Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
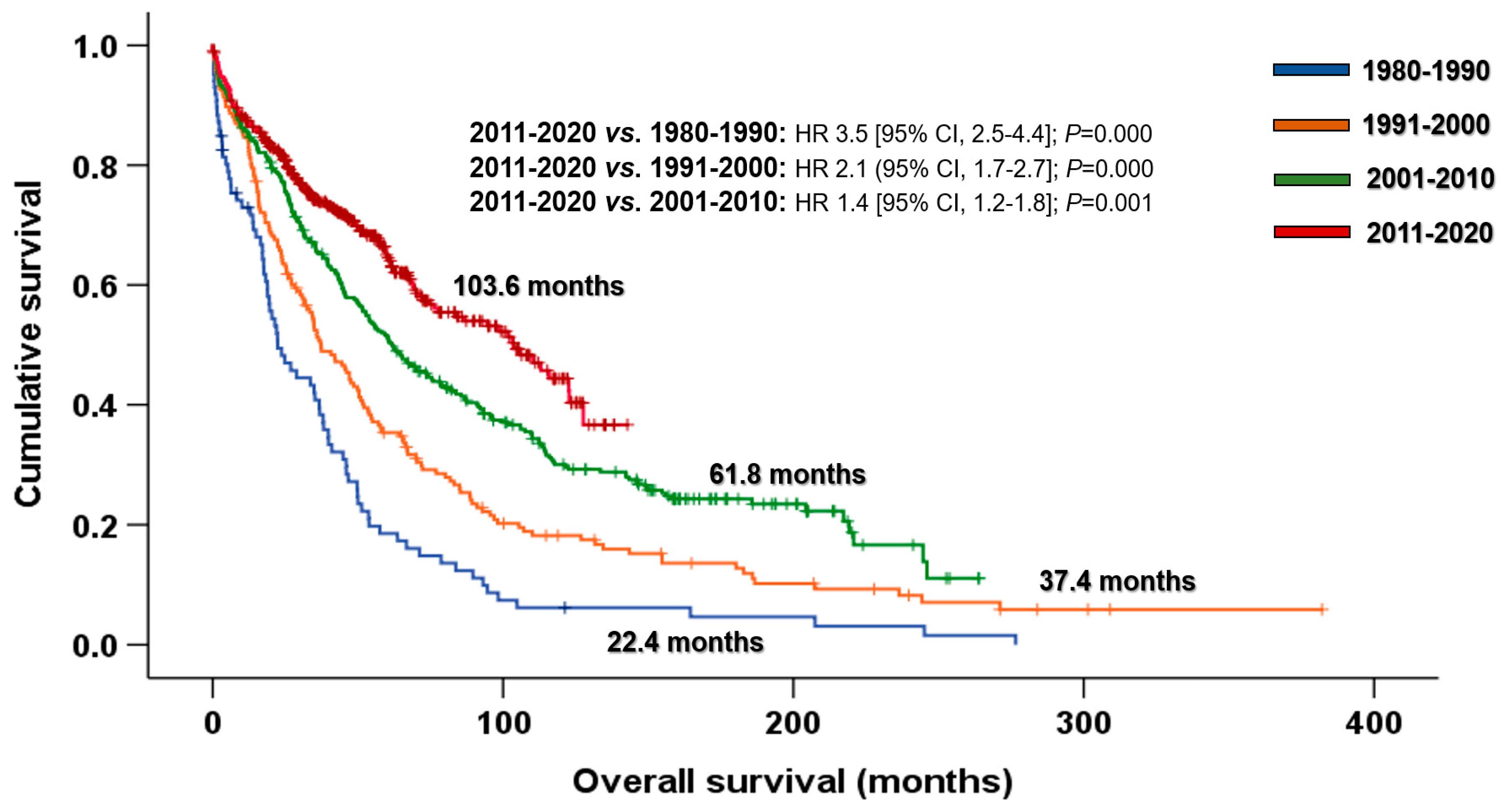
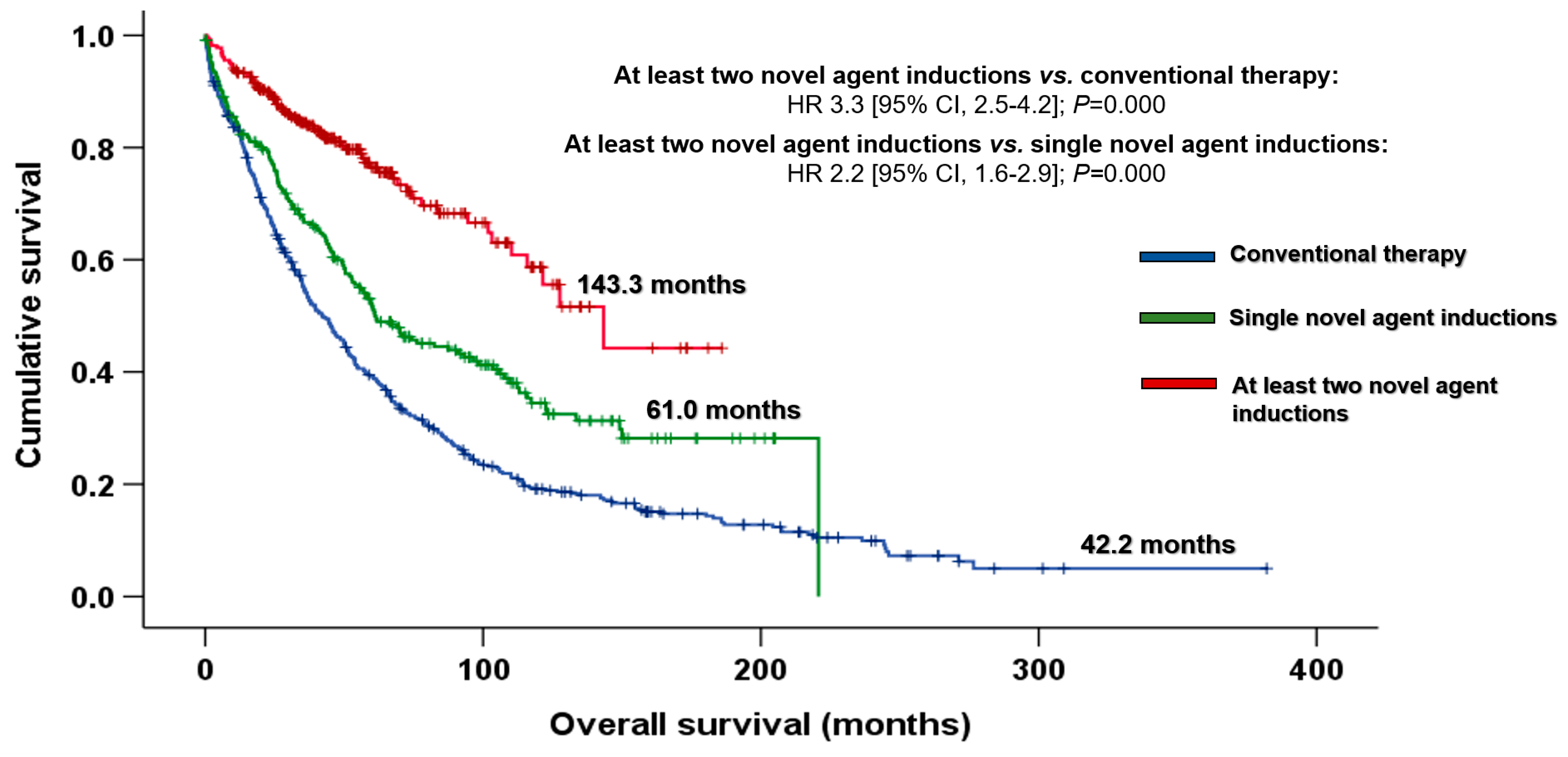
3.1. Impact of the Introduction of Novel Agents on Outcomes
3.2. Long-Term Survivors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mateos, M.-V.; Nooka, A.K.; Larson, S.M. Moving Toward a Cure for Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Barlogie, B.; Alexanian, R.; Dicke, K.A.; Zagars, G.; Spitzer, G.; Jagannath, S.; Horwitz, L. High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood 1987, 70, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Barlogie, B.; Gahrton, G. Bone marrow transplantation in multiple myeloma. Bone Marrow Transplant. 1991, 7, 71–79. [Google Scholar] [PubMed]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef]
- Kastritis, E.; Zervas, K.; Symeonidis, A.; Terpos, E.; Delimbassi, S.; Anagnostopoulos, N.; Michali, E.; Zomas, A.; Katodritou, E.; Gika, D.; et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): An analysis of the Greek Myeloma Study Group (GMSG). Leukemia 2009, 23, 1152–1157. [Google Scholar] [CrossRef]
- Turesson, I.; Velez, R.; Kristinsson, S.Y.; Landgren, O. Patterns of Improved Survival in Patients with Multiple Myeloma in the Twenty-First Century: A Population-Based Study. J. Clin. Oncol. 2010, 28, 830–834. [Google Scholar] [CrossRef]
- Liwing, J.; Uttervall, K.; Lund, J.; Aldrin, A.; Blimark, C.; Carlson, K.; Enestig, J.; Flogegård, M.; Forsberg, K.; Gruber, A.; et al. Improved survival in myeloma patients: Starting to close in on the gap between elderly patients and a matched normal population. Br. J. Haematol. 2014, 164, 684–693. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.C.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef]
- Ríos-Tamayo, R.; Sánchez, M.J.; Puerta, J.M.; Sáinz, J.; Chang-Chan, D.Y.-L.; Rodríguez, T.; López, P.; de Pablos, J.M.; Navarro, P.; de Veas, J.L.G.; et al. Trends in survival of multiple myeloma: A thirty-year population-based study in a single institution. Cancer Epidemiol. 2015, 39, 693–699. [Google Scholar] [CrossRef]
- Blimark, C.H.; Turesson, I.; Genell, A.; Ahlberg, L.; Björkstrand, B.; Carlson, K.; Forsberg, K.; Juliusson, G.; Linder, O.; Mellqvist, U.-H.; et al. Outcome and survival of myeloma patients diagnosed 2008–2015. Real-world data on 4904 patients from the Swedish Myeloma Registry. Haematologica 2018, 103, 506–513. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Cavo, M.; Blade, J.; Dimopoulos, M.A.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet 2020, 395, 132–141. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Rosiñol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernández, M.T.; Martínez-Martínez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M.; et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, S.V. The multiple myelomas—Current concepts in cytogenetic classification and therapy. Nat. Rev. Clin. Oncol. 2018, 15, 409–421. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Hoering, A.; Cavo, M.; Miguel, J.S.; Goldschimdt, H.; Hajek, R.; Turesson, I.; Lahuerta, J.J.; Attal, M.; Barlogie, B.; et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma—An IMWG Research Project. Blood Cancer J. 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Larocca, A.; Wijermans, P.; Cavallo, F.; Rossi, D.; Schaafsma, R.; Genuardi, M.; Romano, A.; Liberati, A.M.; Siniscalchi, A.; et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: Analysis of 1175 patients. Blood 2011, 117, 3025–3031. [Google Scholar] [CrossRef]
- Martín-Mateos, M.-L.; Oriol, A.; Martinez-Lopez, J.; Teruel, A.-I.; De La Guía, A.L.; López, J.; Bengoechea, E.; Pérez, M.; Martínez, R.; Palomera, L.; et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: Do we still need alkylators? Blood 2014, 124, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Otero, P.; Mateos, M.V.; Martínez-López, J.; Hernández, M.-T.; Ocio, E.M.; Rosiñol, L.; Martínez, R.; Teruel, A.-I.; Gutiérrez, N.C.; Bargay, J.; et al. Predicting long-term disease control in transplant-ineligible patients with multiple myeloma: Impact of an MGUS-like signature. Blood Cancer J. 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, N.C.; Castellanos, M.V.; Martín, M.L.; Mateos, M.V.; Hernández, J.M.; Fernández, M.; Carrera, D.; Rosiñol, L.; Ribera, J.M.; Ojanguren, J.M.; et al. Prognostic and biological implications of genetic abnormalities in multiple myeloma undergoing autologous stem cell transplantation: T(4;14) is the most relevant adverse prognostic factor, whereas RB deletion as a unique abnormality is not associated with adverse prognosis. Leukemia 2007, 21, 143–150. [Google Scholar] [CrossRef]
- López-Corral, L.; Gutiérrez, N.C.; Vidriales, M.B.; Mateos, M.V.; Rasillo, A.; García-Sanz, R.; Paiva, B.; Miguel, J.F.S. The Progression from MGUS to Smoldering Myeloma and Eventually to Multiple Myeloma Involves a Clonal Expansion of Genetically Abnormal Plasma Cells. Clin. Cancer Res. 2011, 17, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.M.; Avet-Loiseau, H.; Ameye, G.; Gutiérrez, N.C.; Liebisch, P.; O’Connor, S.; Dalva, K.; Fabris, S.; Testi, A.M.; Jarosova, M.; et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica 2012, 97, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Bergsagel, P.L.; Drach, J.; Shaughnessy, J.; Gutierrez, N.; Stewart, A.K.; Morgan, G.; Van Ness, B.; Chesi, M.; Minvielle, S.; et al. International Myeloma Working Group molecular classification of multiple myeloma: Spotlight review. Leukemia 2009, 23, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Ozaki, S.; Harada, T.; Saitoh, T.; Shimazaki, C.; Itagaki, M.; Asaoku, H.; Kuroda, Y.; Chou, T.; Yoshiki, Y.; Suzuki, K.; et al. Survival of Multiple Myeloma Patients Aged 65-70 Years in the Era of Novel Agents and Autologous Stem Cell Transplantation. Acta Haematol. 2014, 132, 211–219. [Google Scholar] [CrossRef]
- Sant, M.; Minicozzi, P.; Mounier, M.; A Anderson, L.; Brenner, H.; Holleczek, B.; Marcos-Gragera, R.; Maynadié, M.; Monnereau, A.; Osca-Gelis, G.; et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: Results of EUROCARE-5, a population-based study. Lancet Oncol. 2014, 15, 931–942. [Google Scholar] [CrossRef]
- Brenner, H.; Gondos, A.; Pulte, D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood 2008, 111, 2521–2526. [Google Scholar] [CrossRef]
- Pulte, D.; Gondos, A.; Brenner, H. Improvement in Survival of Older Adults with Multiple Myeloma: Results of an Updated Period Analysis of SEER Data. Oncologist 2011, 16, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Anderson, W.F.; Landgren, O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia 2014, 28, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Dickman, P.W.; Landgren, O.; Blimark, C.; Hultcrantz, M.; Turesson, I.; Björkholm, M.; Kristinsson, S.Y. Dramatically improved survival in multiple myeloma patients in the recent decade: Results from a Swedish population-based study. Haematologica 2018, 103, e412–e415. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lobato, L.G.; Pereira, A.; de Larrea, C.F.; Cibeira, M.T.; Tovar, N.; Jiménez-Segura, R.; Moreno, D.F.; Oliver-Caldés, A.; Rosiñol, L.; Bladé, J. Real-world data on survival improvement in patients with multiple myeloma treated at a single institution over a 45-year period. Br. J. Haematol. 2022, 196, 649–659. [Google Scholar] [CrossRef]
- Pozzi, S.; Marcheselli, L.; Bari, A.; Liardo, E.V.; Marcheselli, R.; Luminari, S.; Quaresima, M.; Cirilli, C.; Ferri, P.; Federico, M.; et al. Survival of multiple myeloma patients in the era of novel therapies confirms the improvement in patients younger than 75 years: A population-based analysis. Br. J. Haematol. 2013, 163, 40–46. [Google Scholar] [CrossRef]
- Langseth, O.; Myklebust, T.A.; Johannesen, T.B.; Hjertner, Ø.; Waage, A. Incidence and survival of multiple myeloma: A population-based study of 10 524 patients diagnosed 1982–2017. Br. J. Haematol. 2020, 191, 418–425. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Bonanad, S.; De la Rubia, J.; Gironella, M.; Persona, E.P.; González, B.; Lago, C.F.; Arnan, M.; Zudaire, M.; Rivas, J.H.; Soler, A.; et al. Development and psychometric validation of a brief comprehensive health status assessment scale in older patients with hematological malignancies: The GAH Scale. J. Geriatr. Oncol. 2015, 6, 353–361. [Google Scholar] [CrossRef]
- Martinez-Lopez, J.; Blade, J.; Mateos, M.-V.; Grande, C.; Alegre, A.; García-Laraña, J.; Sureda, A.; de la Rubia, J.; Conde, E.; Martinez, R.; et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood 2011, 118, 529–534. [Google Scholar] [CrossRef]
- Barlogie, B.; Mitchell, A.; Van Rhee, F.; Epstein, J.; Morgan, G.; Crowley, J. Curing myeloma at last: Defining criteria and providing the evidence. Blood 2014, 124, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- van de Velde, H.J.; Liu, X.; Chen, G.; Cakana, A.; Deraedt, W.; Bayssas, M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica 2007, 92, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.; Raab, M.S.; et al. Multiple myeloma: Patient outcomes in real-world practice. Br. J. Haematol. 2016, 175, 252–264. [Google Scholar] [CrossRef] [PubMed]


| All Patients (n = 1001) | Group 1 (1980–1990) (n = 93) | Group 2 (1991–2000) (n = 178) | Group 3 (2001–2010) (n = 314) | Group 4 (2011–2020) (n = 416) | p Value | |
|---|---|---|---|---|---|---|
| Follow-up in months, median (range) | 65.1 (2.4–382.1) | 121.3 (3.0–121.7) | 119.0 (15.0–382.1) | 157.8 (10.3–263.8) | 51.4 (2.4–142.9) | |
| Age at diagnosis, median (range) a | 64 (28–93) | 68 (38–86) | 64 (31–88) | 63 (28–89) | 65 (30–93) | 0.153 |
| Age at diagnosis ≤70, n (%) Older than 70, n (%) | 662 (69.0) 297 (31.0) | 49 (66.2) 25 (26.9) | 114 (72.6) 43 (27.4) | 215 (68.9) 97 (31.1) | 284 (68.3) 132 (31.7) | 0.722 |
| Gender, male, n (%) | 567 (56.6) | 44 (47.3) | 96 (53.9) | 173 (55.1) | 254 (61.1) | 0.059 |
| Ig isotype, n (%) b IgG IgA IgM IgD Light chains only Non-secretory | 557 (56.6) 252 (25.6) 2 (0.2) 7 (0.7) 145 (14.7) 21 (2.1) | 45 (49.5) 33 (36.3) 0 (0.0) 0 (0.0) 13 (14.3) 0 (0.0) | 87 (49.7) 54 (30.9) 0 (0.0) 7 (4.0) 27 (15.4) 0 (0.0) | 187 (59.9) 72 (23.1) 1 (0.3) 0 (0.0) 37 (11.9) 15 (4.8) | 238 (58.6) 93 (22.9) 1 (0.2) 0 (0.0) 68 (16.7) 6 (1.5) | 0.061 0.014 - - 0.328 - |
| Light chain isotype Kappa, n (%) c | 575 (59.1) | 39 (46.4) | 100 (57.8) | 187 (60.9) | 249 (60.9) | 0.026 |
| ECOG PS 0–1, n (%) d | 497 (64.0) | 27 (34.6) | 53 (44.5) | 132 (64.7) | 285 (75.8) | 0.000 |
| Hb ≤ 10 g/dL, n (%) e | 366 (39.6) | 51 (56.0) | 75 (46.9) | 113 (38.6) | 127 (33.3) | 0.000 |
| Cr ≥ 2 mg/dL, n (%) f | 185 (19.6) | 25 (27.8) | 33 (20.4) | 50 (16.9) | 77 (19.4) | 0.157 |
| Ca ≥ 11 mg/dL, n (%) g | 144 (16.2) | 27 (30.7) | 25 (16.4) | 36 (13.2) | 56 (14.8) | 0.001 |
| Lytic lesions, n (%) h | 617 (69.2) | 68 (76.4) | 106 (66.3) | 164 (59.9) | 279 (75.8) | 0.000 |
| Elevated LDH, n (%) i | 230 (43.5) | No data | 24 (72.7) | 136 (72.0) | 70 (22.8) | 0.000 |
| Albumin, g/dL, mean (SD) | 3.6 (±0.7) | 3.6 (±0.7) | 3.7 (±0.7) | 3.5 (±0.7) | 3.6 (±0.7) | 0.476 |
| β2 microglobulin, mg/dL, mean (SD) | 5.8 (±5.4) | 5.5 (±4.1) | 6.6 (±7.6) | 4.7 (±4.2) | 6.5 (±5.2) | 0.000 |
| ISS, n (%) j I II III | 280 (33.5) 280 (33.5) 277 (33.0) | 15 (38.5) 10 (25.6) 14 (35.9) | 62 (42.5) 37 (25.3) 47 (32.2) | 94 (34.6) 115 (42.3) 63 (23.2) | 109 (28.7) 118 (31.1) 153 (40.3) | 0.020 0.001 0.000 |
| High-risk cytogenetic k,*, n (%) | 116 (18.3) | No data | 4 (25.0) | 39 (16.0) | 73 (19.8) | 0.396 |
| Age ≤ 70 Years (n = 662) | Age Older than 70 Years (n = 279) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1980–1990 (n = 49) | 1991–2000 (n = 114) | 2001–2010 (n = 215) | 2011–2020 (n = 284) | 1980–1990 (n = 21) a | 1991–2000 (n = 40) b | 2001–2010 (n = 94) c | 2011–2020 (n = 124) d | |
| Lines of therapy, median (range) | 1 (1–4) | 2 (1–7) | 2 (1–14) | 1 (1–9) | 1 (1–2) | 1 (1–2) | 2 (1–5) | 2 (1–8) |
| Chemotherapy (CyP, MP) | 25 (51.0) | 15 (13.1) | 6 (2.8) | 2 (0.7) | 13 (61.9) | 32 (80.0) | 57 (60.6) | 18 (14.5) |
| Polychemotherapy (VBCMP, VBAD, VAD) | 24 (49.0) | 97 (85.1) | 116 (54.0) | 9 (3.2) | 8 (38.1) | 8 (20.0) | 4 (4.3) | 0 (0.0) |
| Novel agents in first line | 0 (0.0) | 0 (0.0) | 93 (43.2) | 273 (96.1) | 0 (0.0) | 0 (0.0) | 32 (34.0) | 105 (84.7) |
| 1 novel agent in first line | 0 (0.0) | 0 (0.0) | 80 (37.2) | 57 (20.1) | 0 (0.0) | 0 (0.0) | 27 (28.7) | 66 (53.2) |
| ≥2 novel agents in first line | 0 (0.0) | 0 (0.0) | 13 (6.0) | 216 (76.1) | 0 (0.0) | 0 (0.0) | 5 (5.3) | 39 (31.5) |
| PI-based scheme (VD, VMP, VCD, PAD…) | 0 (0.0) | 0 (0.0) | 69 (32.1) | 54 (19.0) | 0 (0.0) | 0 (0.0) | 22 (23.4) | 58 (46.8) |
| IMID-based scheme (TD, TCD, TAD, Rd…) | 0 (0.0) | 0 (0.0) | 11 (5.2) | 3 (1.1) | 0 (0.0) | 0 (0.0) | 5 (5.3) | 11 (8.9) |
| PI plus IMID (VTD, VRD…) | 0 (0.0) | 0 (0.0) | 13 (6.0) | 195 (68.7) | 0 (0.0) | 0 (0.0) | 5 (5.3) | 10 (8.1) |
| Anti-CD38-based scheme (Any combination which included anti-CD38) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 19 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 26 (21.0) |
| Others | 0 (0.0) | 2 (1.8) | 0 (0.0) | 2 (0.7) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1 (0.8) |
| ASCT | 5 (10.2) | 68 (59.6) | 179 (83.3) | 237 (83.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (2.3) |
| Long Survivors (n = 132) | Early-Death (n = 252) | OR (95% CI), p Value | |
|---|---|---|---|
| Age at diagnosis > 65 years, n (%) | 22/132 (16.7) | 163/224 (72.8) | 13.4 (7.8–23.0), 0.000 |
| Male, n (%) | 66/132 (50.0) | 136/252 (54.0) | 1.2 (0.8–1.8), 0.460 |
| IgG subtype, n (%) | 82/129 (63.6) | 122/248 (49.2) | 1.8 (1.2–2.8), 0.008 |
| IgA subtype, n (%) | 21/129 (16.3) | 167/248 (67.3) | 2.5 (1.5–4.3), 0.001 |
| Bence-Jones subtype, n (%) | 18/129 (14.0) | 36/248 (14.5) | 1.0 (0.6–1.9), 0.882 |
| Paraprotein ≥ 3 g/dL, n (%) | 57/102 (55.9) | 120/225 (53.3) | 0.9 (1.6–1.4), 0.668 |
| PCs in BM ≥ 30, n (%) | 50/109 (45.9) | 134/226 (59.3) | 1.7 (1.1–2.7), 0.021 |
| ECOG PS 2–4, n (%) | 12/71 (16.9) | 133/220 (60.5) | 7.5 (3.8–14.8), 0.000 |
| Hb ≤ 10 g/dL, n (%) | 34/120 (28.3) | 126/244 (51.6) | 2.7 (1.7–4.3), 0.000 |
| Cr ≥ 2 mg/dL, n (%) | 10/126 (7.9) | 84/244 (34.4) | 6.1 (3.0–12.2), 0.000 |
| Ca ≥ 11 mg/dL, n (%) | 12/108 (11.1) | 63/240 (26.3) | 2.8 (1.5–5.5), 0.002 |
| Lytic lesions, n (%) | 82/125 (65.6) | 151/223 (67.7) | 1.1 (0.7–1.8), 0.688 |
| Albumine < 3.5 g/dL, n (%) | 37/112 (33.0) | 125/240 (52.1) | 2.2 (1.4–3.5), 0.001 |
| β2 microglobulin ≥ 3.5 mg/dL, n (%) | 28/98 (28.6) | 144/190 (75.8) | 7.8 (4.5–13.7), 0.000 |
| β2 microglobulin ≥ 5 mg/dL, n (%) | 11/98 (11.2) | 106/190 (55.8) | 10.0 (5.0–19.9), 0.000 |
| Elevated LDH, n (%) | 27/64 (42.2) | 76/136 (55.9) | 1.7 (1.0–3.2), 0.072 |
| High-risk cytogenetic *, n (%) | 11/97 (11.3) | 30/107 (28.0) | 3.0 (1.4–6.5), 0.004 |
| ISS-1, n (%) | 55/106 (51.9) | 33/200 (16.5) | 5.5 (3.2–9.3), 0.000 |
| ISS-2. n (%) | 40/106 (37.7) | 56/200 (28.0) | 0.6 (0.4–1.1), 0.082 |
| ISS-3, n (%) | 11/106 (10.4) | 111/200 (55.5) | 10.8 (5.4–21.3), 0.000 |
| Long Survivors (n = 43) | Early Death (n = 82) | OR (95% CI), p Value | |
|---|---|---|---|
| Age at diagnosis > 65 years, n (%) | 29/43 (67.4) | 19/82 (23.2) | 12.2 (3.6–41.5), 0.000 |
| Male, n (%) | - | - | - |
| IgG subtype, n (%) | - | - | - |
| IgA subtype, n (%) | 5/82 (11.6) | 26/82 (31.7) | 5.3 (1.2–23.4), 0.028 |
| Bence-Jones subtype, n (%) | - | - | - |
| Paraprotein ≥ 3 g/dL, n (%) | - | - | - |
| PCs in BM ≥ 30, n (%) | 22/43 (51.2) | 52/82 (63.2) | 1.1 (0.3–3.3); 0.904 |
| ECOG PS 2–4, n (%) | 7/43 (16.3) | 39/82 (47.6) | 4.0 (1.1–14.2), 0.030 |
| Hb ≤ 10 g/dL, n (%) | 11/43 (25.6) | 41/82 (50.0) | 1.5 (0.5–4.8), 0.492 |
| Cr ≥ 2 mg/dL, n (%) | 2/43 (4.7) | 23/82 (28.0) | 3.5 (0.6–21.3), 0.170 |
| Ca ≥ 11 mg/dL, n (%) | 2/43 (4.7) | 18/82 (22.0) | 7.1 (1.2–23.4), 0.074 |
| Lytic lesions, n (%) | - | - | - |
| Albumine < 3.5 g/dL, n (%) | - | - | - |
| β2 microglobulin ≥ 3.5 mg/dL, n (%) | - | - | - |
| β2 microglobulin ≥ 5 mg/dL, n (%) | - | - | - |
| Elevated LDH, n (%) | - | - | - |
| High-risk cytogenetic *, n (%) | 5/43 (11.6) | 25/82 (30.5) | 6.1 (1.2–31.0), 0.028 |
| ISS-1, n (%) | 24/43 (55.8) | 8/82 (9.8) | 4.8 (1.4–16.6), 0.012 |
| ISS-2. n (%) | - | - | - |
| ISS-3, n (%) | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puertas, B.; González-Calle, V.; Sobejano-Fuertes, E.; Escalante, F.; Queizán, J.A.; Bárez, A.; Labrador, J.; Alonso-Alonso, J.M.; García de Coca, A.; Cantalapiedra, A.; et al. Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma. Cancers 2023, 15, 1558. https://doi.org/10.3390/cancers15051558
Puertas B, González-Calle V, Sobejano-Fuertes E, Escalante F, Queizán JA, Bárez A, Labrador J, Alonso-Alonso JM, García de Coca A, Cantalapiedra A, et al. Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma. Cancers. 2023; 15(5):1558. https://doi.org/10.3390/cancers15051558
Chicago/Turabian StylePuertas, Borja, Verónica González-Calle, Eduardo Sobejano-Fuertes, Fernando Escalante, José A. Queizán, Abelardo Bárez, Jorge Labrador, José María Alonso-Alonso, Alfonso García de Coca, Alberto Cantalapiedra, and et al. 2023. "Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma" Cancers 15, no. 5: 1558. https://doi.org/10.3390/cancers15051558
APA StylePuertas, B., González-Calle, V., Sobejano-Fuertes, E., Escalante, F., Queizán, J. A., Bárez, A., Labrador, J., Alonso-Alonso, J. M., García de Coca, A., Cantalapiedra, A., Villaescusa, T., Aguilar-Franco, C., Alejo-Alonso, E., Rey-Bua, B., López-Corral, L., García-Sanz, R., Puig, N., Gutiérrez, N. C., & Mateos, M.-V. (2023). Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma. Cancers, 15(5), 1558. https://doi.org/10.3390/cancers15051558










