Role of Anti-B-Cell Maturation Antigen (BCMA) in the Management of Multiple Myeloma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Multiple Myeloma
2.1. Burden of Disease
2.2. Pathogenesis
2.3. Therapy
3. Role of BCMA in MM Pathogenesis
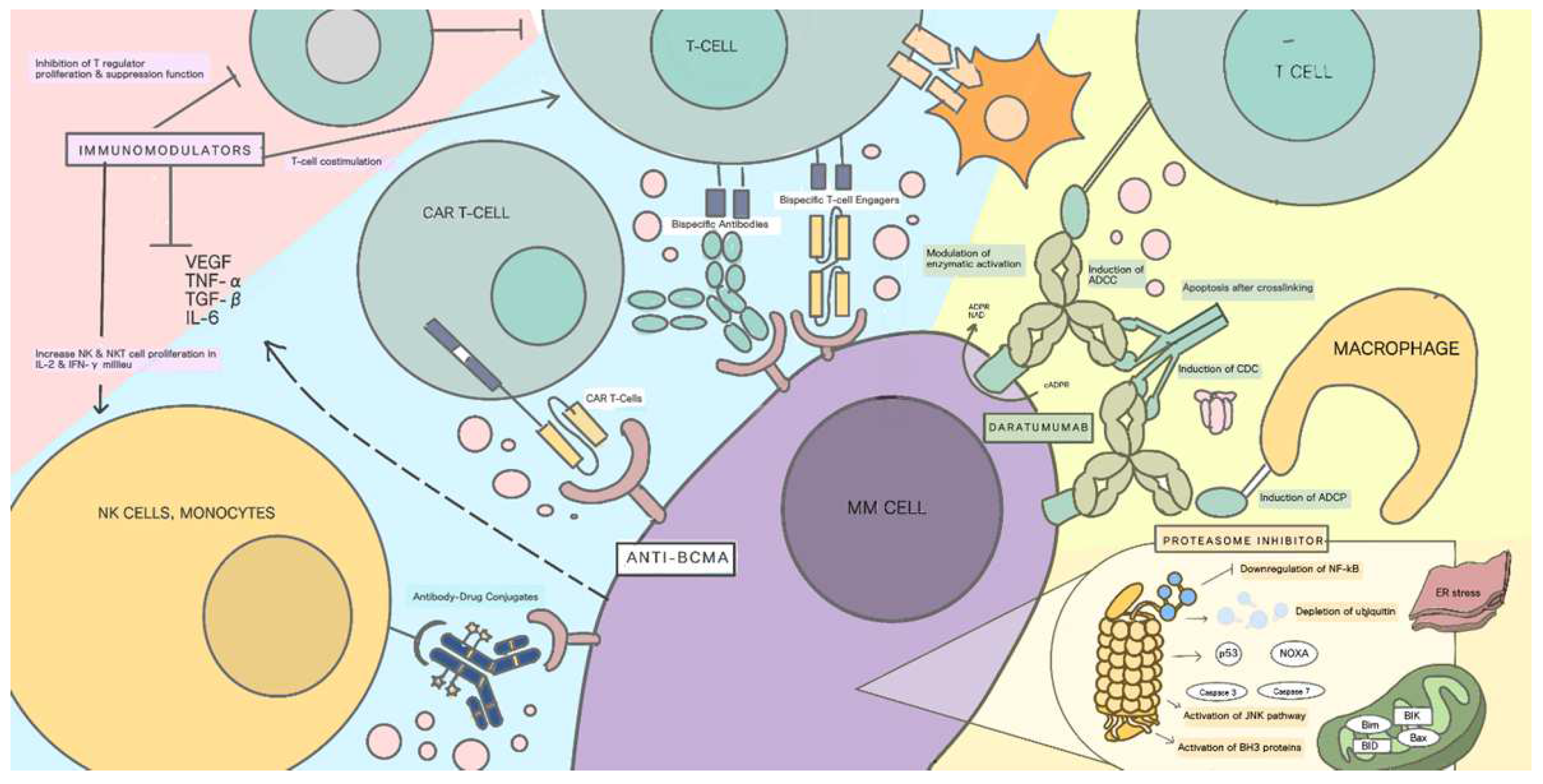
4. Anti-BCMA
5. BCMA-ADC (Antibody-Drug Conjugate)
6. Bispecific T-Cell Engager (BiTEs)
7. Chimeric Antigen Receptor (CAR) T Cells
8. Comparison of the Mechanism of Action to Currently Available Therapies
Mechanism of Action
9. Anti-BCMA Potential for Multiple Myeloma
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abramson, H.N. B-Cell Maturation Antigen (BCMA) as a Target for New Drug Development in Relapsed and/or Refractory Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 5192. [Google Scholar] [CrossRef] [PubMed]
- Multiple Myeloma—Global Cancer Observatory. Available online: https://gco.iarc.fr/today (accessed on 5 April 2022).
- Albagoush, S.A.; Shumway, C.; Azevedo, A.M. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; Multiple Myeloma. [Google Scholar]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia 2020, 34, 985–1005. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. Proteasome Inhibitors for the Treatment of Multiple Myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.F.; Anderson, K.C.; Tai, Y.T. Targeting B cell maturation antigen (BCMA) in multiple myeloma: Potential uses of BCMA-based immunotherapy. Front. Immunol. 2018, 10, 1821. [Google Scholar] [CrossRef]
- Nishimura, K.K.; Barlogie, B.; van Rhee, F.; Zangari, M.; Walker, B.A.; Rosenthal, A.; Schinke, C.; Thanendrarajan, S.; Davies, F.E. Long-term outcomes after autologous stem cell transplantation for multiple myeloma. Blood Adv. 2020, 4, 422–431. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y. Survival analysis of multiple myeloma patients after autologous stem cell transplantation. Stem. Cell. Investig. 2019, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Kaweme, N.M.; Changwe, G.J.; Zhou, F. Approaches and Challenges in the Management of Multiple Myeloma in the Very Old: Future Treatment Prospects. Front. Med. 2021, 8, 612696. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.A.; Boyd, K. Multiple myeloma: An overview of management. Palliat. Care. Soc. Pract. 2019, 13, 1178224219868235. [Google Scholar] [CrossRef]
- Roy, P.; Sarkar, U.A.; Basak, S. The NF-κB Activating Pathways in Multiple Myeloma. Biomedicines 2018, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Seipel, K.; Porret, N.; Wiedemann, G.; Jeker, B.; Bacher, V.U.; Pabst, T. sBCMA Plasma Level Dynamics and Anti-BCMA CAR-T-Cell Treatment in Relapsed Multiple Myeloma. Curr. Issues Mol. Biol. 2022, 44, 1463–1471. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hajek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Jiang, T.; Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Darce, J.R.; Arendt, B.K.; Wu, X.; Jelinek, D.F. Regulated expression of BAFF-binding receptors during human B cell differentiation. J. Immunol. 2007, 179, 7276–7286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dispenzieri, A.; Soof, C.M.; Rajkumar, S.V.; Gertz, M.A.; Kumar, S.; Bujarski, S.; Kyle, R.A.; Berenson, J.R. Serum BCMA levels to predict outcomes for patients with MGUS and smoldering multiple myeloma (SMM). J. Clin. Oncol. 2019, 37, 8020. [Google Scholar] [CrossRef]
- Sanchez, E.; Li, M.; Kitto, A.; Li, J.; Wang, C.S.; Kirk, D.T.; Yellin, O.; Nichols, C.M.; Dreyner, M.P.; Ahles, C.P.; et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br. J. Haematol. 2012, 158, 727–738. [Google Scholar] [CrossRef]
- Ghermezi, M.; Li, M.; Vardanyan, S.; Harutyunyan, N.M.; Gottlieb, J.; Berenson, A.; Spektor, T.M.; Andreu-Vieyra, C.; Gottlieb, J.; Berenson, A.; et al. Serum B-cell maturation antigen: A novel biomarker to predict outcomes for multiple myeloma patients. Haematologica 2017, 102, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.T.; Anderson, K.C. B cell maturation antigen (BCMA)-based Immunotherapy for Multiple Myeloma. Expert. Opin. Biol. Ther. 2019, 19, 1143. [Google Scholar] [CrossRef]
- Palma, B.D.; Marchica, V.; Catarozzo, M.T.; Giuliani, N.; Accardi, F. Monoclonal and Bispecific Anti-BCMA Antibodies in Multiple Myeloma. J. Clin. Med. 2020, 9, 1–11. [Google Scholar]
- Feng, D.; Sun, J. Overview of anti-BCMA CAR-T immunotherapy for multiple myeloma and relapsed/refractory multiple myeloma. Scand. J. Immunol. 2020, 92, e12910. [Google Scholar] [CrossRef]
- Nunes, A.T.; Annunziata, C.M. Proteasome Inhibitors: Structure and Function. Semin. Oncol. 2017, 44, 377. [Google Scholar] [CrossRef]
- Dou, Q.P.; Zonder, J.A. Overview of Proteasome Inhibitor-Based Anti-cancer Therapies: Perspective on Bortezomib and Second Generation Proteasome Inhibitors versus Future Generation Inhibitors of Ubiquitin-Proteasome System. Curr. Cancer. Drug. Targets 2014, 14, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knopf, K.B.; Duh, M.S.; Lafeuille, M.H.; Gravel, J.; Lefebvre, P.; Niculescu, L.; Ba-Mancini, A.; Ma, E.; Shi, H.; Comenzo, R.L. Meta-analysis of the efficacy and safety of bortezomib re-treatment in patients with multiple myeloma. Clin. Lymphoma. Myeloma. Leuk. 2014, 14, 380–388. [Google Scholar] [CrossRef]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010, 24, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holstein, S.A.; Suman, V.J.; McCarthy, P.L. Update on the role of lenalidomide in patients with multiple myeloma. Ther. Adv. Hematol. 2018, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Qiao, S.K.; Guo, X.N.; Ren, J.H.; Ren, H.Y. Efficacy and Safety of Lenalidomide in the Treatment of Multiple Myeloma: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Chin. Med. J. 2015, 128, 1215. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Yamada, T. Monoclonal Antibody Therapies in Multiple Myeloma: A Challenge to Develop Novel Targets. J. Oncol 2019, 2019, 6084012. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Janmaat, M.L.; Mutis, T.; van Bueren, J.J.L.; Ahmadi, T.; Sasser, A.K.; Lokhorst, H.M.; Parren, P.W.H.I. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol. Rev. 2016, 270, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Nooka, A.K.; Manteca, M.V.M.; Bahlis, N.; Weisel, K.; Oriol, A.; Alonso, A.A.; Suvannasankha, A.; Holkova, B.; Luptakova, K.; Fecteau, D.; et al. DREAMM-4: Evaluating safety and clinical activity of belantamab mafodotin (belamaf) in combination with pembrolizumab in patients with relapsed/refractory multiple myeloma. Hematol. Rep. 2020, 12. [Google Scholar]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Kroenke, J.; Facon, T.; Salnikov, A.; Lesley, R.; et al. Evaluation of AMG 420, an anti-BCMA bispecific T-cell engager (BiTE) immunotherapy, in R/R multiple myeloma (MM) patients: Updated results of a first-in-human (FIH) phase I dose escalation study. J. Clin. Oncol. 2019, 37, 8007. [Google Scholar] [CrossRef]
- Costa, L.J.; Wong, S.W.; Bermúdez, A.; de la Rubia, J.; Mateos, M.V.; Ocio, E.M.; Otero, P.R.; Miguel, J.S.; Li, S.; Sarmiento, R.; et al. First Clinical Study of the B-Cell Maturation Antigen (BCMA) 2+1 T Cell Engager (TCE) CC-93269 in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Interim Results of a Phase 1 Multicenter Trial. Blood 2019, 134, 143. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Ma, T.; Shi, J.; Xiao, Y.; Bian, T.; Wang, J.; Hui, L.; Wang, M.; Liu, H. Study on the Relationship Between the Expression of B Cell Mature Antigen and the Classification, Stage, and Prognostic Factors of Multiple Myeloma. Front. Immunol. 2021, 18, 4638. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.S.; Jakubowiak, A.; Gasparetto, C.; Cornell, R.F.; Krupka, H.I.; Navarro, D.; Forgie, A.J.; Udata, C.; Basu, C.; Chou, J.; et al. Safety, Clinical Activity, Pharmacokinetics, and Pharmacodynamics from a Phase I Study of PF-06863135, a B-Cell Maturation Antigen (BCMA)-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 1869. [Google Scholar] [CrossRef]
- Cooper, D.; Madduri, D.; Lentzsch, S.; Jagannath, S.; Li, J.; Boyapati, A.; Adriaens, L.; Chokshi, D.; Zhu, M.; Lowy, I.; et al. Safety and Preliminary Clinical Activity of REGN5458, an Anti-Bcma x Anti-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2019, 134, 3176. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Jiang, S.; Jin, J.; Hao, S.; Yang, M.; Chen, L.; Ruan, H.; Xiao, J.; Wang, W.; Li, Z.; Yu, K. Low Dose of Human scFv-Derived BCMA-Targeted CAR-T Cells Achieved Fast Response and High Complete Remission in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2018, 132, 960. [Google Scholar] [CrossRef]
- Chunrui, L.; Zhou, J.; Wang, J.; Hu, G.; Du, A.; Zhou, X.; Meng, L.; Hong, Z.; Chen, L.; Mao, X. Clinical responses and pharmacokinetics of fully human BCMA targeting CAR T-cell therapy in relapsed/refractory multiple myeloma. J. Clin. Oncol. 2019, 37, 8013. [Google Scholar]
- Shah, N.; Alsina, M.; Siegel, D.S.; Jagannath, S.; Madduri, D.; Kaufman, J.L.; Turka, A.; Lam, L.P.; Massaro, M.; Hege, K.; et al. Initial Results from a Phase 1 Clinical Study of bb21217, a Next-Generation Anti Bcma CAR T Therapy. Blood 2018, 132, 488. [Google Scholar] [CrossRef]
- Fan, X.; Zhuang, Q.; Yang, L.; Hao, J.; Zhao, D.; Zhao, Y.; Wang, P.Y.; Geng, D. Preclinical assessment of LCAR-B38M, a novel BCMA-targeting chimeric antigen receptor (CAR)-T cell therapy in relapsed/refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, e160. [Google Scholar] [CrossRef]
- Li, C.; Mei, H.; Hu, Y.; Guo, T.; Liu, L.; Jiang, H.; Tang, L.; Wu, Y.; Ai, L.; Deng, J.; et al. A Bispecific CAR-T Cell Therapy Targeting Bcma and CD38 for Relapsed/Refractory Multiple Myeloma: Updated Results from a Phase 1 Dose-Climbing Trial. Blood 2019, 134, 930. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Lai, J.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet. Haematol. 2019, 6, e521–e529. [Google Scholar] [CrossRef]
- Mailankody, S.; Ghosh, A.; Staehr, M.; Purdon, T.J.; Roshal, M.; Halton, E.; Diamonte, C.; Pineda, J.; Anant, P.; Bernal, Y.; et al. Clinical Responses and Pharmacokinetics of MCARH171, a Human-Derived Bcma Targeted CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma: Final Results of a Phase I Clinical Trial. Blood 2018, 132, 959. [Google Scholar] [CrossRef]

| Class | Drugs | Target of Action | Mechanism of Action | Reported Toxicities | Indication |
|---|---|---|---|---|---|
| Proteasome inhibitor | Bortezomib Carfilzomib Ixazomib | Proteasomes of malignant plasma cells | Inhibition of IκBα (classical pathway) degradation in proteasome [11,22]; Activation of JNK pathway and caspases [11,22]; Inhibition of pro-apoptotic protein degradation [11,22] | Peripheral neuropathy, nausea, vomiting, diarrhea, cytopenia, infection, fatigue, headache, peripheral edema, and back pain [23,24] | Initial induction therapy (bortezomib) [13,23,24]; Recurrent/relapsed therapy (carfilzomib, ixazomib) [23,24]. |
| Immunomodulators | Thalidomide Lenalidomide | B and T lymphocytes Malignant plasma cells | Augmentation of T-cell costimulation [25]; Inhibition of plasma-cell-derived cytokines [25]; Inhibition of T-regulator proliferation and suppressor function [25]; Increasing NK- and NKT-cell proliferation in IL2- and IFN-gamma milieu [25]; Direct cytotoxicity to malignant cells through apoptosis pathways [26] | Cytopenia, infection, fatigue, and peripheral neuropathy [27]; Deep-vein thrombosis (in combination with dexamethasone [27] | Induction and maintenance therapy [25,26]; Recurrent/relapsed [25,26] |
| Monoclonal antibodies | Daratumumab Elotuzumab | Surface antigens of malignant plasma cells, CD38 (daratumumab), and CS1/SLAMF7 (elotuzumab) | Antibody-dependent cellular cytotoxicity [28,29]; Complement-dependent cellular cytotoxicity [28,29] | Induction therapy [13]; Recurrent/relapsed MM [24,27] | |
| Anti-BCMA | ADC (belantamab mafodotin) | BCMA | Coupling to MMFA [20,22]; Direct cytotoxicity [20,22] | Thrombocytopenia, anemia, and corneal events [30] | Recurrent/relapsed MM [13] |
| BiTEs | BCMA | Binding to T cells and induction of apoptosis through perforin [20,22]; Direct cytotoxicity [20,22] | No serious adverse events reported yet [31] | ||
| CAR T cells | BCMA | Conversion of patient-derived cytotoxic T cells into specific killers of cancer cells using recombinant DNA mutation process [25,26] | Neurotoxicity, nephrotoxicity [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, I.; Muthalib, A.; Edina, B.C.; Wiyono, L.; Winston, K. Role of Anti-B-Cell Maturation Antigen (BCMA) in the Management of Multiple Myeloma. Cancers 2022, 14, 3507. https://doi.org/10.3390/cancers14143507
Rinaldi I, Muthalib A, Edina BC, Wiyono L, Winston K. Role of Anti-B-Cell Maturation Antigen (BCMA) in the Management of Multiple Myeloma. Cancers. 2022; 14(14):3507. https://doi.org/10.3390/cancers14143507
Chicago/Turabian StyleRinaldi, Ikhwan, Abdul Muthalib, Brenda Cristie Edina, Lowilius Wiyono, and Kevin Winston. 2022. "Role of Anti-B-Cell Maturation Antigen (BCMA) in the Management of Multiple Myeloma" Cancers 14, no. 14: 3507. https://doi.org/10.3390/cancers14143507
APA StyleRinaldi, I., Muthalib, A., Edina, B. C., Wiyono, L., & Winston, K. (2022). Role of Anti-B-Cell Maturation Antigen (BCMA) in the Management of Multiple Myeloma. Cancers, 14(14), 3507. https://doi.org/10.3390/cancers14143507






