Pseudoprogression of Vestibular Schwannoma after Stereotactic Radiosurgery with Cyberknife®: Proposal for New Response Criteria
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Radiosurgery Technique
2.3. Tumor Imaging and Volumetric Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients and Treatments
3.2. Clinical Outcome
3.3. Volumetric and Tumor Characteristics
4. Discussion
4.1. Morphological Changes after SRS of Vestibular Schwannoma
4.2. Risk Factors and Complications of Pseudoprogression
4.3. Development of RANO Criteria for vs. after SRS
4.4. Conclusion and Practical Implications of Proposed RANO Criteria for VS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldbrunner, R.; Weller, M.; Regis, J.; Lund-Johansen, M.; Stavrinou, P.; Reuss, D.; Evans, D.G.; Lefranc, F.; Sallabanda, K.; Falini, A.; et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro-Oncology 2020, 22, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Ruess, D.; Pohlmann, L.; Grau, S.; Hamisch, C.; Hellerbach, A.; Treuer, H.; Kocher, M.; Ruge, M.I. Long-term follow-up after stereotactic radiosurgery of intracanalicular acoustic neurinoma. Radiat. Oncol. 2017, 12, 68. [Google Scholar] [CrossRef]
- Ruess, D.; Pohlmann, L.; Hellerbach, A.; Hamisch, C.; Hoevels, M.; Treuer, H.; Grau, S.; Jablonska, K.; Kocher, M.; Ruge, M.I. Acoustic Neuroma Treated with Stereotactic Radiosurgery: Follow-up of 335 Patients. World Neurosurg. 2018, 116, e194–e202. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yamamoto, M.; Kawabe, T.; Koiso, T.; Yamamoto, T.; Matsumura, A.; Kasuya, H. Stereotactic radiosurgery for vestibular schwannomas: Average 10-year follow-up results focusing on long-term hearing preservation. J. Neurosurg. 2016, 125, 64–72. [Google Scholar] [CrossRef]
- Germano, I.M.; Sheehan, J.; Parish, J.; Atkins, T.; Asher, A.; Hadjipanayis, C.G.; Burri, S.H.; Green, S.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Radiosurgery and Radiation Therapy in the Management of Patients With Vestibular Schwannomas. Neurosurgery 2018, 82, E49–E51. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Jokura, H.; Takahashi, K.; Boku, N.; Akabane, A.; Yoshimoto, T. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am. J. Neuroradiol. 2000, 21, 1540–1546. [Google Scholar] [PubMed]
- Hasegawa, T.; Kida, Y.; Yoshimoto, M.; Koike, J.; Goto, K. Evaluation of tumor expansion after stereotactic radiosurgery in patients harboring vestibular schwannomas. Neurosurgery 2006, 58, 1119–1128, discussion 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Lunsford, L.D.; Kondziolka, D.; Sekula, R.; Subach, B.R.; Foote, R.L.; Flickinger, J.C. Vestibular schwannoma management. Part II. Failed radiosurgery and the role of delayed microsurgery. J. Neurosurg. 1998, 89, 949–955. [Google Scholar] [CrossRef]
- Fukuoka, S.; Oka, K.; Seo, Y.; Tokanoshi, M.; Sumi, Y.; Nakamura, H.; Nakamura, J.; Ikawa, F. Apoptosis following gamma knife radiosurgery in a case of acoustic schwannoma. stereotact. Funct. Neurosurg. 1998, 70 (Suppl. 1), 88–94. [Google Scholar] [CrossRef]
- Mindermann, T.; Schlegel, I. How to distinguish tumor growth from transient expansion of vestibular schwannomas following Gamma Knife radiosurgery. Acta Neurochir. 2014, 156, 1121–1123. [Google Scholar] [CrossRef]
- Hayhurst, C.; Zadeh, G. Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro-Oncology 2012, 14, 87–92. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef] [PubMed]
- Rueß, D.; Fritsche, F.; Grau, S.; Treuer, H.; Hoevels, M.; Kocher, M.; Baues, C.; Ruge, M.I. Stereotactic Radiosurgery of Cavernous Sinus Meningiomas. J. Neurol. Surg. Part B Skull Base 2020, 81, 158–164. [Google Scholar] [CrossRef]
- Sorensen, A.G.; Patel, S.; Harmath, C.; Bridges, S.; Synnott, J.; Sievers, A.; Yoon, Y.H.; Lee, E.J.; Yang, M.C.; Lewis, R.F.; et al. Comparison of diameter and perimeter methods for tumor volume calculation. J. Clin. Oncol. 2001, 19, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the Response Assessment in Neuro-Oncology Working Group. Neuro-Oncology 2019, 21, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Breshears, J.D.; Chang, J.; Molinaro, A.M.; Sneed, P.K.; McDermott, M.W.; Tward, A.; Theodosopoulos, P.V. Temporal Dynamics of Pseudoprogression After Gamma Knife Radiosurgery for Vestibular Schwannomas-A Retrospective Volumetric Study. Neurosurgery 2019, 84, 123–131. [Google Scholar] [CrossRef]
- Fouard, O.; Daisne, J.F.; Wanet, M.; Regnier, M.; Gustin, T. Long-term volumetric analysis of vestibular schwannomas following stereotactic radiotherapy: Practical implications for follow-up. Clin. Transl. Radiat. Oncol. 2022, 33, 1–6. [Google Scholar] [CrossRef]
- Yu, C.P.; Cheung, J.Y.; Leung, S.; Ho, R. Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by gamma knife radiosurgery. J. Neurosurg. 2000, 93 (Suppl. 3), 82–89. [Google Scholar] [CrossRef]
- Nagano, O.; Higuchi, Y.; Serizawa, T.; Ono, J.; Matsuda, S.; Yamakami, I.; Saeki, N. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J. Neurosurg. 2008, 109, 811–816. [Google Scholar] [CrossRef]
- Nagano, O.; Serizawa, T.; Higuchi, Y.; Matsuda, S.; Sato, M.; Yamakami, I.; Okiyama, K.; Ono, J.; Saeki, N. Tumor shrinkage of vestibular schwannomas after Gamma Knife surgery: Results after more than 5 years of follow-up. J. Neurosurg. 2010, 113, 122–127. [Google Scholar] [CrossRef]
- van de Langenberg, R.; Dohmen, A.J.; de Bondt, B.J.; Nelemans, P.J.; Baumert, B.G.; Stokroos, R.J. Volume changes after stereotactic LINAC radiotherapy in vestibular schwannoma: Control rate and growth patterns. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 343–349. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, D.G.; Han, J.H.; Chung, H.T.; Kim, I.K.; Song, S.W.; Park, J.H.; Kim, J.W.; Kim, Y.H.; Park, C.K.; et al. Hearing outcomes after stereotactic radiosurgery for unilateral intracanalicular vestibular schwannomas: Implication of transient volume expansion. Int J. Radiat. Oncol. Biol. Phys. 2013, 85, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Okunaga, T.; Kamada, K.; Izumo, T.; Hayashi, N.; Nagata, I. Long-term follow-up results of linear accelerator-based radiosurgery for vestibular schwannoma using serial three-dimensional spoiled gradient-echo MRI. J. Clin. Neurosci. 2015, 22, 320–325. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, H.H.; Chang, J.H.; Chang, J.W.; Park, Y.G.; Chang, W.S. Predictive Factors of Unfavorable Events After Gamma Knife Radiosurgery for Vestibular Schwannoma. World Neurosurg. 2017, 107, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Lunsford, L.D.; McLaughlin, M.R.; Flickinger, J.C. Long-term outcomes after radiosurgery for acoustic neuromas. N. Engl. J. Med. 1998, 339, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Sughrue, M.E.; Han, S.J.; Fang, S.; Aranda, D.; Cheung, S.W.; Pitts, L.H.; Parsa, A.T. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J. Neuro-Oncol. 2009, 93, 41–48. [Google Scholar] [CrossRef]
- Benghiat, H.; Heyes, G.; Nightingale, P.; Hartley, A.; Tiffany, M.; Spooner, D.; Geh, J.I.; Cruickshank, G.; Irving, R.M.; Sanghera, P. Linear accelerator stereotactic radiosurgery for vestibular schwannomas: A UK series. Clin. Oncol. 2014, 26, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Delsanti, C.; Roche, P.H.; Thomassin, J.M.; Regis, J. Morphological changes of vestibular schwannomas after radiosurgical treatment: Pitfalls and diagnosis of failure. Prog. Neurol. Surg. 2008, 21, 93–97. [Google Scholar] [CrossRef]
- Iwai, Y.; Yamanaka, K.; Yamagata, K.; Yasui, T. Surgery after radiosurgery for acoustic neuromas: Surgical strategy and histological findings. Neurosurgery 2007, 60, ONS75-82. [Google Scholar] [CrossRef]
- Prasad, D.; Steiner, M.; Steiner, L. Gamma surgery for vestibular schwannoma. J. Neurosurg. 2000, 92, 745–759. [Google Scholar] [CrossRef]
- Witham, T.F.; Okada, H.; Fellows, W.; Hamilton, R.L.; Flickinger, J.C.; Chambers, W.H.; Pollack, I.F.; Watkins, S.C.; Kondziolka, D. The characterization of tumor apoptosis after experimental radiosurgery. Stereotact. Funct. Neurosurg. 2005, 83, 17–24. [Google Scholar] [CrossRef]
- Meijer, O.W.; Vandertop, W.P.; Lagerwaard, F.J.; Slotman, B.J. Linear accelerator-based stereotactic radiosurgery for bilateral vestibular schwannomas in patients with neurofibromatosis type 2. Neurosurgery 2008, 62, A37–A42. [Google Scholar] [CrossRef] [PubMed]
- Langenhuizen, P.; Sebregts, S.H.P.; Zinger, S.; Leenstra, S.; Verheul, J.B.; de With, P.H.N. Prediction of transient tumor enlargement using MRI tumor texture after radiosurgery on vestibular schwannoma. Med. Phys. 2020, 47, 1692–1701. [Google Scholar] [CrossRef]
- Aoyama, H.; Onodera, S.; Takeichi, N.; Onimaru, R.; Terasaka, S.; Sawamura, Y.; Shirato, H. Symptomatic outcomes in relation to tumor expansion after fractionated stereotactic radiation therapy for vestibular schwannomas: Single-institutional long-term experience. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: Treatment recommendations based on a 15 year experience. Neurosurgery 2006, 58, 241–248, discussion 241–248. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Miyamori, T.; Yamano, J. Hydrocephalus after Gamma Knife Radiosurgery for Schwannoma. Asian J. Neurosurg. 2019, 14, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.H.; Chang, K.W.; Park, S.H.; Jung, H.H.; Chang, J.H.; Chang, J.W.; Chang, W.S. Pseudoprogression and peritumoral edema due to intratumoral necrosis after Gamma knife radiosurgery for meningioma. Sci. Rep. 2022, 12, 13663. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.N.; Sahgal, A.; Xu, W.; De Salles, A.; Hayashi, M.; Levivier, M.; Ma, L.; Martinez, R.; Regis, J.; Ryu, S.; et al. Stereotactic radiosurgery for vestibular schwannoma: International Stereotactic Radiosurgery Society (ISRS) Practice Guideline. J. Radiosurgery. SBRT 2017, 5, 5–24. [Google Scholar]
- Snell, J.W.; Sheehan, J.; Stroila, M.; Steiner, L. Assessment of imaging studies used with radiosurgery: A volumetric algorithm and an estimation of its error. Technical note. J. Neurosurg. 2006, 104, 157–162. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, S.; Kwak, D.W.; Lee, H.S.; Kang, M.K.; Lee, D.K.; Hur, W.J. Maximum diameter versus volumetric assessment for the response evaluation of vestibular schwannomas receiving stereotactic radiotherapy. Radiat. Oncol. J. 2018, 36, 114–121. [Google Scholar] [CrossRef]
- van de Langenberg, R.; de Bondt, B.J.; Nelemans, P.J.; Baumert, B.G.; Stokroos, R.J. Follow-up assessment of vestibular schwannomas: Volume quantification versus two-dimensional measurements. Neuroradiology 2009, 51, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Walz, P.C.; Bush, M.L.; Robinett, Z.; Kirsch, C.F.; Welling, D.B. Three-dimensional segmented volumetric analysis of sporadic vestibular schwannomas: Comparison of segmented and linear measurements. Otolaryngol.-Head Neck Surg. 2012, 147, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tsimpas, A.; Germanwala, A.V. Analysis of vestibular schwannoma size: A literature review on consistency with measurement techniques. Clin. Neurol. Neurosurg. 2015, 138, 72–77. [Google Scholar] [CrossRef] [PubMed]

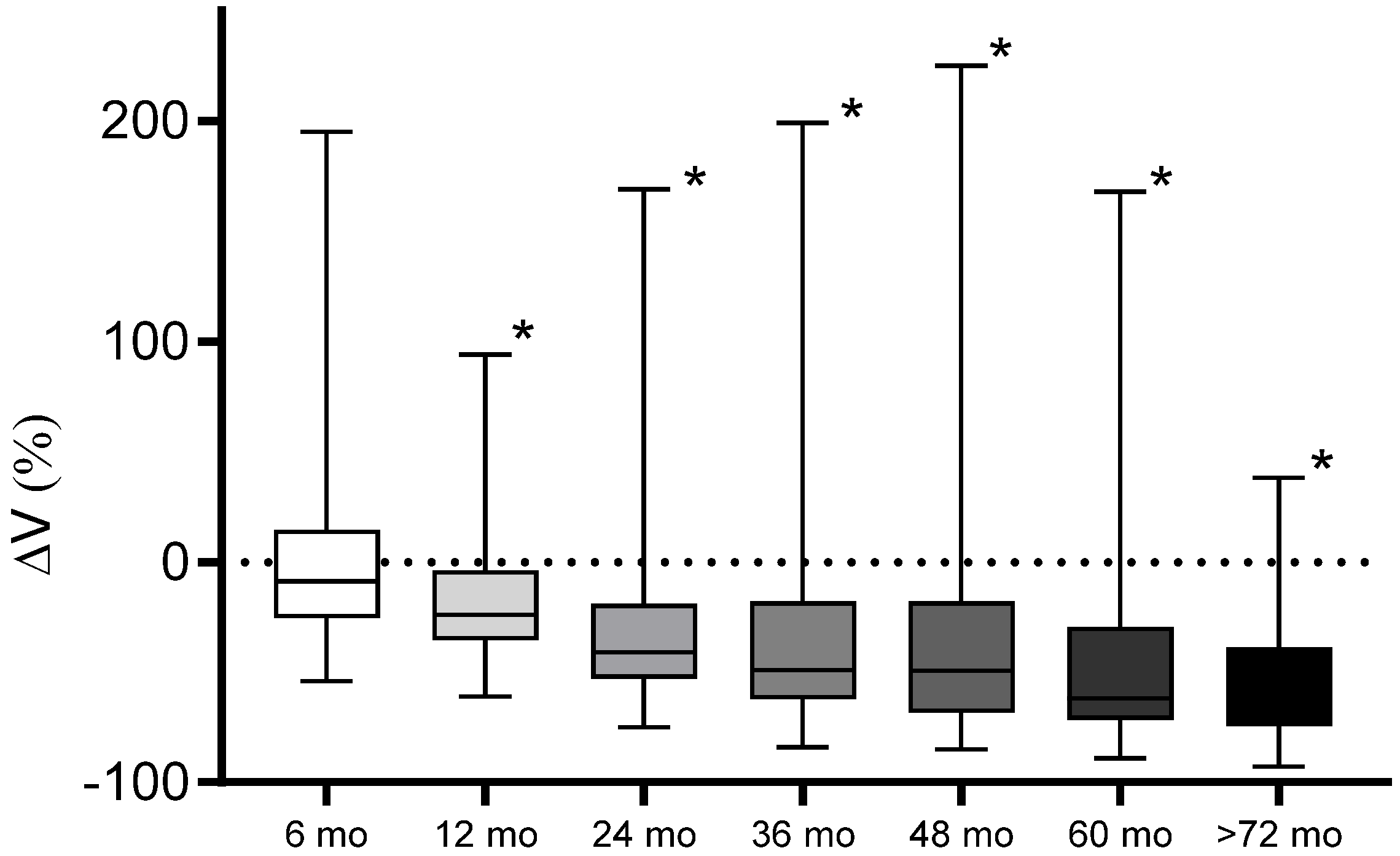
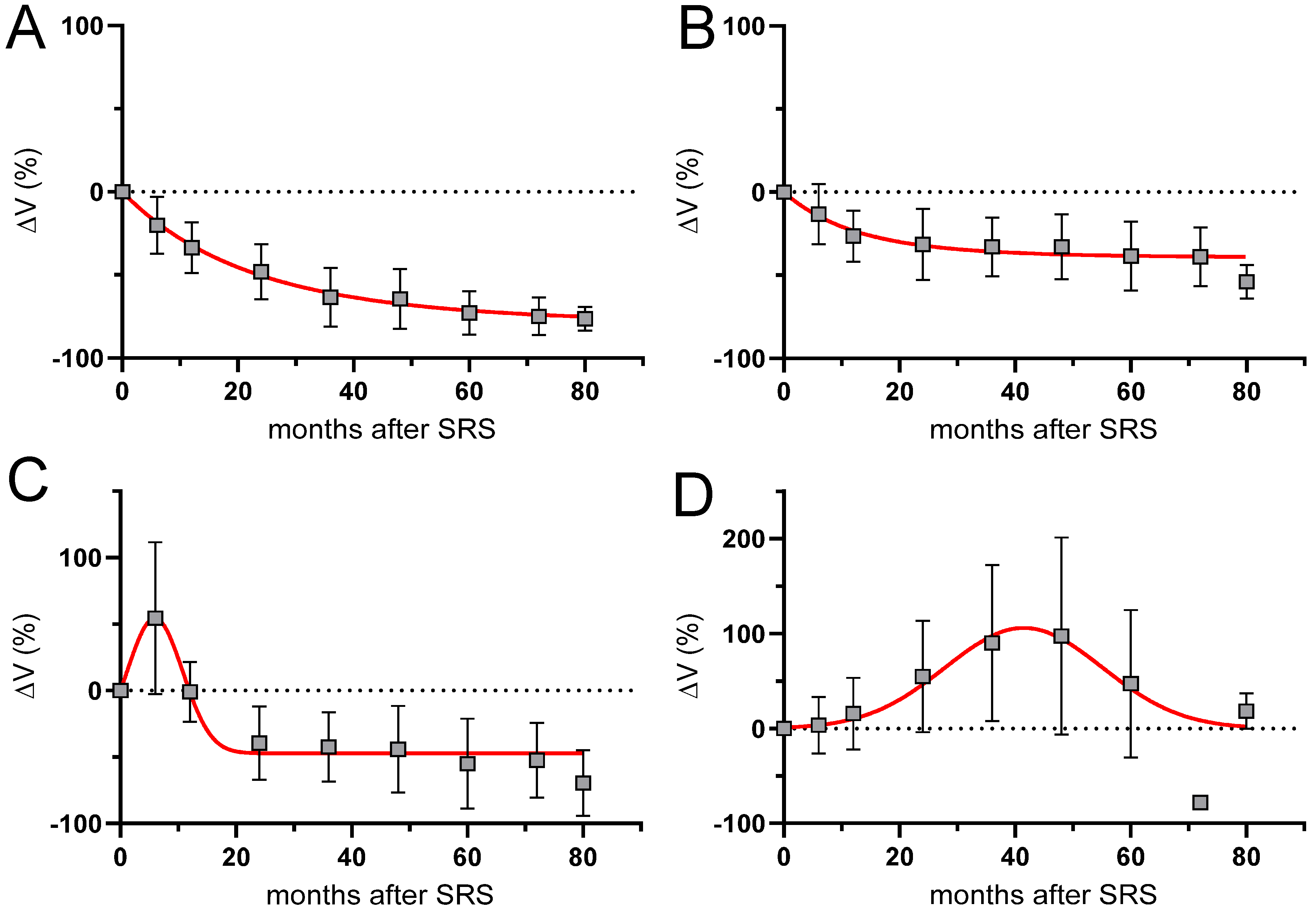

| Criterion | CR Complete Response | PR Partial Response | SD Stable Disease | PP Pseudoprogression | PD Progressive Disease |
|---|---|---|---|---|---|
| (1) Target lesion | None | ≥65% decrease in volume relative to baseline | <65% decrease relative to baseline but <20% increase in volume relative to nadir | >20% increase in volume relative to baseline followed by decrease within 24–36 months | ≥40% increase in volume relative to baseline and/or change of Koos grade I or II to III or IV |
| (2) Onset of volume increase after SRS | n.a. | n.a. | n.a. | Early peak: <12 months Late peak: >12 months | >36–48 months |
| (3) Clinical status (besides hearing deterioration and vertigo) | Stable or improved | Stable or improved | Stable or improved | Stable or improved | Stable or deteriorated |
| Requirement for response | All or (1) alone | All or (1) alone | All | All | All |
| Patient Characteristics | |
| Total no. of patients | 63 |
| Gender (m:f) | 25:38 |
| No. of Koos-Grade | KoosI: 6 KoosII: 45 KoosIII: 11 KoosIV: 1 |
| Age (years) | 56 ± 14 (range: 20–82) |
| Tumor volume (cm3) | 1.5 ± 1.4 (range: 0.1–8.6) |
| Median radiological and clinical FU (months) | 66 (range: 24–103) |
| Radiation Parameters | |
| Marginal dose (Gy) | 13.0 ± 0.2 (range: 12–13) |
| Dose prescription, isodose (%) | 80 ± 3.5 (range: 65–81) |
| Coverage (%) | 99.6 ± 0.9 (range: 94.7–100) |
| Dmax | 16.25 ± 0.7 (range: 15–18.6) |
| Dmean | 14.89 ± 0.3 (range: 14–15.8) |
| Dmin | 12.94 ± 0.4 (range: 11–13.2) |
| nCI | 1.19 ± 0.07 (range: 1.08–1.44) |
| Response/Pseudoresponse Criteria | |
| Loss of central contrast enhancement | 41 (65%) |
| CR—complete response | 0 |
| ePP—early pseudoprogression | 10 (16%) |
| lPP—late pseudoprogression | 8 (13%) |
| SD—stable disease | 22 (35%) |
| PR—partial response | 23 (36%) |
| PD—progressive disease | 0 |
| Patient Characteristics | PR + SD | PP | p-Value |
|---|---|---|---|
| No. of patients | 45 | 18 | |
| Age (years) | 57 ± 14.3 | 54 ± 13.2 | p = 0.731 |
| Tumor volume (cm3) | 1.62 ± 1.5 | 1.15 ± 0.7 | p = 0.057 |
| follow-up (months) | 61.5 ± 21.6 | 64.3 ± 21.1 | p = 0.083 |
| Radiation Parameters | |||
| Marginal dose (Gy) | 13 | 13 | |
| Dose prescription, isodose (%) | 78.02 ± 3.7 | 78.9 ± 2.7 | p = 0.082 |
| Coverage (%) | 99.36 ± 1.1 | 98.8 ± 1.4 | p = 0.087 |
| Dmax | 16.6 ± 0.7 | 16.5 ± 0.6 | p = 0.223 |
| Dmean | 14.9 ± 0.2 | 14.8 ± 0.3 | p = 0.111 |
| Dmin | 12.8 ± 0.4 | 12.8 ± 0.2 | p = 0.158 |
| nCI | 1.17 ± 0.63 | 1.22 ± 0.09 | p = 0.051 |
| Study | n | Mean FU (months) | Radiation Technique | Tumor Volume (ml) | Incidence of PP (%) | Definition of PP | Mean Volume Increase (%) | Median Yime to Peak for PP (months) | Late Peak (% of Collective @ Median Ttime to Peak) | Duration of PP |
|---|---|---|---|---|---|---|---|---|---|---|
| Yu, 2000 [18] | 91 | 22 | GK | 63 | NA | 20 | 6 | NA | ||
| Nakamura, 2000 [6] | 78 | 34 | GK | 0.6 | 41 | Volume increase of more than 20% | NA | 12 | 6.4% @ 24–36 months | 24 mo |
| Nagano, 2008, 2010 [19,20] | 87 | 65 | GK | 2.5 | 77 | Volume increase > 10% | 58 | 8.6 | NA | 90% resolved after 5 yr |
| Van de Langenberg, 2011 [21] | 17 | 40 | LINAC | 2.09 | 54 | Volume increase >19.7% | NA | 5 (3-17) | NA | 15 (8–27) mo |
| Hayhurst, 2012 [11] | 75 | 29 | GK | 1.7 | 23 | Volume increase > 10% | 23 | NA | NA | NA |
| Kim, 2013 [22] | 60 | 42 | GK | 0.34 | 47 | Volume increase > 10% within a year | NA | NA | NA | NA |
| Mindermann, 2014 [10] | 235 | 62 | GK | 1.85 | NA | Volume increase > 20% within 24 months | NA | 6-18 | NA | 12-18 |
| Matsuo, 2015 [23] | 44 | 165 | LINAC | 2.38 | 54.5 | Volume increase > 20% within 24 months | 88 | 9 | 7.1% @ 39.6months | |
| Kim, 2017 [24] | 235 | 34 | GK | 2.2 | 18 | Volume increase > 20% within a year | NA | 7 (4–55) | NA | NA |
| Breshears, 2019 [16] | 18 | 49 | GK | 0.74 | 42 | Volume increase at any time during FU followed by reduction | 49 | 12.5 | 10% @36–48 months | 28.8 mo, 90% resolved at 6.9 yr |
| Fouard, 2021 [17] | 42 | 83 | GK | 0.69 | 63.5 | Volume increase > 13% at any time during FU followed by reduction | 64 | 6-12 mo | 17% @ 36–48 months | 24 mo, |
| Our study | 63 | 66 | CK | 1.5 | 29 | Volume increase > 20% at any time during FU followed by reduction | 57 | 18 | 12.7% @ 36 months | 12.5 mo, 90% resolved after 4 yr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueß, D.; Schütz, B.; Celik, E.; Baues, C.; Jünger, S.T.; Neuschmelting, V.; Hellerbach, A.; Eichner, M.; Kocher, M.; Ruge, M.I. Pseudoprogression of Vestibular Schwannoma after Stereotactic Radiosurgery with Cyberknife®: Proposal for New Response Criteria. Cancers 2023, 15, 1496. https://doi.org/10.3390/cancers15051496
Rueß D, Schütz B, Celik E, Baues C, Jünger ST, Neuschmelting V, Hellerbach A, Eichner M, Kocher M, Ruge MI. Pseudoprogression of Vestibular Schwannoma after Stereotactic Radiosurgery with Cyberknife®: Proposal for New Response Criteria. Cancers. 2023; 15(5):1496. https://doi.org/10.3390/cancers15051496
Chicago/Turabian StyleRueß, Daniel, Betina Schütz, Eren Celik, Christian Baues, Stephanie T. Jünger, Volker Neuschmelting, Alexandra Hellerbach, Markus Eichner, Martin Kocher, and Maximilian I. Ruge. 2023. "Pseudoprogression of Vestibular Schwannoma after Stereotactic Radiosurgery with Cyberknife®: Proposal for New Response Criteria" Cancers 15, no. 5: 1496. https://doi.org/10.3390/cancers15051496
APA StyleRueß, D., Schütz, B., Celik, E., Baues, C., Jünger, S. T., Neuschmelting, V., Hellerbach, A., Eichner, M., Kocher, M., & Ruge, M. I. (2023). Pseudoprogression of Vestibular Schwannoma after Stereotactic Radiosurgery with Cyberknife®: Proposal for New Response Criteria. Cancers, 15(5), 1496. https://doi.org/10.3390/cancers15051496





