COVID-19 Severity and Survival over Time in Patients with Hematologic Malignancies: A Population-Based Registry Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
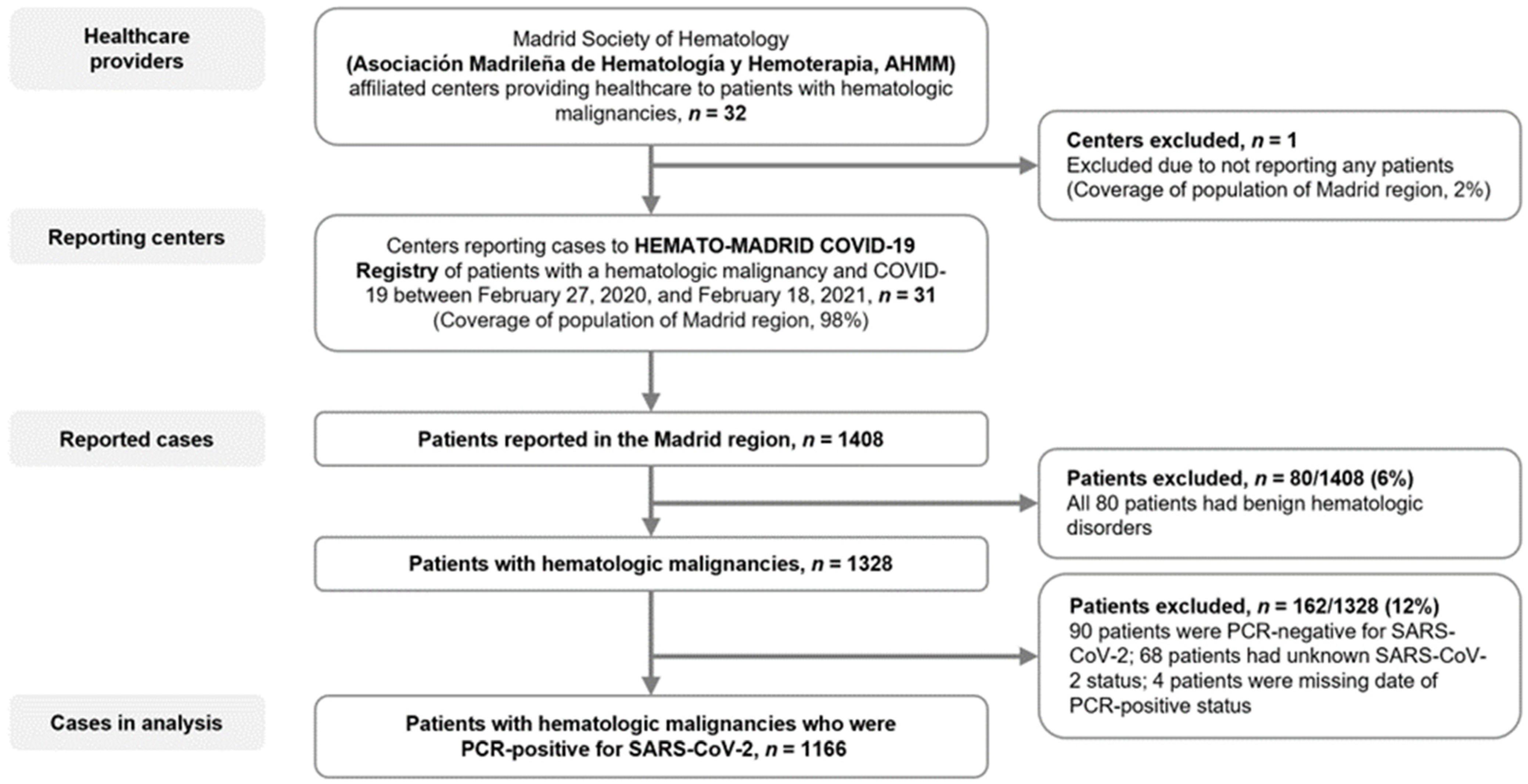
2.1. Study Design and Participants
2.2. Data Collection and End Points
2.3. Statistical Analyses
3. Results
3.1. Patients
3.2. COVID-19 Diagnosis and Treatment
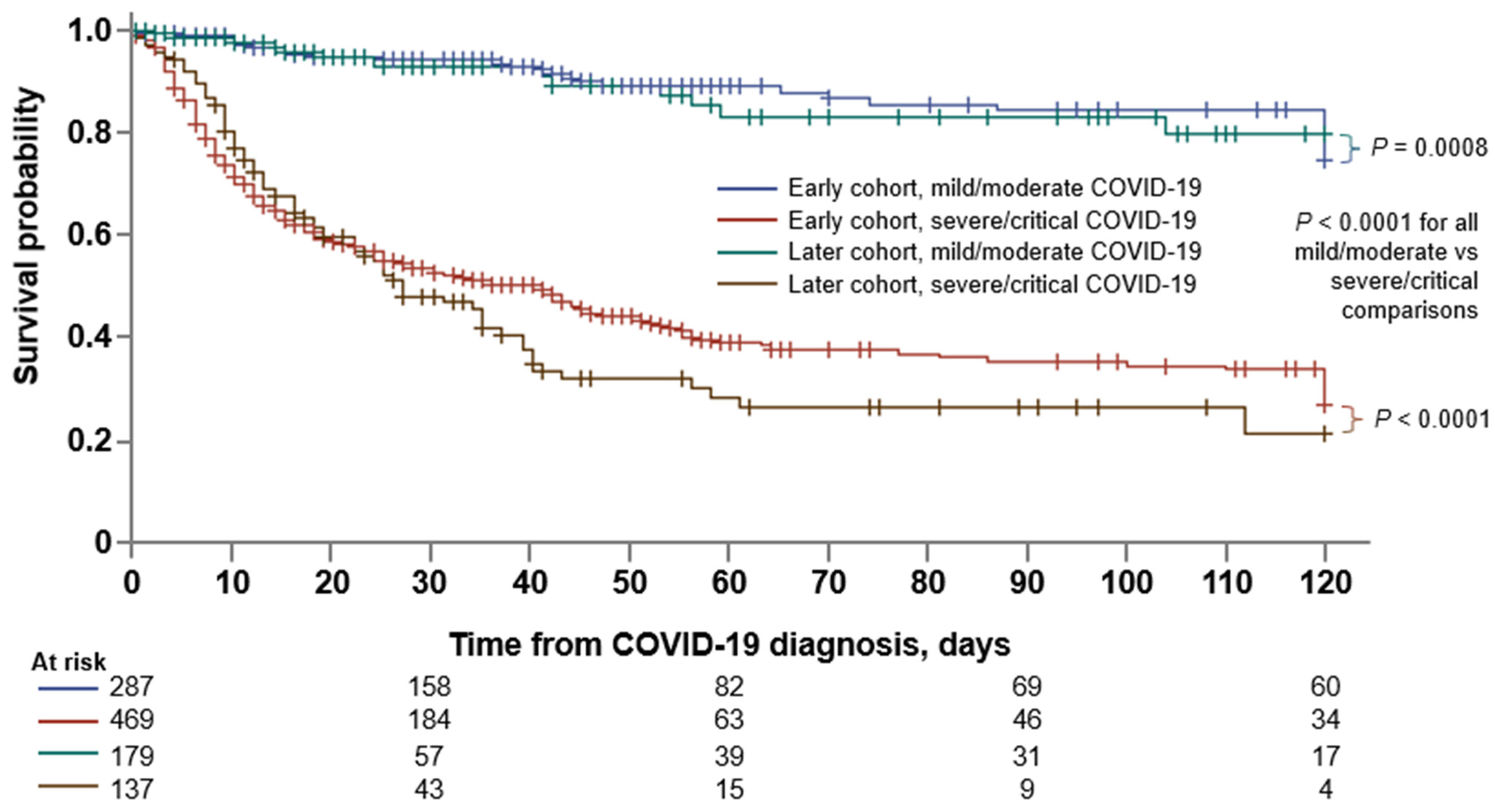
3.3. Comparison between the Early and Later Cohorts
3.4. Factors Associated with COVID-19 Severity
3.5. Factors Associated with Mortality
3.6. Comparison between HM and Non-Cancer Inpatients with COVID-19
3.7. Post COVID-19 Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 7 September 2022).
- Spanish Ministry of Health Social Services and Equality. Madrid—The Evolution of the Coronavirus by Region. Available online: https://www.epdata.es/datos/evolucion-coronavirus-cada-comunidad/518/madrid/304 (accessed on 6 September 2022).
- Statistical Information for the Analysis of the Impact of the COVID-19 Crisis. Available online: https://www.ine.es/en/covid/covid_inicio_en.htm (accessed on 6 September 2022).
- Dennis, J.M.; McGovern, A.P.; Vollmer, S.J.; Mateen, B.A. Improving survival of critical care patients with coronavirus disease 2019 in England: A national cohort study, March to June. Crit. Care Med. 2020, 49, 209. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.K.; Navaratnam, A.V.; Day, J.; Wendon, J.; Briggs, T.W. Changes in COVID-19 in-hospital mortality in hospitalised adults in England over the first seven months of the pandemic: An observational study using administrative data. Lancet Reg. Health Eur. 2021, 5, 100104. [Google Scholar] [CrossRef] [PubMed]
- I Horwitz, L.; A Jones, S.; Cerfolio, R.J.; Francois, F.; Greco, J.; Rudy, B.; Petrilli, C.M. Trends in COVID-19 Risk-Adjusted Mortality Rates. J. Hosp. Med. 2020, 16, 90–92. [Google Scholar] [CrossRef]
- The Royal Society. Available online: https://royalsociety.org/-/media/policy/projects/set-c/set-c-long-covid.pdf (accessed on 6 September 2022).
- Asch, D.A.; Sheils, N.E.; Islam, M.N.; Chen, Y.; Werner, R.M.; Buresh, J.; Doshi, J.A. Variation in US Hospital Mortality Rates for Patients Admitted With COVID-19 During the First 6 Months of the Pandemic. JAMA Intern. Med. 2021, 181, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.E.; Paul, P.; MacCannell, D.R.; Johansson, M.A.; Brooks, J.T.; MacNeil, A.; Slayton, R.B.; Tong, S.; Silk, B.J.; Armstrong, G.L.; et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage - United States, December 29, 2020-January 12, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 95–99. [Google Scholar] [CrossRef]
- Booth, S.; Curley, H.M.; Varnai, C.; Arnold, R.; Lee, L.Y.W.; Campton, N.A.; Cook, G.; Purshouse, K.; Aries, J.; Innes, A.; et al. Key findings from the UKCCMP cohort of 877 patients with haematological malignancy and COVID-19: Disease control as an important factor relative to recent chemotherapy or anti-CD20 therapy. Br. J. Haematol. 2022, 196, 892–901. [Google Scholar] [CrossRef]
- García-Suárez, J.; de la Cruz, J.; De La Cruz, J.; Cedillo, Á.; Llamas, P.; Duarte, R.; Jiménez-Yuste, V.; Hernández-Rivas, J.Á.; Gil-Manso, R.; Kwon, M.; et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: Lessons from a large population-based registry study. J. Hematol. Oncol. 2020, 13, 133. [Google Scholar] [CrossRef]
- Passamonti, F.; Cattaneo, C.; Arcaini, L.; Bruna, R.; Cavo, M.; Merli, F.; Angelucci, E.; Krampera, M.; Cairoli, R.; Della Porta, M.G.; et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: A retrospective, multicentre, cohort study. Lancet Haematol. 2020, 7, e737–e745. [Google Scholar] [CrossRef]
- Piñana, J.L.; Martino, R.; García-García, I.; Parody, R.; Morales, M.D.; Benzo, G.; Gómez-Catalan, I.; Coll, R.; Fuente, I.D.L.; Luna, A.; et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp. Hematol. Oncol. 2020, 9, 21. [Google Scholar] [CrossRef]
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martín-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892. [Google Scholar] [CrossRef]
- Johns Hopkins University of Medicine—Coronavirus Resource Center. Mortality Analyses. Available online: https://coronavirus.jhu.edu/data/mortality (accessed on 6 September 2022).
- Lee, L.Y.; Cazier, J.B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Roeker, L.E.; Eyre, T.A.; Thompson, M.C.; Lamanna, N.; Coltoff, A.R.; Davids, M.S.; Baker, P.O.; Leslie, L.A.; Rogers, K.A.; Allan, J.N.; et al. COVID-19 in patients with CLL: Improved survival outcomes and update on management strategies. Blood 2021, 138, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.A.; Neuberg, D.S.; Thompson, J.C.; Tallman, M.S.; Sekeres, M.A.; Sehn, L.H.; Anderson, K.; Goldberg, A.D.; Pennell, N.A.; Niemeyer, C.M.; et al. Outcomes of Patients with Hematologic Malignancies and COVID-19 Infection: A Report from the ASH Research Collaborative Data Hub. Blood 2020, 136, 7–8. [Google Scholar] [CrossRef]
- Wood, W.A.; Neuberg, D.S.; Thompson, J.C.; Tallman, M.S.; Sekeres, M.A.; Sehn, L.H.; Anderson, K.C.; Goldberg, A.D.; Pennell, N.A.; Niemeyer, C.M.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A report from the ASH Research Collaborative Data Hub. Blood Adv. 2020, 4, 5966–5975. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 6 October 2021).
- Pinato, D.J.; Tabernero, J.; Bower, M.; Scotti, L.; Patel, M.; Colomba, E.; Dolly, S.; Loizidou, A.; Chester, J.; Mukherjee, U.; et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: Evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021, 22, 1669–1680. [Google Scholar] [CrossRef]
- Tang, Y.W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512–e00520. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO COVID-19: Case Definitions, Updated in Public Health Surveillance for COVID-19. Available online: https://apps.who.int/iris/bitstream/handle/10665/333912/WHO-2019-nCoV-Surveillance_Case_Definition-2020.1-eng.pdf (accessed on 7 August 2020).
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (nCoV) Infection is Suspected. Available online: https://www.who.int/publications/i/item/10665-332299 (accessed on 12 January 2020).
- Casas-Rojo, J.; Antón-Santos, J.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.; Vargas-Núñez, J.; et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev. Clín. Esp. 2020, 220, 480–494. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Lei, X.; Zhao, H.; Scheet, P.; Giordano, S.H. Evaluation of COVID-19 Mortality and Adverse Outcomes in US Patients with or Without Cancer. JAMA Oncol. 2022, 8, 69. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet. 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Shah, V.; Ko Ko, T.; Zuckerman, M.; Vidler, J.; Sharif, S.; Mehra, V.; Gandhi, S.; Kuhnl, A.; Yallop, D.; Avenoso, D.; et al. Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with haematological malignancies; King’s College Hospital experience. Br. J. Haematol. 2020, 190, e279–e282. [Google Scholar] [CrossRef] [PubMed]
- Recovery Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Westblade, L.F.; Brar, G.; Pinheiro, L.C.; Paidoussis, D.; Rajan, M.; Martin, P.; Goyal, P.; Sepulveda, J.L.; Zhang, L.; George, G.; et al. SARS-CoV-2 Viral Load Predicts Mortality in Patients with and without Cancer Who Are Hospitalized with COVID-19. Cancer Cell 2020, 38, 661–671.e2. [Google Scholar] [CrossRef]
- Barbui, T.; Iurlo, A.; Masciulli, A.; Carobbio, A.; Ghirardi, A.; Rossi, G.; Harrison, C.; Alvarez-Larran, A.; Elli, E.M.; Kiladjian, J.-J.; et al. Long-term follow-up of recovered MPN patients with COVID-19. Blood Cancer J. 2021, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhang, X.; Chen, C.; Jiang, D.; Liu, X.; Zhou, Y.; Huang, C.; Zhou, Y.; Guan, Z.; Ding, C.; et al. Characteristics of Viral Shedding Time in SARS-CoV-2 Infections: A Systematic Review and Meta-Analysis. Front Public Health 2021, 9, 652842. [Google Scholar] [CrossRef]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Patients with Hematologic Malignancies, N = 1166 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Lymphoid Malignancies | Myeloid Malignancies | ||||||||
| NHL | MM | CLL | HL | ALL | MDS | AML | CML | Ph-MPN | ||
| Patients, n (%) | 1166 (100) | 325 (27.9) | 263 (22.6) | 175 (15.0) | 50 (4.3) | 26 (2.2) | 115 (9.9) | 92 (7.9) | 33 (2.8) | 87 (7.5) |
| Age, y | N = 1147 | n = 322 | n = 253 | n = 174 | n = 49 | n = 26 | n = 113 | n = 91 | n = 32 | n = 87 |
| Median (IQR) | 71 (59–79) | 69 (57–76) | 72 (63–79) | 74 (63–82) | 57 (42–71) | 47 (33–59) | 79 (71–84) | 65 (50–75) | 64 (54–84) | 72 (65–82) |
| Sex, n (%) | N = 1152 | n = 321 | n = 261 | n = 175 | n = 49 | n = 26 | n = 113 | n = 91 | n = 33 | n = 83 |
| Female | 464 (40.3) | 136 (42.4) | 108 (41.4) | 61 (34.9) | 18 (36.7) | 8 (30.8) | 41 (36.3) | 46 (50.5) | 8 (24.2) | 28 (34) |
| Male | 688 (59.7) | 185 (57.6) | 153 (58.6) | 114 (65.1) | 31 (63.3) | 18 (69.2) | 72 (63.7) | 45 (49.5) | 25 (75.8) | 55 (66) |
| Comorbidity count | N = 1166 | n = 325 | n = 263 | n = 175 | n = 50 | n = 26 | n = 115 | n = 92 | n = 33 | n = 87 |
| 0 | 314 (26.9) | 96 (29.4) | 62 (23.6) | 44 (25.1) | 13 (26.0) | 15 (57.7) | 22 (19.1) | 33 (35.9) | 11 (33.3) | 18 (20.7) |
| 1 | 484 (41.5) | 140 (43.1) | 102 (38.8) | 77 (44.0) | 18 (36.0) | 8 (30.8) | 50 (43.5) | 40 (43.5) | 9 (27.3) | 40 (46.0) |
| ≥2 | 368 (31.6) | 89 (27.4) | 99 (37.6) | 54 (30.9) | 19 (38) | 3 (11.6) | 43 (37.4) | 19 (20.7) | 13 (39.4) | 29 (33.3) |
| Stem cell transplantation, * n (%) | N = 1127 | n = 312 | n = 259 | n = 168 | n = 47 | n = 24 | n = 112 | n = 92 | n = 32 | n = 81 |
| Autologous | 100 (8.9) | 23 (7.4) | 66 (25.5) | 0 | 7 (14.9) | 1 (4.2) | 1 (0.9) | 2 (2.2) | 0 | 0 |
| Allogeneic | 56 (5.0) | 5 (1.6) | 3 (1.2) | 1 (0.6) | 4 (8.5) | 9 (37.5) | 9 (8.0) | 25 (27.2) | 0 | 0 |
| No | 971 (86.2) | 284 (91.0) | 190 (73.4) | 167 (99.4) | 36 (76.6) | 14 (58.3) | 102 (91.1) | 65 (70.7) | 32 (100) | 81 (100) |
| Cancer therapy, within 30 d, † n (%) | N = 1162 | n = 325 | n = 260 | n = 175 | n = 50 | n = 26 | n = 115 | n = 92 | n = 33 | n = 86 |
| Active therapy | 679 (58.4) | 178 (54.8) | 190 (73.1) | 55 (31.4) | 24 (48.0) | 16 (61.5) | 50 (43.5) | 61 (66.3) | 29 (87.9) | 76 (88.4) |
| Conventional chemotherapy | 260 (22.4) | 112 (34.5) | 82 (31.5) | 3 (1.7) | 17 (34.0) | 13 (50.0) | 2 (1.7) | 31 (33.7) | 0 | 0 |
| Low-intensity chemotherapy | 71 (6.1) | 4 (1.2) | 8 (3.1) | 3 (1.7) | 0 | 1 (3.8) | 3 (2.6) | 0 | 4 (12.1) | 48 (55.8) |
| Molecular-targeted therapy | 130 (11.2) | 13 (4.0) | 20 (7.7) | 43 (24.6) | 0 | 1 (3.8) | 1 (0.9) | 5 (5.4) | 24 (72.7) | 23 (26.7) |
| Immunotherapy, mAb only ‡ | 56 (4.8) | 38 (11.7) | 10 (3.8) | 3 (1.7) | 4 (8.0) | 1 (3.8) | 0 | 0 | 0 | 0 |
| Immunomodulator drugs | 71 (6.1) | 1 (0.3) | 69 (26.5) | 0 | 0 | 0 | 1 (0.9) | 0 | 0 | 0 |
| Hypomethylating agents | 49 (4.2) | 1 (0.3) | 0 | 0 | 0 | 0 | 24 (20.9) | 24 (26.1) | 0 | 0 |
| Supportive therapy | 31 (2.7) | 5 (1.5) | 0 | 2 (1.1) | 0 | 0 | 19 (16.5) | 0 | 0 | 5 (5.8) |
| Active, not detailed | 11 (0.9) | 4 (1.2) | 1 (0.4) | 1 (0.6) | 3 (6.0) | 0 | 0 | 1 (1.1) | 1 (3.0) | 0 |
| No active therapy | 483 (41.6) | 147 (45.2) | 70 (26.9) | 120 (68.6) | 26 (52.0) | 10 (38.5) | 65 (56.5) | 31 (33.7) | 4 (12.1) | 10 (11.6) |
| Survival Estimate, % (95% CI) | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|
| 30-d | 60-d | Unadjusted | Adjusted * | |
| All patients † | 68.4 (65.3–71.3) | 56.3 (52.6–59.9) | ||
| Age | ||||
| Age 18–49 y | 93.7 (86.6–97.1) | 90.1 (80.8–95.1) | reference | reference |
| Age 50–79 y | 72.8 (68.9–76.2) | 58.8 (54.0–63.3) | 3.59 (2.01–6.42) | 3.01 (1.67–5.43) |
| Age ≥ 80 y | 46.3 (39.8–52.5) | 35.1 (28.3–42.0) | 7.86 (4.36–14.2) | 6.24 (3.39–11.49) |
| Sex | ||||
| Female | 69.5 (64.5–74.0) | 60.0 (54.2–65.4) | reference | reference |
| Male | 67.4 (63.3–71.2) | 53.6 (48.6–58.3) | 1.12 (0.91–1.38) | 1.15 (0.93–1.41) |
| Comorbidities | ||||
| 0 | 79.3 (73.5–84.0) | 69.7 (62.3–76.0) | reference | reference |
| 1 | 70.9 (66.1–75.1) | 56.3 (50.4–61.7) | 1.49 (1.12–1.98) | 1.10 (0.83–1.48) |
| 2 | 61.4 (53.7–68.2) | 49.7 (41.0–57.9) | 1.89 (1.37–2.61) | 1.15 (0.82–1.61) |
| ≥3 | 50.4 (41.4–58.7) | 41.6 (32.0–51.0) | 2.57 (1.84–3.59) | 1.43 (1.01–2.03) |
| Hematologic malignancy | ||||
| NHL | 71.0 (64.9–76.2) | 57.2 (49.9–63.8) | reference | reference |
| MM | 69.7 (62.9–75.5) | 57.3 (49.1–64.7) | 0.98 (0.73–1.32) | 0.83 (0.61–1.12) |
| CLL | 59.1 (50.5–66.8) | 49.8 (39.8–59.0) | 1.37 (1.00–1.87) | 1.02 (0.74–1.41) |
| HL | 73.0 (55.6–84.4) | 68.1 (49.0–81.3) | 0.77 (0.43–1.41) | 0.89 (0.49–1.64) |
| ALL | 78.4 (52.2–91.3) | 47.0 (17.6–72.1) | 0.99 (0.46–2.13) | 2.31 (1.04–5.12) |
| MDS | 59.4 (48.9–68.4) | 48.7 (37.2–59.3) | 1.46 (1.04–2.05) | 0.96 (0.68–1.37) |
| AML | 63.8 (52.2–73.2) | 53.6 (41.3–64.3) | 1.41 (0.99–2.01) | 1.68 (1.17–2.40) |
| CML | 81.8 (58.5–92.8) | 81.8 (58.5–92.8) | 0.58 (0.24–1.43) | 0.44 (0.18–1.08) |
| Ph-MPN | 82.4 (71.0–89.6) | 66.4 (50.9–78.0) | 0.70 (0.43–1.13) | 0.51 (0.31–0.82) |
| Stem cell transplantation ‡ | ||||
| No | 65.7 (62.2–68.9) | 53.9 (49.8–57.8) | reference | reference |
| Autologous | 85.3 (75.1–91.6) | 80.8 (68.7–88.6) | 0.58 (0.34–0.99) | 0.54 (0.31–0.95) |
| Allogeneic | 86.2 (71.8–93.6) | 69.6 (51.2–82.2) | 0.35 (0.20–0.59) | 1.15 (0.64–2.07) |
| Cancer therapy, within 30 d ¶ | ||||
| No active therapy | 67.4 (62.3–71.9) | 58.7 (51.8–63.3) | reference | reference |
| Active therapy | 69.0 (65.0–72.7) | 55.4 (50.6–60.1) | 1.08 (0.88–1.33) | 1.10 (0.89–1.36) |
| Conventional chemotherapy | 66.4 (59.6–72.3) | 51.8 (44.0–59.2) | 1.16 (0.90–1.50) | 1.49 (1.14–1.93) |
| Low-intensity chemotherapy | 76.8 (63.4–85.8) | 63.1 (46.1–76.1) | 0.80 (0.48–1.32) | 0.65 (0.39–1. 08) |
| Molecular targeted therapy | 69.2 (59.4–77.1) | 60.4 (48.9–70.0) | 1.05 (0.75–1.47) | 1.02 (0.73–1.44) |
| Immunotherapy, mAb only | 68.9 (53.2–80.2) | 47.7 (30.2–63.2) | 1.14 (0.72–1.80) | 1.40 (0.88–2.24) |
| Immunomodulator drugs | 70.4 (56.8–80.5) | 58.2 (42.3–71.1) | 0.88 (0.56–1.39) | 0.84 (0.53–1.33) |
| Hypomethylating agents | 64.6 (47.6–77.3) | 60.5 (42.8–74.3) | 1.46 (0.94–2.27) | 1.07 (0.68–1.67) |
| Supportive therapy | 64.7 (43.1–79.8) | 41.2 (19.9–61.4) | 1.39 (0.79–2.46) | 0.90 (0.51–1.61) |
| Time period of COVID-19 diagnosis | ||||
| Early cohort (1st wave, February–June 2020) | 67.4 (63.7–70.8) | 56.3 (52.0–60.4) | reference | reference |
| Later cohort (2nd/3rd wave, July 2020–February 2021) | 70.9 (64.9–76.1) | 55.8 (48.0–62.9) | 0.93 (0.73–1.17) | 0.99 (0.79–1.26) |
| Care setting of COVID-19 treatment | ||||
| Outpatient | 99.4 (95.5–99.9) | 93.8 (85.3–97.4) | reference | reference |
| Inpatient | 62.1 (58.6–65.4) | 49.3 (45.2–53.2) | 10.8 (5.37–21.8) | 8.81 (4.37–17.8) |
| Intensive care unit | 45.2 (38.9–51.2) | 28.0 (22.1–34.1) | 2.21 (1.80–2.71) | 2.42 (1.97–2.99) |
| Pharmacologic therapies for COVID-19 ¶¶ | ||||
| (Hydroxy)chloroquine | 67.7 (63.6–71.4) | 56.9 (52.2–61.2) | 1.00 (0.81–1.23) | 0.96 (0.77–1.19) |
| Azithromycin | 67.7 (62.5–72.5) | 56.6 (50.3–62.3) | 1.06 (0.86–1.30) | 1.03 (0.83–1.27) |
| Lopinavir/darunavir | 66.1 (60.9–70.7) | 56.1 (50.3–61.5) | 1.03 (0.84–1.26) | 1.09 (0.88–1.35) |
| Remdesivir | 80.5 (68.2–88.4) | 61.5 (46.3–73.6) | 0.71 (0.46–1.08) | 0.84 (0.55–1.28) |
| Tocilizumab | 66.3 (58.3–73.2) | 52.0 (43.1–60.1) | 1.07 (0.83–1.37) | 1.25 (0.96–1.62) |
| Corticosteroids | 59.8 (55.4–63.8) | 45.2 (40.4–49.9) | 2.15 (1.71–2.69) | 2.06 (1.64–2.59) |
| Oxygen support during COVID-19 treatment | ||||
| No | 98 (94–99) | 91 (85–95) | reference | reference |
| Low-flow oxygen support | 67.5 (63.0–71.6) | 57.7 (52.4–62.6) | 4.9 (3.09–7.62) | 3.68 (2.33–5.81) |
| High-flow oxygen support or mechanical ventilation | 44.3 (37.9–50.4) | 26.8 (20.9–33.0) | 10.1 (6.4–15.9) | 8.52 (5.38–13.5) |
| Clinical severity of COVID-19 | ||||
| Mild | 96.1 (91.6–98.2) | 90.5 (82.3–95.7) | reference | reference |
| Moderate | 92.9 (88.2–95.7) | 85.8 (78.9–90.6) | 1.45 (0.77–2.74) | 1.17 (0.62–2.22) |
| Severe | 57.8 (52.5–62.8) | 46.8 (40.8–52.5) | 7.34 (4.26–12.7) | 5.64 (3.26–9.79) |
| Critical | 40.9 (34.0–47.7) | 21.7 (15.7–28.4) | 12.4 (7.17–21.5) | 11.0 (6.32–19.2) |
| Patients with COVID-19 Severity Data, N = 1131 | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|
| Total, N = 1131* | Mild/Moderate, n = 508 | Severe/Critical, n = 623 | Unadjusted | Adjusted † | |
| Age, y | n = 1112 | n = 491 | n = 621 | ||
| Median (IQR) | 71 (60–79) | 66 (54–76) | 73 (65–82) | n/a | n/a |
| Age 18–49 y, n (%) | 129 (11.6) | 88 (17.9) | 41 (6.6) | reference | reference |
| Age 50–79 y, n (%) | 718 (64.6) | 318 (64.8) | 400 (64.4) | 2.70 (1.81–4.02) | 2.19 (1.44–3.33) |
| Age ≥80 y, n (%) | 265 (23.8) | 85 (17.3) | 180 (29.0) | 4.54 (2.89–7.14) | 3.37 (2.08–5.46) |
| Sex, n (%) | N = 1117 | n = 501 | n = 616 | ||
| Female | 448 (40.1) | 212 (42.3) | 236 (38.3) | reference | reference |
| Male | 669 (59.9) | 289 (57.7) | 380 (61.7) | 1.18 (0.93–1.50) | 1.16 (0.90–1.50) |
| Comorbidity count | N = 1131 | n = 508 | n = 623 | ||
| 0 | 305 (27.0) | 181 (35.6) | 124 (19.9) | reference | reference |
| 1 | 469 (41.5) | 199 (39.2) | 270 (43.3) | 1.98 (1.48–2.65) | 1.53 (1.12–2.10) |
| ≥2 | 357 (31.6) | 128 (25.2) | 229 (36.8) | 2.61 (1.91–3.58) | 1.62 (1.14–2.30) |
| Hematologic malignancy, n (%) | N = 1131 | n = 508 | n = 623 | ||
| Lymphoid malignancy | 816 (72.1) | 386 (76.0) | 430 (69.0) | reference | reference |
| Myeloid malignancy | 315 (27.9) | 122 (24.0) | 193 (31.0) | 1.42 (1.09–1.85) | 1.30 (0.98–1.72) |
| NHL | 315 (27.9) | 165 (32.5) | 150 (24.1) | reference | reference |
| MM | 258 (22.8) | 130 (25.6) | 128 (20.5) | 1.08 (0.78–1.51) | 0.97 (0.69–1.38) |
| CLL | 169 (14.9) | 55 (10.8) | 114 (18.3) | 2.28 (1.54–3.37) | 2.02 (1.34–3.05) |
| HL | 48 (4.2) | 24 (4.7) | 24 (3.9) | 1.10 (0.60–2.02) | 1.61 (0.82–3.16) |
| ALL | 26 (2.3) | 12 (2.4) | 14 (2.2) | 1.28 (0.58–2.86) | 2.88 (1.22–6.82) |
| MDS | 111 (9.8) | 41 (8.1) | 70 (11.2) | 1.81 (1.18–2.76) | 1.31 (0.82–2.11) |
| AML | 91 (8.0) | 29 (5.7) | 62 (10.0) | 2.35 (1.44–3.85) | 3.13 (1.83–5.34) |
| CML | 31 (2.7) | 21 (4.1) | 10 (1.6) | 0.52 (0.24–1.15) | 0.57 (0.24–1.32) |
| Ph-MPN | 82 (7.3) | 31 (6.1) | 51 (8.2) | 1.81 (1.10–2.98) | 1.43 (0.84–2.42) |
| Stem cell transplantation, ‡ n (%) | N = 1098 | n = 494 | n = 604 | ||
| Autologous | 100 (9.1) | 65 (13.2) | 35 (5.8) | 0.39 (0.26–0.61) | 0.56 (0.35–0.90) |
| Allogeneic | 56 (5.1) | 31 (6.3) | 25 (4.1) | 0.59 (0.34–1.02) | 1.02 (0.56–1.84) |
| No | 942 (85.8) | 398 (80.6) | 544 (90.1) | reference | reference |
| Cancer therapy, within 30 d, ¶ n (%) | N = 1131 | n = 508 | n = 623 | ||
| No active therapy | 461 (41.8) | 208 (40.9) | 253 (40.6) | reference | reference |
| Active therapy | 670 (59.2) | 300 (59.1) | 370 (59) | 1.01 (0.80–1.29) | 1.02 (0.79–1.31) |
| Conventional chemotherapy | 255 (23) | 125 (24.6) | 130 (20.9) | 0.86 (0.63–1.16) | 1.05 (0.76–1.46) |
| Low-intensity chemotherapy | 67 (6) | 28 (5.5) | 39 (6.3) | 1.15 (0.68–1.92) | 0.88 (0.51–1.52) |
| Molecular-targeted therapy | 129 (11.4) | 56 (11.0) | 73 (11.7) | 1.07 (0.72–1.59) | 1.06 (0.70–1.61) |
| Immunotherapy, mAb only | 56 (5) | 24 (4.7) | 32 (8.7) | 1.10 (0.63–1.92) | 1.21 (0.67–2.21) |
| Immunomodulator drugs | 70 (6) | 33 (6.6) | 37 (5.9) | 0.92 (0.56–1.53) | 0.83 (0.49–1.41) |
| Hypomethylating agents | 49 (4) | 16 (3.2) | 33 (5.3) | 1.70 (0.91–3.17) | 1.23 (0.65–2.33) |
| Supportive therapy | 30 (2.7) | 11 (2.2) | 19 (3.1) | 1.42 (0.66–3.05) | 0.98 (0.51–1.52) |
| Active, not detailed | 14 (1.2) | 7 (1.4) | 7 (1.1) | 0.69 (0.21; 2.2) | 0.60 (0.16–2.24) |
| Early Cohort | Later Cohort | |||||
|---|---|---|---|---|---|---|
| Patients with Hematologic Malignancies n = 681 | Non-Cancer Inpatients n = 5227 | Odds Ratio (95% CI) | Patients with Hematologic Malignancies n = 215 | Non-Cancer Inpatients n = 5312 | Odds Ratio (95% CI) | |
| Age, y | n = 207 | |||||
| Median (IQR) | 72 (62–80) | 66 (53–68) | – | 72 (64–80) | 70 (57–81) | – |
| Age ≥ 65 y, n (%) | 483 (70.9) | 2829 (54.1) | 2.68 (1.74–2.46) | 151 (73.0) | 3248 (61.1) | 1.71 (1.25–2.34) |
| Sex, n (%) | n = 669 | n = 5218 | ||||
| Female | 259 (38.7) | 2213 (42.4) | reference | 89 (41.4) | 2272 (42.8) | reference |
| Male | 410 (61.3) | 3005 (57.6) | 1.17 (0.99–1.37) | 126 (58.6) | 3040 (57.2) | 1.06 (0.80–1.40) |
| Patients matched by characteristics | n = 669 | n = 669 | n = 207 | n = 207 | ||
| Age, y | ||||||
| Median (IQR) | 72 (62–80) | 72 (62–80) | – | 72 (64–80) | 72 (64–80) | – |
| Sex, n (%) | ||||||
| Female | 259 (38.7) | 259 (38.7) | – | 84 (40.6) | 84 (40.6) | – |
| Male | 410 (61.3) | 410 (61.3) | – | 123 (59.4) | 123 (59.4) | – |
| Comorbidities | ||||||
| Cardiac disease | 148 (22.1) | 420 (62.8) | 0.17 (0.13- 0.21) | 46 (22.2) | 138 (66.7) | 0.14 (0.09–0.22) |
| Respiratory disease | 102 (15.2) | 143 (21.4) | 0.66 (0.50–0.88) | 34 (16.4) | 49 (23.7) | 0.63 (0.39–1.03) |
| Renal disease | 81 (12.1) | 41 (6.1) | 2.11 (1.43–3.12) | 28 (13.5) | 7 (3.4) | 4.47 (1.91–10.5) |
| Diabetes | 126 (18.8) | 145 (21.7) | 0.80 (0.62–1.05) | 45 (21.7) | 57 (27.5) | 0.73 (0.47–1.15) |
| Hypertension | 291 (43.5) | 391 (58.4) | 0.55 (0.44–0.68) | 84 (40.6) | 129 (62.3) | 0.41 (0.28–0.61) |
| Pharmacologic therapies for COVID-19 | ||||||
| (Hydroxy)chloroquine | 590 (88.2) | 567 (84.8) | 1.34 (0.98–1.84) | 0 | 0 | n/a |
| Azithromycin | 318 (47.5) | 343 (51.3) | 0.86 (0.69–1.07) | 27 (13.0) | 43 (20.8) | 0.57 (0.34–0.97) |
| Lopinavir/darunavir | 383 (57.2) | 424 (63.4) | 0.77 (0.62–0.96) | 0 | 0 | n/a |
| Remdesivir | 22 (3.3) | 3 (0.4) | 7.55 (2.25–25.3) | 53 (25.6) | 54 (26.1) | 0.98 (0.63–1.51) |
| Tocilizumab | 131 (19.6) | 44 (6.6) | 3.46 (2.41–4.96) | 41 (19.8) | 35 (16.9) | 1.21 (0.74–2.00) |
| Corticosteroids | 283 (42.3) | 172 (25.7) | 2.12 (1.68–2.67) | 191 (92.3) | 182 (87.9) | 1.64 (0.85–3.17) |
| Oxygen support during COVID-19 treatment | ||||||
| No or Low-flow oxygen support | 511 (76.4) | 575 (86.0) | reference | 107 (51.7) | 126 (60.9) | reference |
| High-flow oxygen support or mechanical ventilation | 158 (23.6) | 94 (14.0) | 1.89 (1.43–2.51) | 100 (48.3) | 81 (39.1) | 1.45 (0.98–2.15) |
| Inpatient 30-d mortality * | 216/669 (32.3) | 198/669 (29.6) | 1.13 (0.90–1.43) | 72/207 (34.8) | 26/207 (12.6) | 3.71 (2.25–6.13) |
| Patients with PCC Data, N = 278 * | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|
| Total, N = 278 | PCC, n = 76 | No PCC, n = 202 | Unadjusted | Adjusted † | |
| Age, y | N = 276 | n = 200 | |||
| Median (IQR) | 67 (54.5–76) | 69 (54–76) | 67 (54.5–75) | n/a | n/a |
| Age 18–49 y, n (%) | 47 (17.0) | 11 (14.5) | 36 (18) | reference | reference |
| Age 50–79 y, n (%) | 191 (69.2) | 54 (71.1) | 142 (71.0) | ||
| Age ≥ 80 y, n (%) | 33 (12.0) | 11 (14.5) | 22 (11) | 1.64 (0.61–4.40) | 1.24 (0.42–3.69) |
| Sex, n (%) | N = 271 | n = 72 | n = 199 | ||
| Female | 149 (55.0) | 42 (58.3) | 107 (53.8) | reference | reference |
| Male | 122 (45.0) | 30 (41.7) | 92 (46.2) | 1.20 (0.70–2.08) | 1.20 (0.69–2.08) |
| Comorbidities | |||||
| Number of 6 specified comorbidities | |||||
| 0 | 78 (28.1) | 16 (21.1) | 62 (30.7) | reference | reference |
| ≥1 | 200 (71.9) | 60 (79.0) | 140 (69.3) | 1.66 (0.89–3.11) | 1.57 (0.78–3.16) |
| Other comorbidities | 144 (51.8) | 44 (57.9) | 100 (49.5) | 1.40 (0.82–2.39) | 1.36 (0.77–2.42) |
| Hematologic malignancy | |||||
| NHL | 83 (29.9) | 16 (21.1) | 67 (33.2) | reference | reference |
| MM | 66 (23.7) | 21 (27.6) | 45 (22.3) | 1.95 (0.92–4.15) | 1.86 (0.85–4.05) |
| CLL | 29 (10.4) | 10 (13.2) | 19 (9.4) | 2.20 (0.86–5.64) | 2.10 (0.81–5.44) |
| HL | 16 (5.8) | 4 (5.3) | 12 (5.9) | 1.40 (0.38–4.90) | 1.06 (0.25–4.41) |
| ALL | 3 (1.1) | 1 (1.3) | 2 (1) | 2.09 (0.18–24.5) | 2.16 (0.17–27.1) |
| MDS | 23 (8.3) | 6 (7.9) | 17 (8.4) | 1.48 (0.50–4.35) | 1.47 (0.48–4.47) |
| AML | 28 (10.1) | 8 (10.5) | 20 (9.9) | 1.68 (0.63–4.84) | 1.58 (0.58–4.30) |
| CML | 7 (2.5) | 1 (1.3) | 6 (3.0) | 0.70 (0.08–6.21) | 0.58 (0.06–5.31) |
| Ph-MPN | 23 (8.3) | 9 (11.8) | 14 (6.9) | 2.69 (0.99–7.31) | 2.02 (0.68–6.02) |
| Stem cell transplantation ‡ | N = 274 | n = 72 | |||
| No | 219 (79.9) | 59 (81.9) | 160 (79.2) | reference | reference |
| Autologous | 35 (12.8) | 7 (9.7) | 28 (13.9) | 0.68 (0.28–1.64) | 0.68 (0.25–1.84) |
| Allogeneic | 20 (7.3) | 6 (8.3) | 14 (6.9) | 2.16 (0.43–3.17) | 1.36 (0.46–4.05) |
| Cancer therapy, within 30 d § | N = 276 | ||||
| No active therapy | 105 (38.0) | 28 (36.8) | 77 (38.1) | reference | reference |
| Active therapy | 173 (62.0) | 48 (63.2) | 125 (61.9) | 1.06 (0.61–1.82) | 0.95 (0.54–1.67) |
| Conventional chemotherapy | 62 (22.5) | 17 (22.7) | 45 (22.4) | 1.04 (0.51–2.11) | 0.99 (0.47–2.08) |
| Low-intensity chemotherapy | 16 (5.8) | 5 (6.7) | 11 (5.5) | 1.25 (0.40–3.92) | 0.98 (0.28–3.50) |
| Molecular targeted therapy | 34 (12.3) | 11 (14.7) | 23 (11.4) | 1.32 (0.57–3.04) | 1.03 (0.42–2.56) |
| Immunotherapy, mAb only | 16 (5.8) | 2 (2.7) | 14 (7.0) | 0.39 (0.08–1.84) | 0.41 (0.09–1.99) |
| Immunomodulator drugs | 19 (6.9) | 4 (5.3) | 15 (7.5) | 0.73 (0.22–2.40) | 0.63 (0.19–2.11) |
| Hypomethylating agents | 16 (5.8) | 4 (5.3) | 12 (6.0) | 0.92 (0.27–3.08) | 0.76 (0.21–2.71) |
| Supportive therapy | 5 (1.8) | 2 (2.7) | 3 (1.5) | 1.83 (0.29–11.6) | 1.35 (0.20–9.02) |
| Time period of COVID-19 diagnosis | |||||
| Early cohort (1st wave, March–June 2020) | 209 (75.2) | 63 (82.9) | 146 (72.3) | reference | reference |
| Later cohort (2nd/3rd wave, July 2020–February 2021) | 69 (24.8) | 13 (17.1) | 56 (27.7) | 0.54 (0.28–1.05) | 0.60 (0.30–1.20) |
| Care setting of COVID-19 treatment | |||||
| Outpatient | 72 (25.9) | 11 (14.5) | 61 (30.2) | reference | reference |
| Inpatient | 206 (74.1) | 65 (85.5) | 141 (69.8) | 2.56 (1.26–5.18) | 2.37 (1.15–4.90) |
| Intensive care unit | 44/206 (21.4) | 17/65 (26.2) | 27/141 (19.1) | 1.50 (0.75–2.99) | 1.59 (0.75–3.31) |
| Pharmacologic therapies for COVID-19 | |||||
| (Hydroxy)chloroquine | 181 (65.1) | 58 (76.3) | 123 (60.9) | 2.07 (1.14–3.80) | 1.91 (1.03–3.53) |
| Azithromycin | 87 (31.3) | 26 (34.2) | 61 (30.2) | 1.20 (0.69–2.11) | 0.99 (0.54–1.80) |
| Lopinavir/darunavir | 132 (47.5) | 46 (60.5) | 86 (42.6) | 2.07 (1.21–3.54) | 2.03 (1.16–3.56) |
| Remdesivir | 31 (11.2) | 13 (17.1) | 18 (8.9) | 2.11 (0.98–4.55) | 2.26 (1.03–4.98) |
| Tocilizumab | 54 (19.4) | 21 (27.6) | 33 (16.3) | 1.96 (1.05–3.66) | 1.97 (1.03–3.77) |
| Corticosteroids | 138 (49.6) | 45 (59.2) | 93 (46.0) | 1.70 (1.00–2.90) | 1.68 (0.97–2.92) |
| Oxygen support during COVID-19 treatment | |||||
| No | 104 (37.4) | 24 (31.6) | 80 (39.6) | reference | reference |
| Low-flow oxygen support | 133 (47.8) | 36 (47.4) | 97 (48.0) | 1.24 (0.68–2.24) | 1.24 (0.66–2.35) |
| High-flow oxygen support or mechanical ventilation | 41 (14.7) | 16 (21.1) | 25 (12.4) | 2.13 (0.98–4.64) | 2.20 (0.98–4.94) |
| Clinical severity of COVID-19 | N = 273 | n = 75 | n = 198 | ||
| Mild | 69 (25.3) | 14 (18.7) | 55 (27.8) | reference | reference |
| Moderate | 89 (32.6) | 22 (29.3) | 67 (33.8) | 1.29 (0.60–2.76) | 1.48 (0.66–3.37) |
| Severe | 83 (30.4) | 24 (32.0) | 59 (29.8) | 1.60 (0.75–3.40) | 1.73 (0.77–3.90) |
| Critical | 32 (11.7) | 15 (20.0) | 17 (8.6) | 3.47 (1.40–8.60) | 3.60 (1.35–9.62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-López, J.; De la Cruz, J.; Gil-Manso, R.; Alegre, A.; Ortiz, J.; Llamas, P.; Martínez, Y.; Hernández-Rivas, J.-Á.; González-Gascón, I.; Benavente, C.; et al. COVID-19 Severity and Survival over Time in Patients with Hematologic Malignancies: A Population-Based Registry Study. Cancers 2023, 15, 1497. https://doi.org/10.3390/cancers15051497
Martínez-López J, De la Cruz J, Gil-Manso R, Alegre A, Ortiz J, Llamas P, Martínez Y, Hernández-Rivas J-Á, González-Gascón I, Benavente C, et al. COVID-19 Severity and Survival over Time in Patients with Hematologic Malignancies: A Population-Based Registry Study. Cancers. 2023; 15(5):1497. https://doi.org/10.3390/cancers15051497
Chicago/Turabian StyleMartínez-López, Joaquín, Javier De la Cruz, Rodrigo Gil-Manso, Adrián Alegre, Javier Ortiz, Pilar Llamas, Yolanda Martínez, José-Ángel Hernández-Rivas, Isabel González-Gascón, Celina Benavente, and et al. 2023. "COVID-19 Severity and Survival over Time in Patients with Hematologic Malignancies: A Population-Based Registry Study" Cancers 15, no. 5: 1497. https://doi.org/10.3390/cancers15051497
APA StyleMartínez-López, J., De la Cruz, J., Gil-Manso, R., Alegre, A., Ortiz, J., Llamas, P., Martínez, Y., Hernández-Rivas, J.-Á., González-Gascón, I., Benavente, C., Estival Monteliu, P., Jiménez-Yuste, V., Canales, M., Bastos, M., Kwon, M., Valenciano, S., Callejas-Charavia, M., López-Jiménez, J., Herrera, P., ... García-Suárez, J., on behalf of the Asociación Madrileña de Hematología y Hemoterapia (AMHH). (2023). COVID-19 Severity and Survival over Time in Patients with Hematologic Malignancies: A Population-Based Registry Study. Cancers, 15(5), 1497. https://doi.org/10.3390/cancers15051497







