, S. Sensitive nonradiometric method for determining thymidine kinase 1 activity. Clin. Chem. 2004, 50, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Oberaigner, W.; Minicozzi, P.; Bielska-Lasota, M.; Allemani, C.; de Angelis, R.; Mangone, L.; Sant, M.; Eurocare Working Group. Eurocare Working Group. Survival for ovarian cancer in Europe: The across-country variation did not shrink in the past decade. Acta Oncol. 2012, 51, 441–453. [Google Scholar] [CrossRef]
- Moore, R.G.; Miller, M.C.; Disilvestro, P.; Landrum, L.M.; Gajewski, W.; Ball, J.J.; Skates, S.J. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet. Gynecol. 2011, 118 Pt 1, 280–288. [Google Scholar] [CrossRef]
- Xi, Q.-P.; Pu, D.-H.; Lu, W.-N. Research on application value of combined detection of serum CA 125, HE4 and TK1 in the diagnosis of ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4536–4541. [Google Scholar]
- Jagarlamudi, K.K.; Hansson, L.O.; Eriksson, S. Breast, and prostate cancer patients differ significantly in their serum thymidine kinase 1 (TK1) specific activities compared with those hemato- logical malignancies and blood donors: Implications of using serum TK1 as a biomarker. BMC Cancer 2015, 15, 66. [Google Scholar] [CrossRef]
- Guo, B.Q.; Lu, W.Q. The prognostic significance of high/positive expression of tissue VEGF in ovarian cancer. Oncotarget 2018, 9, 30552–30560. [Google Scholar] [CrossRef]

| Characteristics | N/% | Mean ± SD (ys) |
|---|---|---|
| Ovarian tumor (borderline and malignant ovarian cancer) | 62 | 57.0 ± 3.7 |
| Menopausal status | ||
| Pre-menopause | 21 | 40.5 ± 3.3 |
| Post-menopause | 41 | 65.5 ± 2.9 |
| Histologic type | ||
| Serous carcinomas | 58% | |
| Mucinous tumors | 15% | |
| Endometrial carcinomas | 15% | |
| Benign ovarian tumor | 72 | 52.01 ± 3.8 |
| Menopausal status | ||
| Pre-menopause | 33 | 37.6 ± 3.4 |
| Post-menopause | 39 | 54.3 ± 2.9 |
| Histologic type | ||
| Endometriosis | 17% | |
| Serous cystadenoma | 15% | |
| Serous systadenofibroma | 13% | |
| Mucinous cystadenoma | 10% | |
| Healthy controls | 64 | 47.2 ± 2.3 |
| Menopausal status | ||
| Pre-menopause | 42 | 42.6 ± 2.5 |
| Post-menopause | 22 | 55.1 ± 2.4 |
| Histopathology and Classification | All Patients (N) | Menopausal Status | |
|---|---|---|---|
| 134 | pre-(N) | post-(N) | |
| Benign ovarian tumor | 72 | 33 | 39 |
| Serous cystadenoma | 11 | 2 | 9 |
| Serous cystadenofibroma | 9 | 1 | 8 |
| Mucinous cystadenoma | 7 | 4 | 3 |
| Mucinous cystadenofibroma | 2 | 0 | 2 |
| Endometriosis | 12 | 12 | 0 |
| Benign Brenner tumor | 1 | 0 | 1 |
| Sclerosing stromal tumor | 1 | 1 | 0 |
| Mature teratoma | 8 | 5 | 3 |
| Follicle cyst | 2 | 2 | 0 |
| Corpus luteum cyst | 1 | 1 | 0 |
| Inclusion cyst | 7 | 2 | 5 |
| Simple cyst | 9 | 3 | 6 |
| Cellular fibroma | 2 | 0 | 2 |
| Ovarian tumor (borderline and malignant ovarian cancer) | 62 | 21 | 41 |
| Serous borderline tumor | 9 | 6 | 3 |
| Low-grade serous carcinoma | 4 | 1 | 3 |
| High-grade serous carcinoma | 17 | 3 | 14 |
| Mucinous borderline tumor | 7 | 5 | 2 |
| Mucinous carcinoma | 2 | 1 | 1 |
| Endometrioid carcinoma | 8 | 2 | 6 |
| Endometrioid borderline tumor | 1 | 0 | 1 |
| Clear cell carcinoma | 2 | 1 | 1 |
| Seromucinous borderline tumor | 3 | 0 | 3 |
| Adult granulosa cell tumor | 2 | 1 | 1 |
| Dysgerminoma | 1 | 1 | 0 |
| High-grade primary peritoneal serous carcinoma | 5 | 0 | 5 |
| Low-grade primary peritoneal Serous carcinoma | 1 | 0 | 1 |
| Healthy controls | 65 | 43 | 22 |
| FIGO stages | 62 | ||
| I | 29 | 13 | 16 |
| II | 5 | 2 | 3 |
| III | 24 | 6 | 18 |
| IV | 4 | 0 | 4 |
| Grade | 62 | ||
| G1 | 35 | 16 | 19 |
| G2 | 6 | 3 | 3 |
| G3 | 21 | 2 | 19 |
| N | TK1p (ng/mL) | p Value | TK1a (U/L) | p Value | CA125 (kU/L) | p Value | HE4 (pmol/L) | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Healthy controls | |||||||||
| All | 64 | 0.21 (0.16–0.27) | 6.22 (5.1–7.1) | 12.16 (9.77–17.7) | 47.2 (42.1–54.9) | ||||
| Pre-menopausal | 42 | 0.22 (0.167–0.25) | 6.07 (5.1–6.9) | 13.17 (10.3–19.8) | 45.4 (38.2–56.9) | ||||
| Post-menopausal | 22 | 0.18 (0.155–0.35) | 0.625 | 6.6 (5.3–7.5) | 0.299 | 10.66 (8.8–16.1) | 0.113 | 49.7 (44.5–53.5) | 0.429 |
| Benign masses | |||||||||
| All | 72 | 0.53 (0.39–0.70) | 6.62 (5.0–8.2) | 17.5 (10.5–41.2) | 50 (42.0–66.4) | ||||
| Pre-menopausal | 33 | 0.53 (0.39–0.62) | 6.43 (4.7–7.7) | 31.9 (15.2–69.1) | 46.8 (39.3–59.0) | ||||
| Post-menopausal | 39 | 0.52 (0.39–0.86) | 0.68 | 5.99 (4.9–8.8) | 0.688 | 13.9 (9.0–25.2) | 0.0005 | 57.8 (44.7–75.6) | 0.0088 |
| Malignant masses | |||||||||
| All | 62 | 0.62 (0.38–1.44) | 7.91 (5.95–11.4) | 103.8 (21.4–444) | 136.5 (62.4–417.8) | ||||
| Pre-menopausal | 21 | 0.61 (0.45–1.65) | 6.52 (5.4–9.1) | 42.7 (19.3–193) | 52.0 (40.5–128.6) | ||||
| Post-menopausal | 41 | 0.63 (0.38–1.40) | 0.64 | 9.02 (6.3–12.8) | 0.045 | 169.2 (27.6–700) | 0.047 | 290.8 (93–972) | < 0.0001 |
| Marker Name | ROC AUC ± SE (AUC) |
|---|---|
| TK1p | 0.88 ± 0.024 |
| TK1a | 0.60 ± 0.04 |
| CA 125 | 0.77 ± 0.032 |
| HE4 | 0.71 ± 0.035 |
| ROMA Index | 0.73 ± 0.034 |
| TK1p + TK1a | 0.89 ± 0.024 |
| TK1p + CA 125 | 0.93 ± 0.020 |
| TK1p + HE4 | 0.88 ± 0.024 |
| TK1p + CA125 + HE4 | 0.94 ± 0.015 |
| TK1p + ROMA Index | 0.91 ± 0.020 |
| TK1a + CA 125 | 0.78 ± 0.032 |
| TK1a + HE4 | 0.71 ± 0.035 |
| TK1a + CA125 + HE4 | 0.80 ± 0.030 |
| TK1a + ROMA Index | 0.73 ± 0.034 |
| Biomarker | Cut-Off | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| TK 210 ELISA | 0.50 | 0.88 | 62.9% | 95.3% | 96.6% | 55.1% |
| TK-Liaison | 9.10 | 0.598 | 27.6% | 95.3% | 90.2% | 38.2% |
| CA 125 | 26.4 | 0.77 | 54.48% | 93.75% | 94.8% | 49.6% |
| HE4 | 65.5 | 0.71 | 47.8% | 93.75% | 94.1% | 46.2% |
| ROMA Index | 16.7 | 0.725 | 42.54% | 95.31% | 95.0% | 44.2% |
| TK 210 + CA 125 | 0.78 | 0.932 | 75.3% | 95.3% | 97.1% | 64.9% |
| TK 210 + HE4 | 0.80 | 0.91 | 73.8% | 93.75% | 96.1% | 63.2% |
| TK 210 + ROMA Index | 0.83 | 0.912 | 70.2% | 95.3% | 96.9% | 60.4% |
| TK-Liason + CA 125 | 0.76 | 0.783 | 49.25% | 95.3% | 95.7% | 47.3% |
| TK-Liason + HE4 | 0.72 | 0.712 | 47.1% | 95.3% | 95.5% | 46.2% |
| TK-Liason + ROMA Index | 0.73 | 0.728 | 44.1% | 95.3% | 95.2% | 44.9% |
| Pre-Menopausal vs. Controls | Cut-Off | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| All stages | ||||||
| TK 210 ELISA | 0.49 | 0.875 | 64.8% | 95.2% | 94.6% | 67.80% |
| TK 210 + CA 125 | 0.68 | 0.945 | 81.5% | 95.2% | 95.7% | 80.0% |
| TK 210 + HE4 | 0.75 | 0.875 | 72.2% | 95.2% | 95.1% | 72.7% |
| TK 210 + ROMA Index | 0.75 | 0.877 | 72.2% | 95.2% | 95.1% | 72.7% |
| Post-menopausal vs. controls | ||||||
| All stages | ||||||
| TK 210 ELISA | 0.55 | 0.886 | 55.0% | 100% | 100% | 37.9% |
| TK 210 + CA 125 | 0.86 | 0.920 | 73.75% | 100% | 100% | 51.2% |
| TK 210 + HE4 | 0.86 | 0.927 | 72.5% | 100% | 100% | 50.0% |
| TK 210 + ROMA Index | 0.83 | 0.936 | 78.75% | 100% | 100% | 56.4% |
| Benign vs. controls | ||||||
| TK 210 ELISA | 0.50 | 0.872 | 59.72% | 95.3% | 93.5% | 68.1% |
| TK 210 + CA 125 | 0.76 | 0.91 | 63.89% | 96.87% | 95.9% | 71.3% |
| TK 210 + HE4 | 0.77 | 0.877 | 61.1% | 95.3% | 93.6% | 68.5% |
| TK 210 + ROMA Index | 0.79 | 0.876 | 59.72% | 95.3% | 93.5% | 67.8% |
| Malignant vs. controls | ||||||
| TK 210 ELISA | 0.50 | 0.904 | 66.13% | 95.3% | 93.2% | 74.7% |
| TK 210 + CA 125 | 0.65 | 0.954 | 80.65% | 100% | 100% | 84.2% |
| TK 210 + HE4 | 0.577 | 0.947 | 80.65 | 100% | 100% | 84.2% |
| TK 210 + ROMA Index | 0.606 | 0.951 | 82.26% | 100% | 100% | 85.1% |
| Malignant vs. Benign | ||||||
| TK 210 ELISA | 1.36 | 0.61 | 27.5% | 95.8% | 85% | 60.5% |
| TK 210 + CA 125 | 0.56 | 0.73 | 43.5% | 95.8% | 90% | 66.3% |
| TK 210 + HE4 | 0.60 | 0.817 | 58.1% | 97.22% | 94.7% | 72.9% |
| TK 210 + ROMA Index | 0.59 | 0.819 | 66.1% | 95.8% | 93.2% | 76.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cviič, D.; Jagarlamudi, K.; Meglič, L.; Škof, E.; Zore, A.; Lukanović, D.; Eriksson, S.; Osredkar, J. A Dual Biomarker TK1 Protein and CA125 or HE4-Based Algorithm as a Better Diagnostic Tool than ROMA Index in Early Detection of Ovarian Cancer. Cancers 2023, 15, 1593. https://doi.org/10.3390/cancers15051593
Cviič D, Jagarlamudi K, Meglič L, Škof E, Zore A, Lukanović D, Eriksson S, Osredkar J. A Dual Biomarker TK1 Protein and CA125 or HE4-Based Algorithm as a Better Diagnostic Tool than ROMA Index in Early Detection of Ovarian Cancer. Cancers. 2023; 15(5):1593. https://doi.org/10.3390/cancers15051593
Chicago/Turabian StyleCviič, Diana, Kiran Jagarlamudi, Leon Meglič, Erik Škof, Andrej Zore, David Lukanović, Staffan Eriksson, and Joško Osredkar. 2023. "A Dual Biomarker TK1 Protein and CA125 or HE4-Based Algorithm as a Better Diagnostic Tool than ROMA Index in Early Detection of Ovarian Cancer" Cancers 15, no. 5: 1593. https://doi.org/10.3390/cancers15051593
APA StyleCviič, D., Jagarlamudi, K., Meglič, L., Škof, E., Zore, A., Lukanović, D., Eriksson, S., & Osredkar, J. (2023). A Dual Biomarker TK1 Protein and CA125 or HE4-Based Algorithm as a Better Diagnostic Tool than ROMA Index in Early Detection of Ovarian Cancer. Cancers, 15(5), 1593. https://doi.org/10.3390/cancers15051593









