Impact of Time to Surgery on Outcome in Wilms Tumor Treated with Preoperative Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
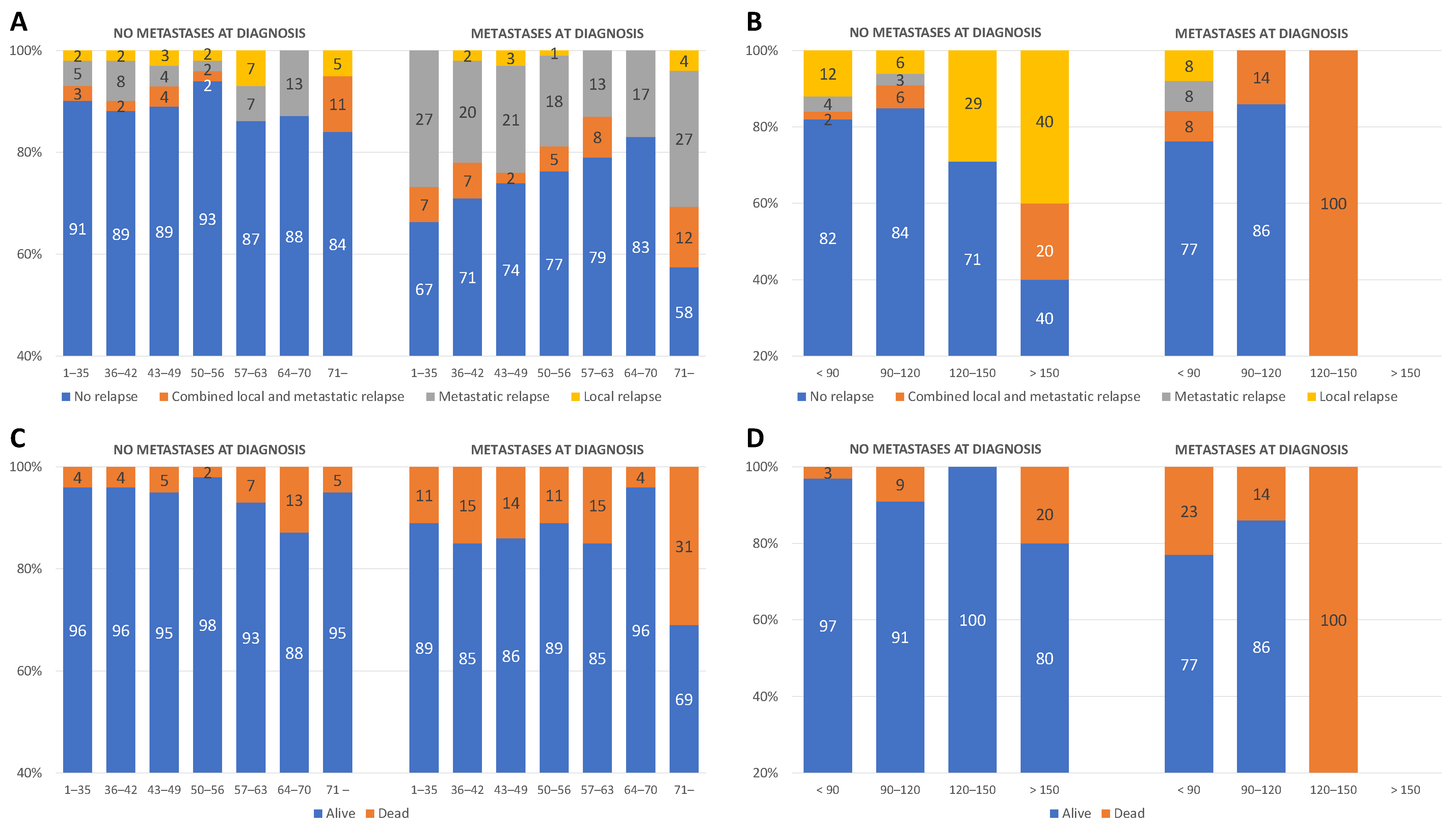
3.1. General Results
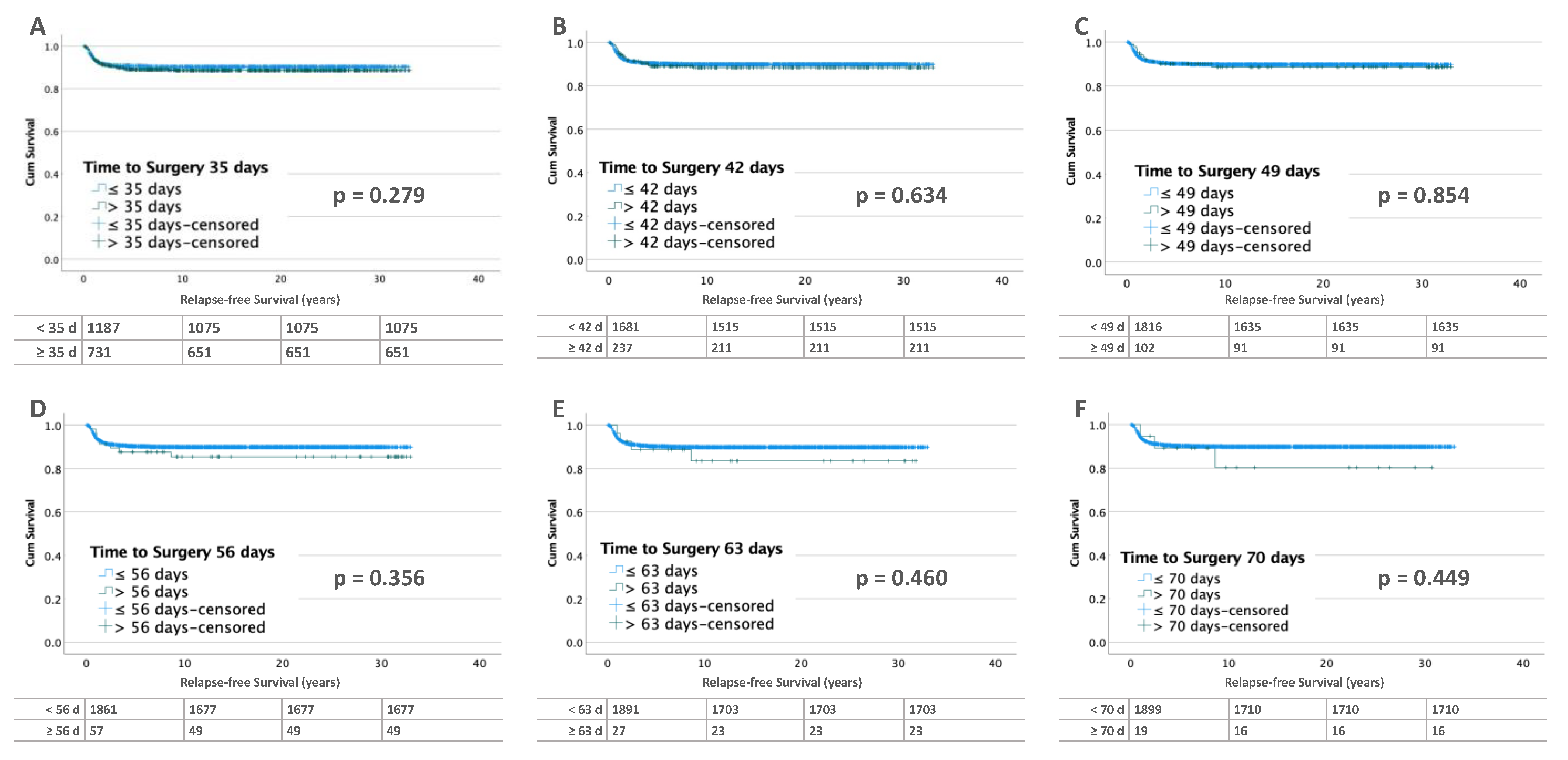
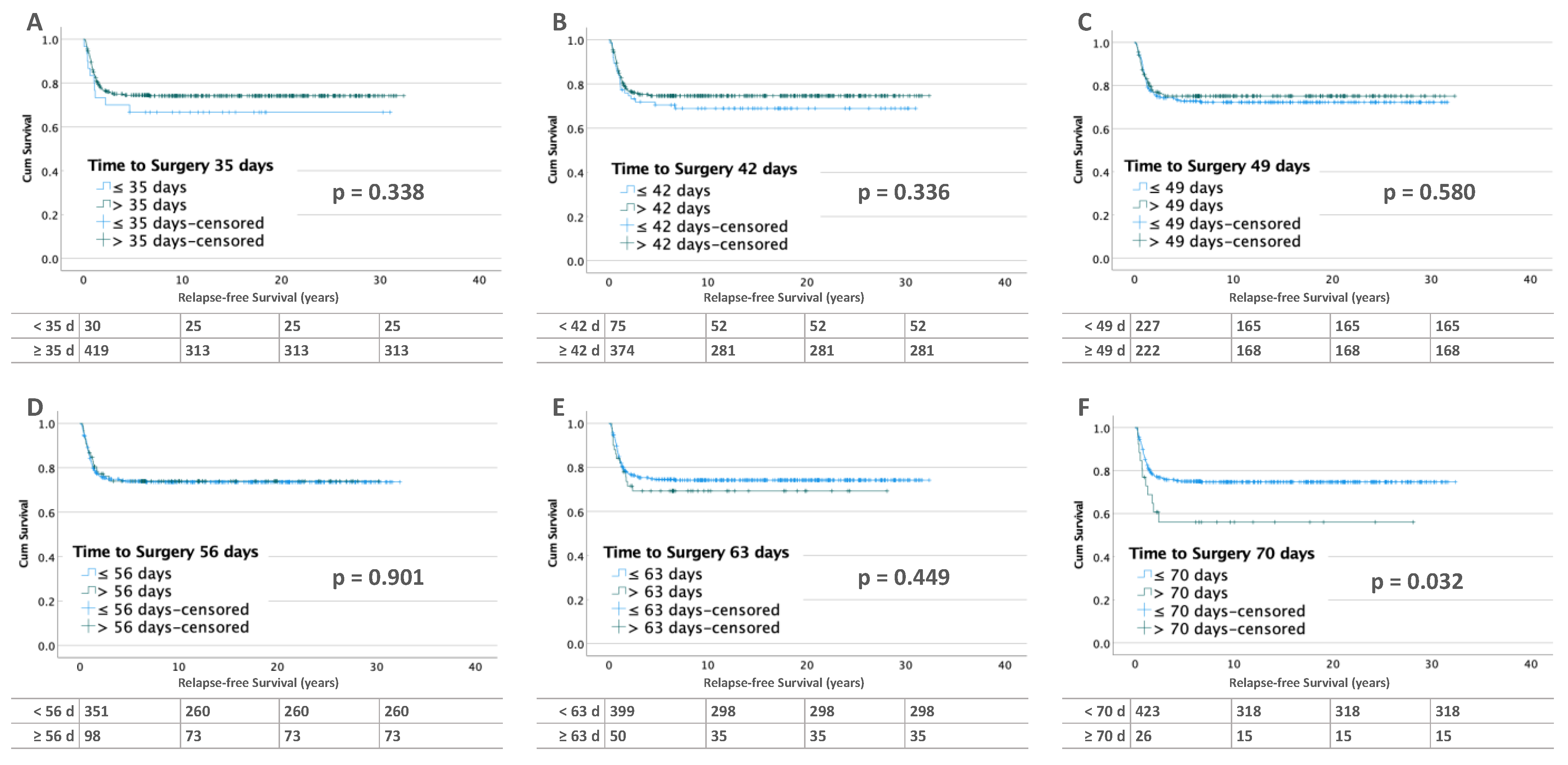
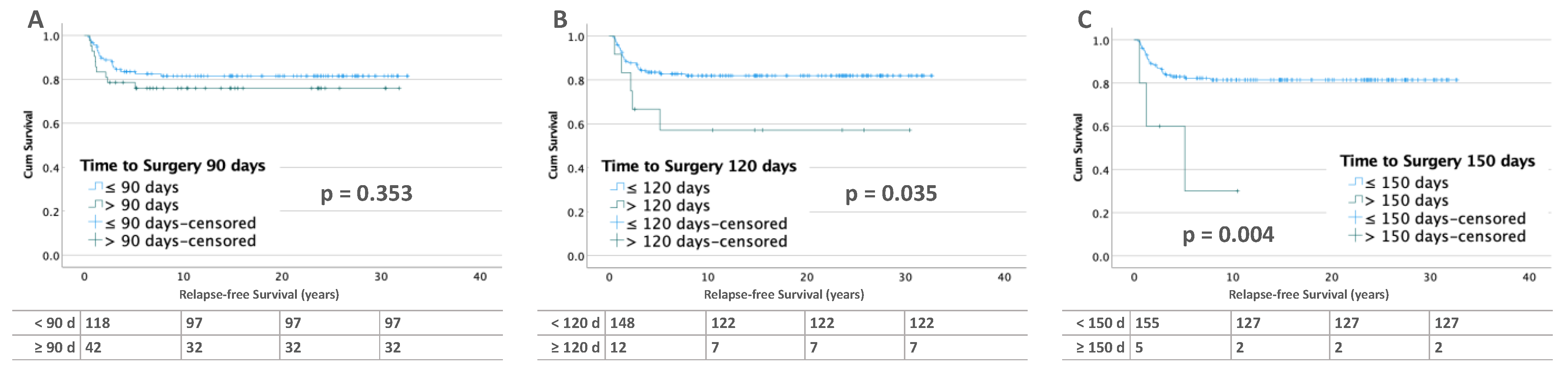
3.2. Influence of Time to Surgery on RFS
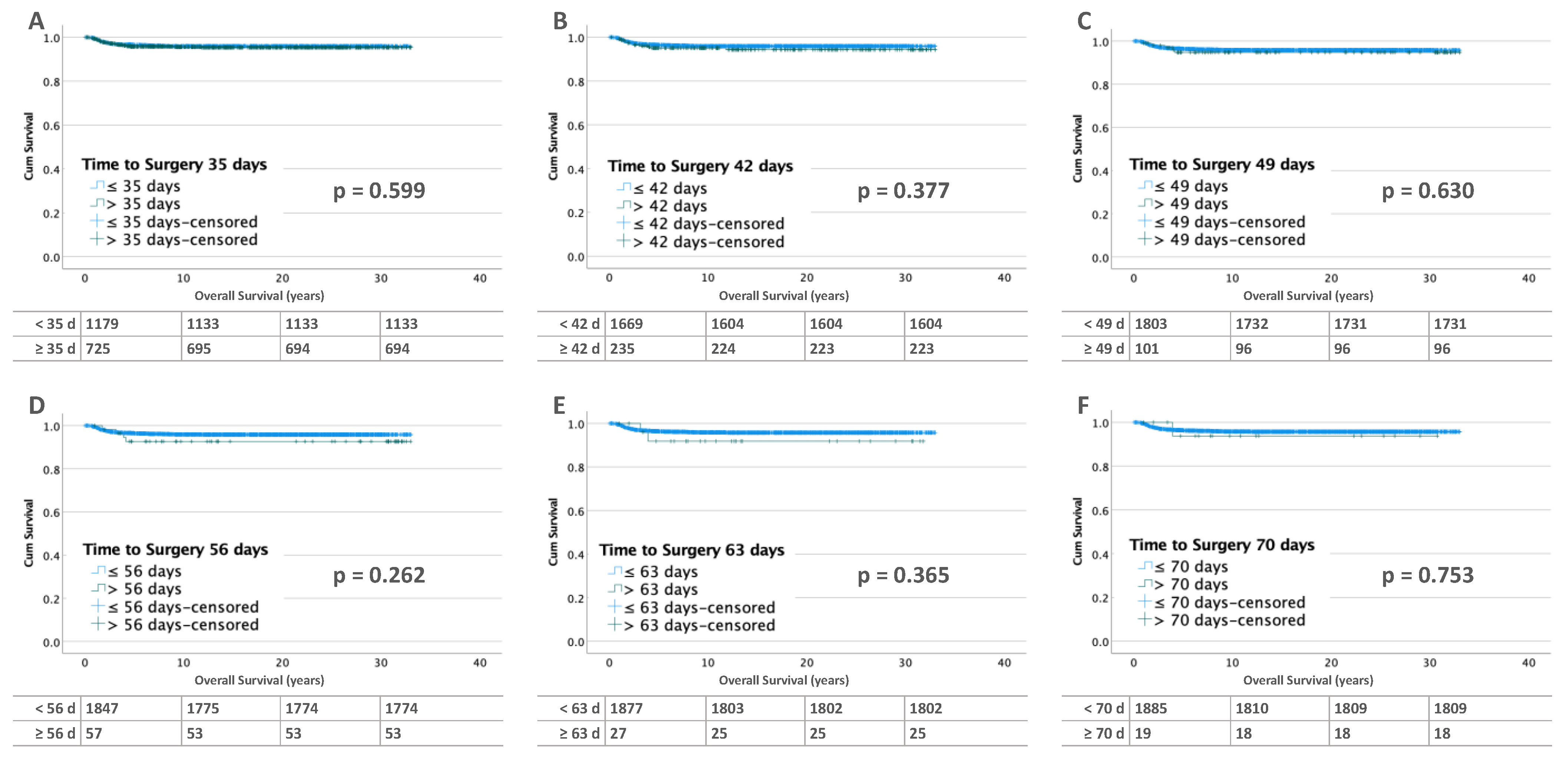
3.2.1. Unilateral Tumors
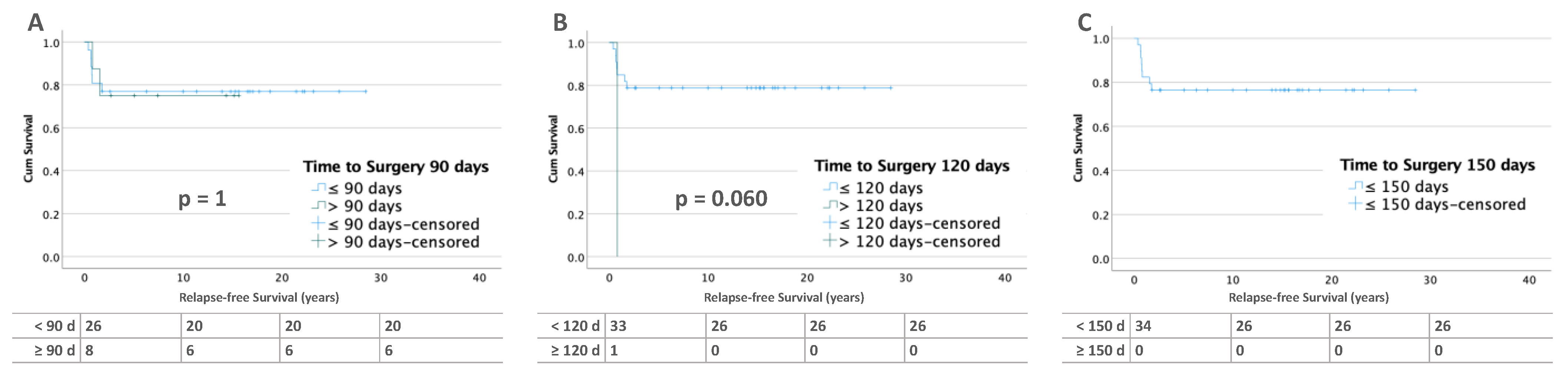
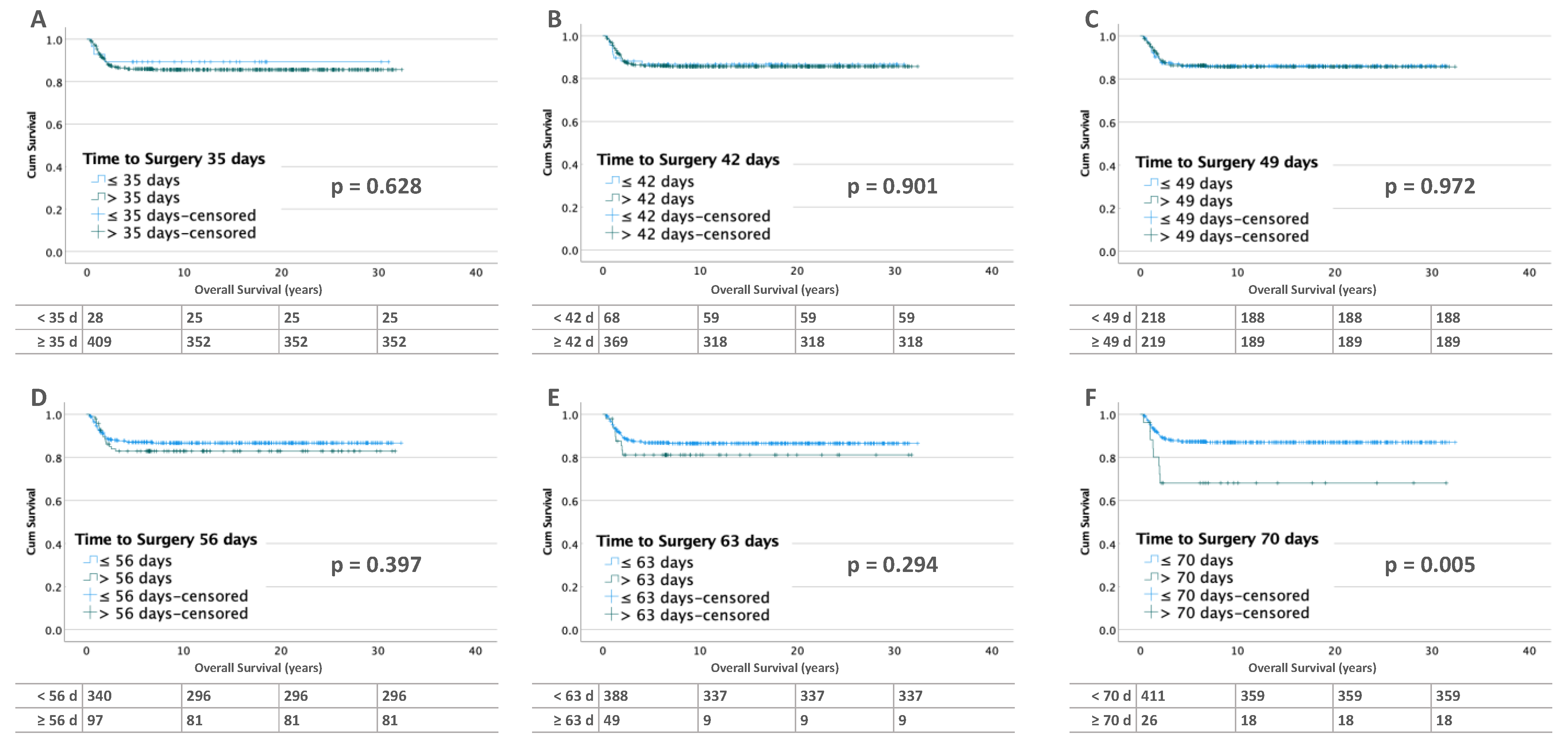
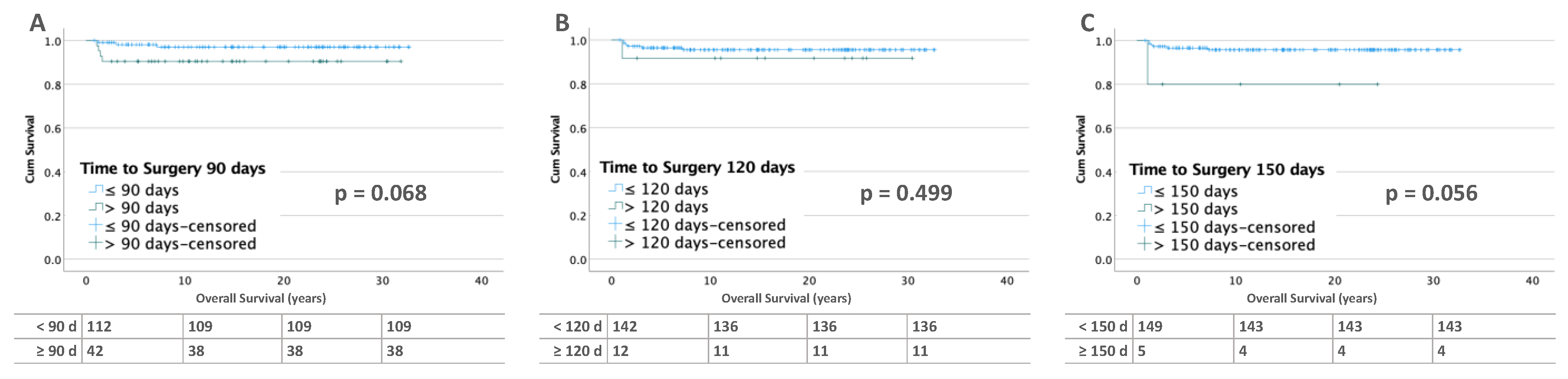
3.2.2. Bilateral Tumors
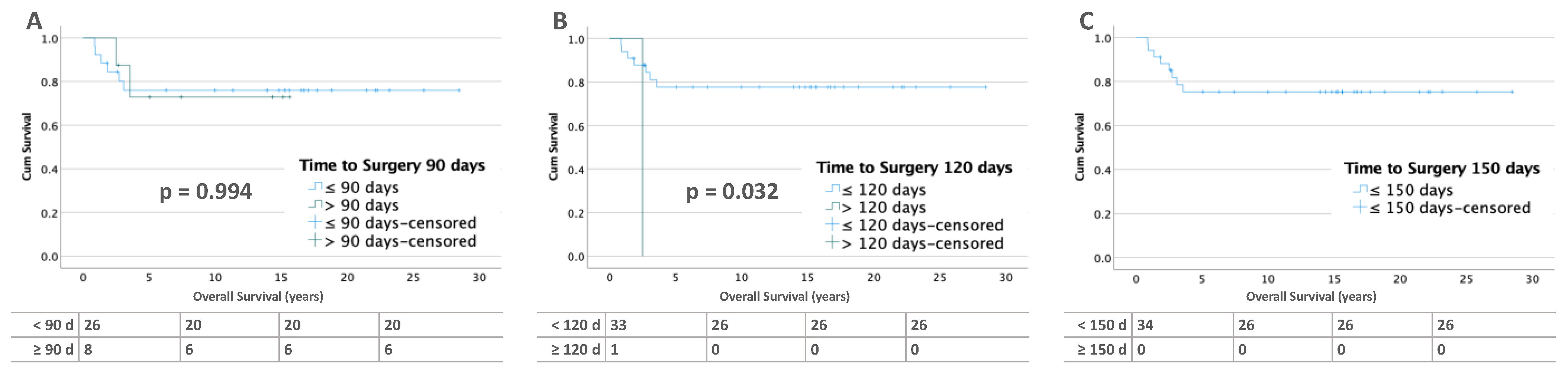
3.3. Influence of Time to Surgery on Overall Survival (OS)
3.3.1. Unilateral Tumors
3.3.2. Bilateral Tumors
4. Discussion
4.1. Unilateral Wilms Tumor
4.2. Bilateral Wilms Tumor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graf, N.; van Tinteren, H.; Bergeron, C.; Pein, F.; van den Heuvel-Eibrink, M.M.; Sandstedt, B.; Schenk, J.P.; Godzinski, J.; Oldenburger, F.; Furtwangler, R.; et al. Characteristics and outcome of stage II and III non-anaplastic Wilms’ tumour treated according to the SIOP trial and study 93-01. Eur. J. Cancer 2012, 48, 3240–3248. [Google Scholar] [CrossRef]
- Green, D.M.; Breslow, N.E.; D’Angio, G.J.; Malogolowkin, M.H.; Ritchey, M.L.; Evans, A.E.; Beckwith, J.B.; Perlman, E.J.; Shamberger, R.C.; Peterson, S.; et al. Outcome of patients with Stage II/favorable histology Wilms tumor with and without local tumor spill: A report from the National Wilms Tumor Study Group. Pediatr. Blood Cancer 2014, 61, 134–139. [Google Scholar] [CrossRef]
- Fuchs, J.; Kienecker, K.; Furtwangler, R.; Warmann, S.W.; Burger, D.; Thurhoff, J.W.; Hager, J.; Graf, N. Surgical aspects in the treatment of patients with unilateral wilms tumor: A report from the SIOP 93-01/German Society of Pediatric Oncology and Hematology. Ann. Surg. 2009, 249, 666–671. [Google Scholar] [CrossRef]
- Cox, S.; Buyukunal, C.; Millar, A.J.W. Surgery for the complex Wilms tumour. Pediatr. Surg. Int. 2020, 36, 113–127. [Google Scholar] [CrossRef]
- Graf, N.; Bergeron, C.; Brok, J.; de Camargo, B.; Chowdhury, T.; Furtwangler, R.; Gessler, M.; Godzinski, J.; Pritchard-Jones, K.; Ramirez-Villar, G.L.; et al. Fifty years of clinical and research studies for childhood renal tumors within the International Society of Pediatric Oncology (SIOP). Ann. Oncol. 2021, 32, 1327–1331. [Google Scholar] [CrossRef]
- Nelson, M.V.; van den Heuvel-Eibrink, M.M.; Graf, N.; Dome, J.S. New approaches to risk stratification for Wilms tumor. Curr. Opin. Pediatr. 2021, 33, 40–48. [Google Scholar] [CrossRef]
- van den Heuvel-Eibrink, M.M.; Hol, J.A.; Pritchard-Jones, K.; van Tinteren, H.; Furtwangler, R.; Verschuur, A.C.; Vujanic, G.M.; Leuschner, I.; Brok, J.; Rube, C.; et al. Position paper: Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat. Rev. Urol. 2017, 14, 743–752. [Google Scholar] [CrossRef]
- Kieran, K.; Ehrlich, P.F. Current surgical standards of care in Wilms tumor. Urol. Oncol. 2016, 34, 13–23. [Google Scholar] [CrossRef]
- Dome, J.S.; Graf, N.; Geller, J.I.; Fernandez, C.V.; Mullen, E.A.; Spreafico, F.; Van den Heuvel-Eibrink, M.; Pritchard-Jones, K. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J. Clin. Oncol. 2015, 33, 2999–3007. [Google Scholar] [CrossRef]
- Tournade, M.F.; Com-Nougue, C.; de Kraker, J.; Ludwig, R.; Rey, A.; Burgers, J.M.; Sandstedt, B.; Godzinski, J.; Carli, M.; Potter, R.; et al. Optimal duration of preoperative therapy in unilateral and nonmetastatic Wilms’ tumor in children older than 6 months: Results of the Ninth International Society of Pediatric Oncology Wilms’ Tumor Trial and Study. J. Clin. Oncol. 2001, 19, 488–500. [Google Scholar] [CrossRef]
- Weirich, A.; Ludwig, R.; Graf, N.; Abel, U.; Leuschner, I.; Vujanic, G.M.; Mehls, O.; Boos, J.; Beck, J.; Royer-Pokora, B.; et al. Survival in nephroblastoma treated according to the trial and study SIOP-9/GPOH with respect to relapse and morbidity. Ann. Oncol. 2004, 15, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Vujanic, G.M.; Gessler, M.; Ooms, A.; Collini, P.; Coulomb-l’Hermine, A.; D’Hooghe, E.; de Krijger, R.R.; Perotti, D.; Pritchard-Jones, K.; Vokuhl, C.; et al. The UMBRELLA SIOP-RTSG 2016 Wilms tumour pathology and molecular biology protocol. Nat. Rev. Urol. 2018, 15, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Vujanic, G.M.; Sandstedt, B. The pathology of Wilms’ tumour (nephroblastoma): The International Society of Paediatric Oncology approach. J. Clin. Pathol. 2010, 63, 102–109. [Google Scholar] [CrossRef]
- Irtan, S.; Ehrlich, P.F.; Pritchard-Jones, K. Wilms tumor: “State-of-the-art” update, 2016. Semin. Pediatr. Surg. 2016, 25, 250–256. [Google Scholar] [CrossRef]
- Mitchell, C.; Pritchard-Jones, K.; Shannon, R.; Hutton, C.; Stevens, S.; Machin, D.; Imeson, J.; Kelsey, A.; Vujanic, G.M.; Gornall, P.; et al. Immediate nephrectomy versus preoperative chemotherapy in the management of non-metastatic Wilms’ tumour: Results of a randomised trial (UKW3) by the UK Children’s Cancer Study Group. Eur. J. Cancer 2006, 42, 2554–2562. [Google Scholar] [CrossRef]
- Kalapurakal, J.A.; Li, S.M.; Breslow, N.E.; Beckwith, J.B.; Macklis, R.; Thomas, P.R.; D’Angio, G.J.; Kim, T.; de Lorimier, A.; Kelalis, P.; et al. Influence of radiation therapy delay on abdominal tumor recurrence in patients with favorable histology Wilms’ tumor treated on NWTS-3 and NWTS-4: A report from the National Wilms’ Tumor Study Group. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 495–499. [Google Scholar] [CrossRef]
- Stokes, C.L.; Stokes, W.A.; Kalapurakal, J.A.; Paulino, A.C.; Cost, N.G.; Cost, C.R.; Garrington, T.P.; Greffe, B.S.; Roach, J.P.; Bruny, J.L.; et al. Timing of Radiation Therapy in Pediatric Wilms Tumor: A Report from the National Cancer Database. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 453–461. [Google Scholar] [CrossRef]
- Meier, C.M.; Fuchs, J.; von Schweinitz, D.; Stein, R.; Wagenpfeil, S.; Kager, L.; Schenk, J.P.; Vokuhl, C.; Melchior, P.; Welter, N.; et al. Surgical Factors Influencing Local Relapse and Outcome in the Treatment of Unilateral Nephroblastoma. Ann. Surg. 2022, in press. [CrossRef]
- Shamberger, R.C.; Guthrie, K.A.; Ritchey, M.L.; Haase, G.M.; Takashima, J.; Beckwith, J.B.; D’Angio, G.J.; Green, D.M.; Breslow, N.E. Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann. Surg. 1999, 229, 292–297. [Google Scholar] [CrossRef]
- Meier, C.M.; Furtwangler, R.; von Schweinitz, D.; Stein, R.; Welter, N.; Wagenpfeil, S.; Kager, L.; Schenk, J.P.; Vokuhl, C.; Melchior, P.; et al. Vena Cava Thrombus in Patients with Wilms Tumor. Cancers 2022, 14, 3924. [Google Scholar] [CrossRef]
- Breslow, N.; Olshan, A.; Beckwith, J.B.; Green, D.M. Epidemiology of Wilms tumor. Med. Pediatr. Oncol. 1993, 21, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Breslow, N.E.; Beckwith, J.B. Epidemiological features of Wilms’ tumor: Results of the National Wilms’ Tumor Study. J. Natl. Cancer Inst. 1982, 68, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Welter, N.; Wagner, A.; Furtwangler, R.; Melchior, P.; Kager, L.; Vokuhl, C.; Schenk, J.P.; Meier, C.M.; Siemer, S.; Gessler, M.; et al. Characteristics of Nephroblastoma/Nephroblastomatosis in Children with a Clinically Reported Underlying Malformation or Cancer Predisposition Syndrome. Cancers 2021, 13, 5016. [Google Scholar] [CrossRef] [PubMed]
- Charlton, J.; Irtan, S.; Bergeron, C.; Pritchard-Jones, K. Bilateral Wilms tumour: A review of clinical and molecular features. Expert Rev. Mol. Med. 2017, 19, e8. [Google Scholar] [CrossRef]
- Furtwangler, R.; Schmolze, M.; Graber, S.; Leuschner, I.; Amann, G.; Schenk, J.P.; Niggli, F.; Kager, L.; von Schweinitz, D.; Graf, N. Pretreatment for bilateral nephroblastomatosis is an independent risk factor for progressive disease in patients with stage V nephroblastoma. Klin. Padiatr. 2014, 226, 175–181. [Google Scholar] [CrossRef]
- Murphy, A.J.; Davidoff, A.M. Bilateral Wilms Tumor: A Surgical Perspective. Children 2018, 5, 134. [Google Scholar] [CrossRef]
- Breslow, N.E.; Collins, A.J.; Ritchey, M.L.; Grigoriev, Y.A.; Peterson, S.M.; Green, D.M. End stage renal disease in patients with Wilms tumor: Results from the National Wilms Tumor Study Group and the United States Renal Data System. J. Urol. 2005, 174, 1972–1975. [Google Scholar] [CrossRef]
- Davidoff, A.M.; Interiano, R.B.; Wynn, L.; Delos Santos, N.; Dome, J.S.; Green, D.M.; Brennan, R.C.; McCarville, M.B.; Krasin, M.J.; Kieran, K.; et al. Overall Survival and Renal Function of Patients with Synchronous Bilateral Wilms Tumor Undergoing Surgery at a Single Institution. Ann. Surg. 2015, 262, 570–576. [Google Scholar] [CrossRef]
- Davidoff, A.M.; Giel, D.W.; Jones, D.P.; Jenkins, J.J.; Krasin, M.J.; Hoffer, F.A.; Williams, M.A.; Dome, J.S. The feasibility and outcome of nephron-sparing surgery for children with bilateral Wilms tumor. The St Jude Children’s Research Hospital experience: 1999–2006. Cancer 2008, 112, 2060–2070. [Google Scholar] [CrossRef]
- Hamilton, T.E.; Ritchey, M.L.; Haase, G.M.; Argani, P.; Peterson, S.M.; Anderson, J.R.; Green, D.M.; Shamberger, R.C. The management of synchronous bilateral Wilms tumor: A report from the National Wilms Tumor Study Group. Ann. Surg. 2011, 253, 1004–1010. [Google Scholar] [CrossRef]
- Kieran, K.; Williams, M.A.; Dome, J.S.; McGregor, L.M.; Krasin, M.J.; Davidoff, A.M. Margin status and tumor recurrence after nephron-sparing surgery for bilateral Wilms tumor. J. Pediatr. Surg. 2013, 48, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Sudour, H.; Audry, G.; Schleimacher, G.; Patte, C.; Dussart, S.; Bergeron, C. Bilateral Wilms tumors (WT) treated with the SIOP 93 protocol in France: Epidemiological survey and patient outcome. Pediatr. Blood Cancer 2012, 59, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.; Chi, Y.Y.; Chintagumpala, M.M.; Hoffer, F.A.; Perlman, E.J.; Kalapurakal, J.A.; Warwick, A.; Shamberger, R.C.; Khanna, G.; Hamilton, T.E.; et al. Results of the First Prospective Multi-institutional Treatment Study in Children with Bilateral Wilms Tumor (AREN0534): A Report from the Children’s Oncology Group. Ann. Surg. 2017, 266, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Rojas, Y.; Jaramillo, S.; Lyons, K.; Mahmood, N.; Wu, M.F.; Liu, H.; Vasudevan, S.A.; Guillerman, R.P.; Louis, C.U.; Russell, H.V.; et al. The optimal timing of surgical resection in high-risk neuroblastoma. J. Pediatr. Surg. 2016, 51, 1665–1669. [Google Scholar] [CrossRef]
- Bleicher, R.J.; Ruth, K.; Sigurdson, E.R.; Beck, J.R.; Ross, E.; Wong, Y.N.; Patel, S.A.; Boraas, M.; Chang, E.I.; Topham, N.S.; et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol. 2016, 2, 330–339. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Shen, C.; Medich, D.S.; Geller, D.A.; Bartlett, D.L.; Tsung, A.; Tohme, S. Time to Surgery and Colon Cancer Survival in the United States. Ann. Surg. 2021, 274, 1025–1031. [Google Scholar] [CrossRef]
| TTS (Days) | Relapse | Univariate Cox Regression | Multiple Cox Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | χ2 | HR | CI | p | HR | CI | p | ||||
| Without Metastases at Diagnosis (n = 1918) | ||||||||||||
| 35 | ≤35 | 1075 | 90.6% | 112 | 9.4% | 0.309 | 1.16 | 0.87–1.55 | 0.319 | 1.02 | 0.76–1.38 | 0.896 |
| >35 | 651 | 89.1% | 80 | 10.9% | ||||||||
| 42 | ≤42 | 1515 | 90.1% | 166 | 9.9% | 0.643 | 1.08 | 0.71–1.64 | 0.706 | 0.95 | 0.62–1.45 | 0.801 |
| >42 | 221 | 89.0% | 26 | 11.0% | ||||||||
| 49 | ≤49 | 1635 | 90.0% | 181 | 10.0% | 0.865 | 1.02 | 0.55–1.89 | 0.947 | 0.92 | 0.49–1.70 | 0.784 |
| >49 | 91 | 89.2% | 11 | 10.8% | ||||||||
| 56 | ≤56 | 1677 | 90.1% | 184 | 9.9% | 0.366 | 1.33 | 0.65–2.73 | 0.441 | 1.09 | 0.53–2.26 | 0.814 |
| >56 | 49 | 86.0% | 8 | 14.0% | ||||||||
| 63 | ≤63 | 1703 | 90.1% | 188 | 9.9% | 0.512 * | 1.34 | 0.48–3.70 | 0.574 | 1.03 | 0.37–2.90 | 0.950 |
| >63 | 23 | 85.2% | 4 | 14.8% | ||||||||
| 70 | ≤70 | 1710 | 90.0% | 189 | 10.0% | 0.428 * | 1.40 | 0.43–4.55 | 0.573 | 1.07 | 0.32–3.52 | 0.918 |
| >70 | 16 | 84.2% | 3 | 15.8% | ||||||||
| With Metastases at Diagnosis (n = 449) | ||||||||||||
| 35 | ≤35 | 20 | 66.7% | 10 | 33.3% | 0.387 | 0.80 | 0.40–1.58 | 0.518 | 1.07 | 0.53–2.15 | 0.853 |
| >35 | 313 | 74.7% | 106 | 25.3% | ||||||||
| 42 | ≤42 | 52 | 69.3% | 23 | 30.7% | 0.313 | 0.82 | 0.51–1.31 | 0.414 | 1.02 | 0.62–1.66 | 0.948 |
| >42 | 281 | 75.1% | 93 | 24.9% | ||||||||
| 49 | ≤49 | 165 | 72.7% | 62 | 27.3% | 0.518 | 0.90 | 0.62–1.30 | 0.566 | 0.84 | 0.56–1.26 | 0.397 |
| >49 | 168 | 75.7% | 54 | 24.3% | ||||||||
| 56 | ≤56 | 260 | 74.1% | 91 | 25.9% | 1 | 0.95 | 0.60–1.50 | 0.816 | 0.82 | 0.49–1.37 | 0.448 |
| >56 | 73 | 74.5% | 25 | 25.5% | ||||||||
| 63 | ≤63 | 298 | 74.7% | 101 | 25.3% | 0.494 | 1.20 | 0.69–2.12 | 0.515 | 0.90 | 0.46–1.77 | 0.751 |
| >63 | 35 | 70.0% | 15 | 30.0% | ||||||||
| 70 | ≤70 | 318 | 75.2% | 105 | 24.8% | 0.063 | 1.96 | 1.03–3.75 | 0.041 | 1.44 | 0.64–3.23 | 0.375 |
| >70 | 15 | 57.7% | 11 | 42.3% | ||||||||
| TTS (Days) | Relapse | Univariate Cox Regression | Multiple Cox Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | χ2 | HR | CI | p | HR | CI | p | ||||
| Without Metastases at Diagnosis (n = 160) | ||||||||||||
| 90 | <90 | 97 | 82.2% | 21 | 17.8% | 0.495 | 1.48 | 0.69–3.15 | 0.313 | 1.22 | 0.56–2.66 | 0.618 |
| ≥90 | 32 | 76.2% | 10 | 23.8% | ||||||||
| 120 | <120 | 122 | 82.4% | 26 | 17.4% | 0.057 * | 2.95 | 1.12–7.77 | 0.029 | 2.87 | 1.19–7.95 | 0.022 |
| ≥120 | 7 | 58.3% | 5 | 41.7% | ||||||||
| 150 | <150 | 127 | 81.9% | 28 | 18.1% | 0.050 * | 5.85 | 1.74–19.64 | 0.004 | 4.62 | 1.17–18.26 | 0.029 |
| ≥150 | 2 | 40% | 3 | 60% | ||||||||
| With Metastases at Diagnosis (n = 34) | ||||||||||||
| 90 | <90 | 20 | 76.9% | 6 | 23.1% | 1 * | 1 | 0.20–4.96 | 1 | 1.04 | 0.16–6.90 | 0.966 |
| ≥90 | 6 | 75.0% | 2 | 25.0% | ||||||||
| 120# | <120 | 26 | 78.8% | 7 | 21.2% | - | - | - | - | - | - | - |
| ≥120 | - | - | 1 | 100% | ||||||||
| 150# | <150 | 26 | 76.5% | 8 | 23.5% | - | - | - | - | - | - | - |
| ≥150 | - | - | - | - | ||||||||
| TTS (Days) | Death | Univariate Cox Regression | Multiple Cox Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | χ2 | HR | CI | p | HR | CI | p | ||||
| Without Metastases at Diagnosis (n = 1904) | ||||||||||||
| 35 | ≤35 | 1133 | 96.1% | 46 | 3.9% | 0.720 | 1.13 | 0.72–1.78 | 0.605 | 1.02 | 0.64–1.63 | 0.944 |
| >35 | 694 | 95.7% | 31 | 4.3% | ||||||||
| 42 | ≤42 | 1604 | 96.1% | 65 | 3.9% | 0.480 | 1.35 | 0.73–2.50 | 0.341 | 0.98 | 0.51–1.89 | 0.960 |
| >42 | 223 | 94.9% | 12 | 5.1% | ||||||||
| 49 | ≤49 | 1731 | 96.0% | 72 | 4.0% | 0.600 * | 1.25 | 0.50–3.09 | 0.633 | 1.04 | 0.42–2.57 | 0.938 |
| >49 | 96 | 95.0% | 5 | 5.0% | ||||||||
| 56 | ≤56 | 1774 | 96.0% | 73 | 4.0% | 0.288 * | 1.76 | 0.64–4.82 | 0.270 | 1.23 | 0.45–3.39 | 0.688 |
| >56 | 53 | 93.0% | 4 | 7.0% | ||||||||
| 63 | ≤63 | 1802 | 96.0% | 75 | 4.0% | 0.624 * | 1.89 | 0.46–7.70 | 0.374 | 1.35 | 0.33–5.51 | 0.681 |
| >63 | 25 | 92.6% | 2 | 7.4% | ||||||||
| 70 | ≤70 | 1809 | 96.0% | 76 | 4.0% | 0.545 * | 1.37 | 0.19–9.85 | 0.755 | 1.06 | 0.15–7.69 | 0.951 |
| >70 | 18 | 94.7 | 1 | 5.3% | ||||||||
| With Metastases at Diagnosis (n = 437) | ||||||||||||
| 35 | ≤35 | 25 | 89.3% | 3 | 10.7% | 0.783 * | 1.33 | 0.42–4.23 | 0.635 | 2.07 | 0.64–6.71 | 0.224 |
| >35 | 352 | 86.1% | 57 | 13.9% | ||||||||
| 42 | ≤42 | 59 | 86.8% | 9 | 13.2% | 1 | 1.04 | 0.51–2.12 | 0.905 | 1.81 | 0.85–3.82 | 0.121 |
| >42 | 318 | 86.2% | 51 | 13.8% | ||||||||
| 49 | ≤49 | 188 | 86.2% | 30 | 13.8% | 1 | 1.01 | 0.61–1.67 | 0.974 | 1.05 | 0.60–1.84 | 0.878 |
| >49 | 189 | 86.3% | 30 | 13.7% | ||||||||
| 56 | ≤56 | 296 | 87.1% | 44 | 12.9% | 0.403 | 1.28 | 0.72–2.27 | 0.399 | 0.88 | 0.45–1.72 | 0.700 |
| >56 | 81 | 83.5% | 16 | 16.5% | ||||||||
| 63 | ≤63 | 337 | 86.9% | 51 | 13.1% | 0.376 | 1.46 | 0.72–2.96 | 0.297 | 0.89 | 0.35–2.29 | 0.813 |
| >63 | 40 | 81.6% | 9 | 18.4% | ||||||||
| 70 | ≤70 | 359 | 87.3% | 52 | 12.7% | 0.017 | 2.77 | 1.31–5.84 | 0.007 | 1.32 | 0.46–3.78 | 0.611 |
| >70 | 18 | 69.2% | 8 | 30.8% | ||||||||
| TTS (Days) | Death | Univariate Cox Regression | Multiple Cox Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | χ2 | HR | CI | p | HR | CI | p | ||||
| Without Metastases at Diagnosis (n = 154) | ||||||||||||
| 90 | <90 | 109 | 97.3% | 3 | 2.7% | 0.089 * | 3.64 | 0.81–16.23 | 0.091 | 1.31 | 0.25–6.91 | 0.754 |
| ≥90 | 38 | 90.5% | 4 | 9.5% | ||||||||
| 120 | <120 | 136 | 95.8% | 6 | 4.2% | 0.440 * | 2.03 | 0.24–16.87 | 0.512 | 9.65 | 0.58–166.11 | 0.115 |
| ≥120 | 11 | 91.7% | 1 | 8.3% | ||||||||
| 150 | <150 | 143 | 96.0% | 6 | 4.0 | 0.210 * | 6.07 | 0.73–50.51 | 0.095 | 12.23 | 0.69–215.48 | 0.087 |
| ≥150 | 4 | 80% | 1 | 20% | ||||||||
| With Metastases at Diagnosis (n = 34) | ||||||||||||
| 90 | <90 | 20 | 76.9% | 6 | 23.1% | 1 * | 1 | 0.20–4.96 | 1 | 1.11 | 0.21–5.72 | 0.905 |
| ≥90 | 6 | 75% | 2 | 25% | ||||||||
| 120# | <120 | 26 | 78.8% | 7 | 21.2% | 0.235 * | - | - | - | - | - | - |
| ≥120 | - | - | 1 | 100% | ||||||||
| 150# | <150 | 26 | 76.5 | 8 | 23.5% | - | - | - | - | - | - | - |
| ≥150 | - | - | - | - | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meier, C.-M.; Furtwängler, R.; Mergen, M.; Welter, N.; Melchior, P.; Schenk, J.-P.; Vokuhl, C.; Kager, L.; Kroiss-Benninger, S.; Wagenpfeil, S.; et al. Impact of Time to Surgery on Outcome in Wilms Tumor Treated with Preoperative Chemotherapy. Cancers 2023, 15, 1494. https://doi.org/10.3390/cancers15051494
Meier C-M, Furtwängler R, Mergen M, Welter N, Melchior P, Schenk J-P, Vokuhl C, Kager L, Kroiss-Benninger S, Wagenpfeil S, et al. Impact of Time to Surgery on Outcome in Wilms Tumor Treated with Preoperative Chemotherapy. Cancers. 2023; 15(5):1494. https://doi.org/10.3390/cancers15051494
Chicago/Turabian StyleMeier, Clemens-Magnus, Rhoikos Furtwängler, Marvin Mergen, Nils Welter, Patrick Melchior, Jens-Peter Schenk, Christian Vokuhl, Leo Kager, Sabine Kroiss-Benninger, Stefan Wagenpfeil, and et al. 2023. "Impact of Time to Surgery on Outcome in Wilms Tumor Treated with Preoperative Chemotherapy" Cancers 15, no. 5: 1494. https://doi.org/10.3390/cancers15051494
APA StyleMeier, C.-M., Furtwängler, R., Mergen, M., Welter, N., Melchior, P., Schenk, J.-P., Vokuhl, C., Kager, L., Kroiss-Benninger, S., Wagenpfeil, S., & Graf, N. (2023). Impact of Time to Surgery on Outcome in Wilms Tumor Treated with Preoperative Chemotherapy. Cancers, 15(5), 1494. https://doi.org/10.3390/cancers15051494






