Combination of Muscle Quantity and Quality Is Useful to Assess the Necessity of Surveillance after a 5-Year Cancer-Free Period in Patients Who Undergo Radical Cystectomy: A Multi-Institutional Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
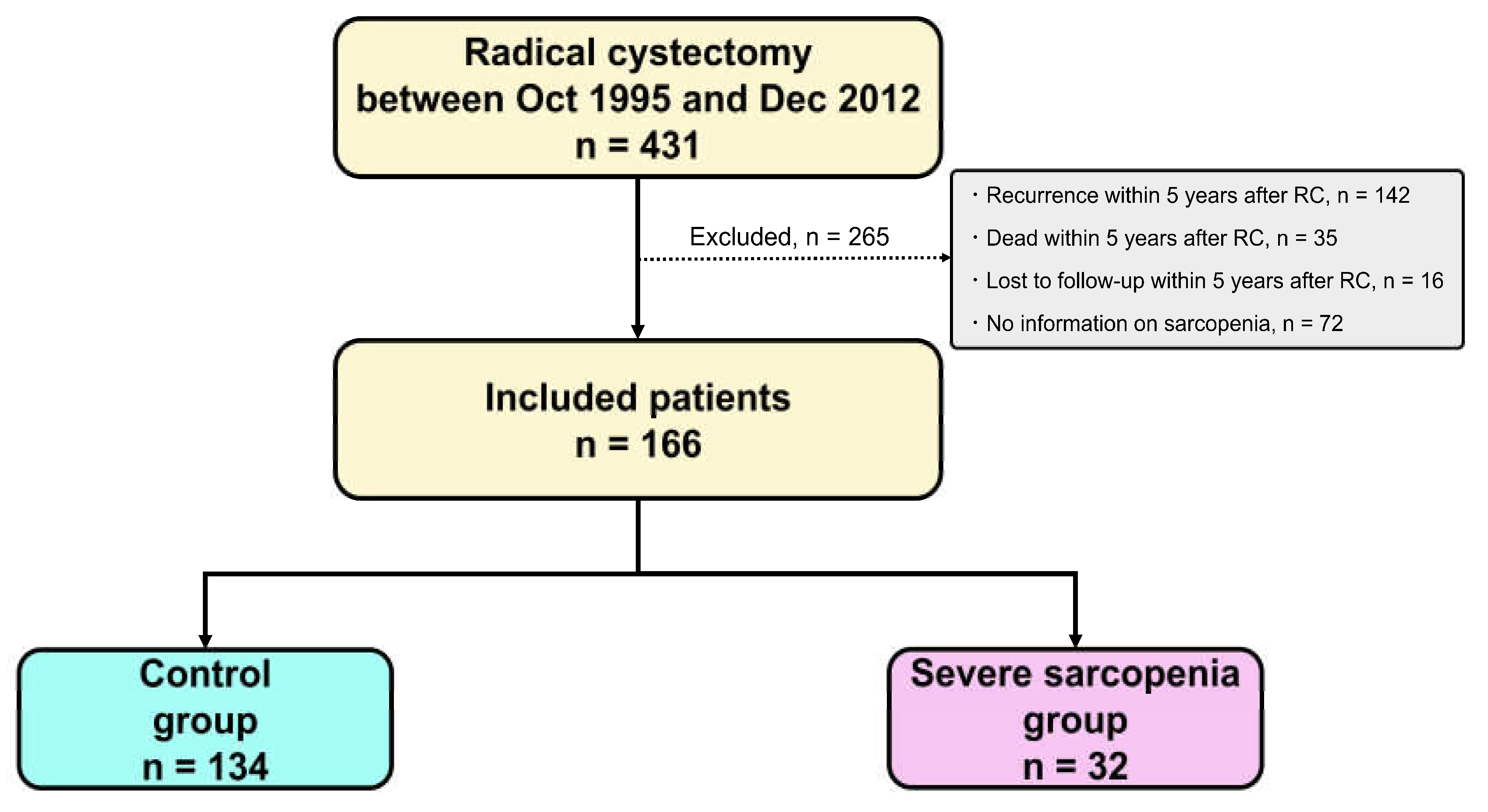
2.2. Patient Selection
2.3. Evaluation of Variables
2.4. NAC and Adjuvant Chemotherapy
2.5. Surgical Procedures
2.6. Follow-Up Schedule
2.7. Evaluation of Muscle Quantity and Quality
2.8. Statistical Analysis
3. Results
3.1. Patients’ Backgrounds
3.2. Evaluation of Muscle Quantity and Quality
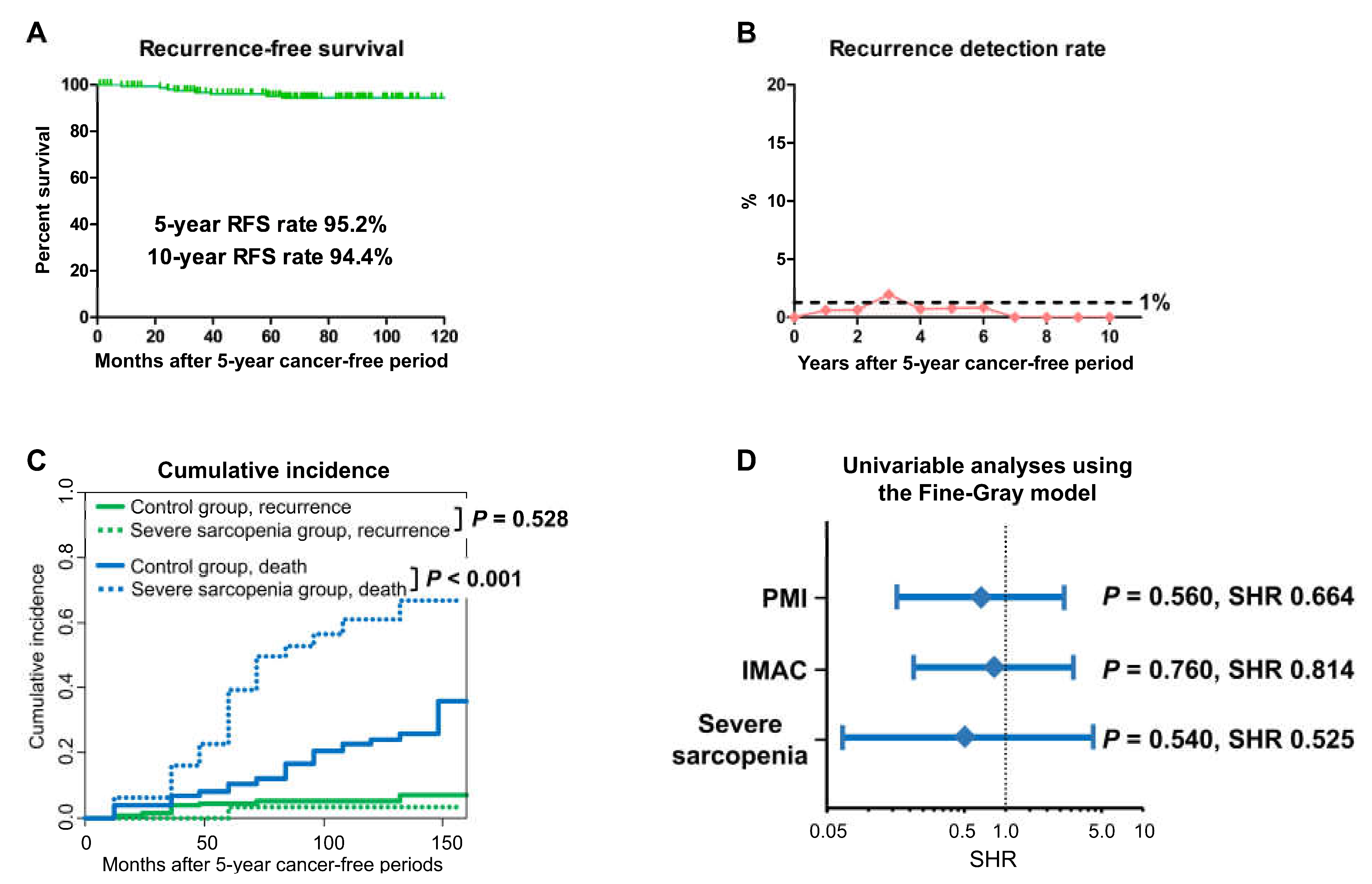
3.3. BC Recurrence
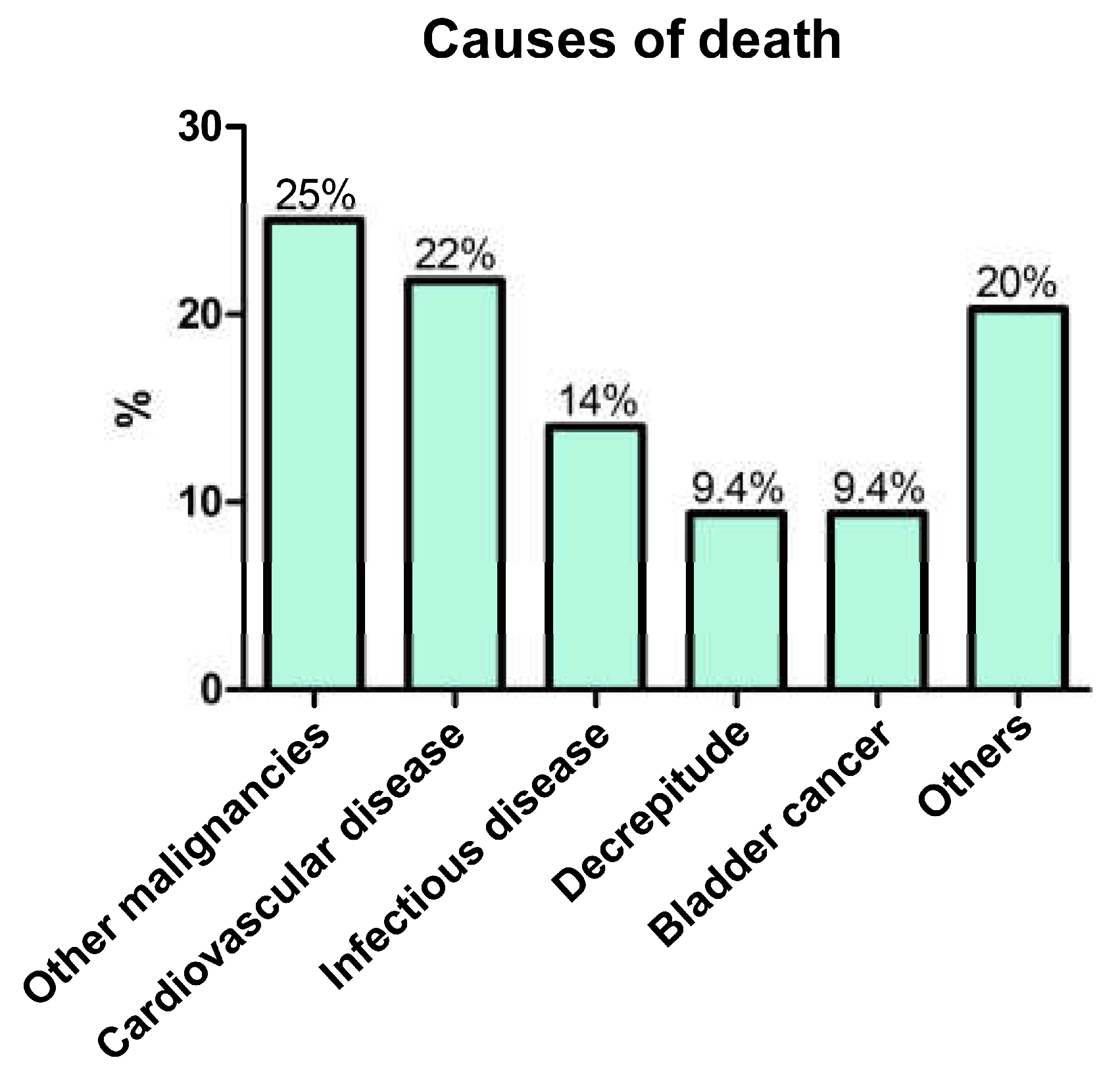
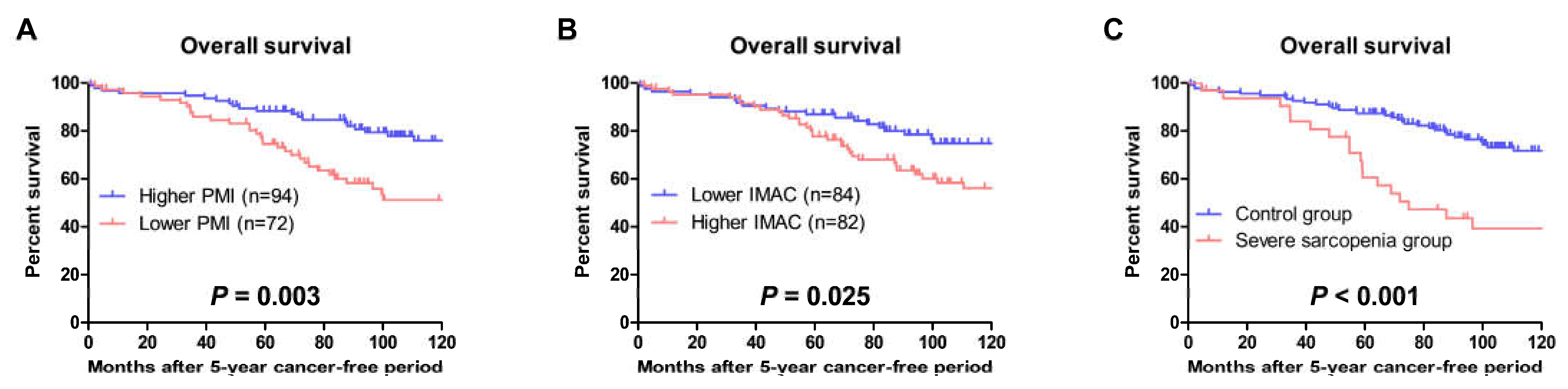
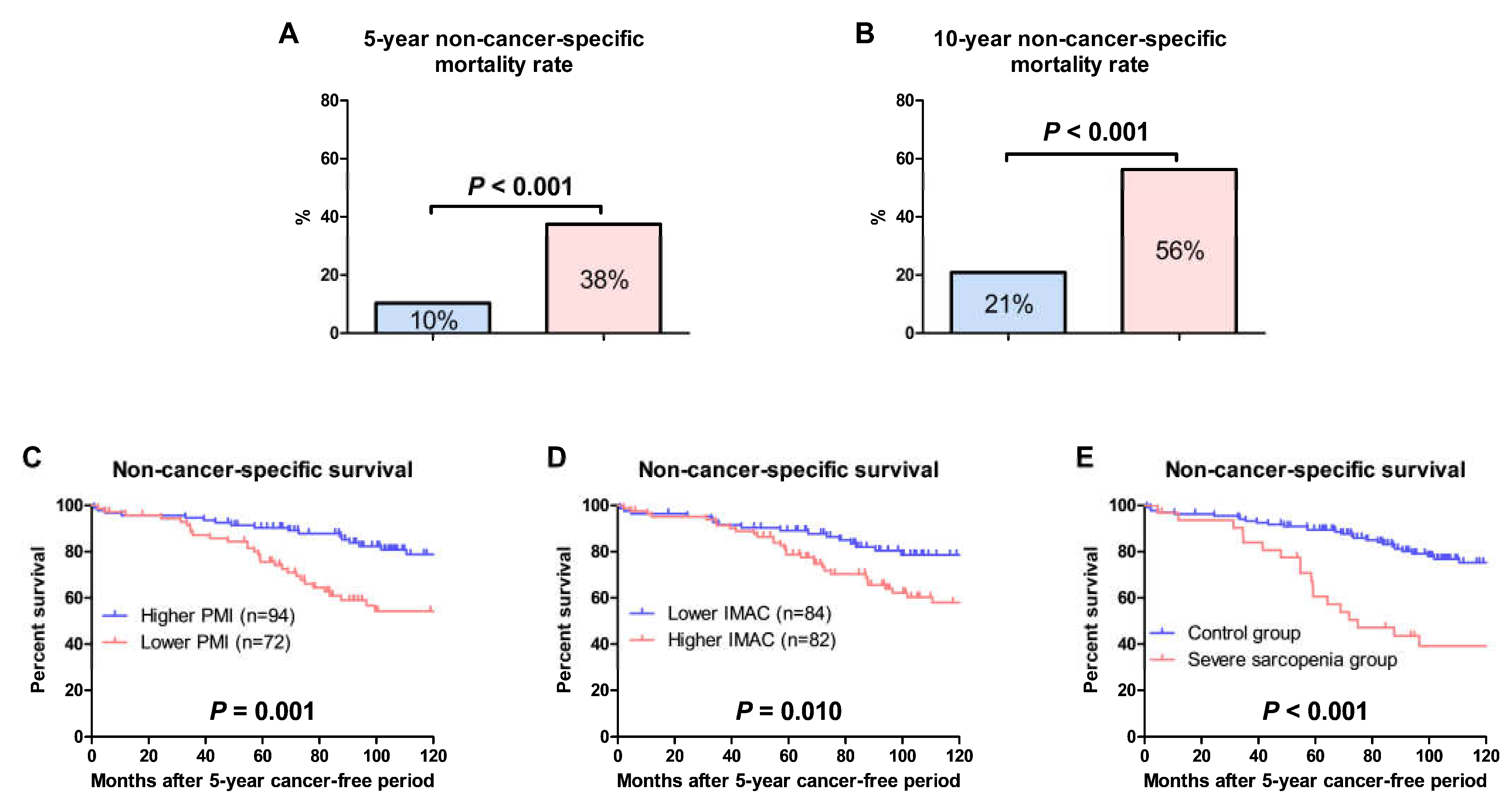
3.4. OS and Non-Cancer-Specific Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Linder, B.J.; Boorjian, S.A.; Hudolin, T.; Cheville, J.C.; Thapa, P.; Tarrell, R.F.; Frank, I. Late Recurrence after Radical Cystectomy: Patterns, Risk Factors and Outcomes. J. Urol. 2014, 191, 1256–1261. [Google Scholar] [CrossRef]
- Solsona, E.; Iborra, I.; Rubio, J.; Casanova, J.; Dumont, R.; Monrós, J. Late Oncological Occurrences Following Radical Cystectomy in Patients with Bladder Cancer. Eur. Urol. 2003, 43, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, T.; Okugawa, Y.; Shimura, T.; Yamashita, S.; Sato, Y.; Goel, A.; Mizuno, N.; Yin, C.; Uratani, R.; Imaoka, H.; et al. Combined assessment of muscle quality and quantity predicts oncological outcome in patients with esophageal cancer. Am. J. Surg. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Prado, C.M.; Meyerhardt, J.A.; Weltzien, E.K.; Xiao, J.; Cespedes Feliciano, E.M.; Caan, B.J. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 2018, 124, 3008–3015. [Google Scholar] [CrossRef] [PubMed]
- Mayr, R.; Gierth, M.; Zeman, F.; Reiffen, M.; Seeger, P.; Wezel, F.; Pycha, A.; Comploj, E.; Bonatti, M.; Ritter, M.; et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, Y.; Nakashima, J.; Yunaiyama, D.; Sugihara, T.; Gondo, T.; Nakagami, Y.; Horiguchi, Y.; Ohno, Y.; Namiki, K.; Ohori, M.; et al. Sarcopenia as a Novel Preoperative Prognostic Predictor for Survival in Patients with Bladder Cancer Undergoing Radical Cystectomy. Ann. Surg. Oncol. 2016, 23, 1048–1054. [Google Scholar] [CrossRef]
- Smith, A.B.; Deal, A.M.; Yu, H.; Boyd, B.; Matthews, J.; Wallen, E.M.; Pruthi, R.S.; Woods, M.E.; Muss, H.; Nielsen, M.E. Sarcopenia as a Predictor of Complications and Survival Following Radical Cystectomy. J. Urol. 2014, 191, 1714–1720. [Google Scholar] [CrossRef]
- Fraisse, G.; Renard, Y.; Lebacle, C.; Masson-Lecomte, A.; Desgrandchamps, F.; Hennequin, C.; Bessede, T.; Irani, J. Is sarcopenia a morbi-mortality factor in the treatment of localized muscle-invasive bladder cancer? Prog. Urol. 2020, 30, 41–50. [Google Scholar] [CrossRef]
- Bayraktar, E.; Tasar, P.T.; Binici, D.N.; Karasahin, O.; Timur, O.; Sahin, S. Relationship between Sarcopenia and Mortality in Elderly Inpatients. Eurasian J. Med. 2020, 52, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Ha, J.; Kim, Y.-J.; Ko, Y.; Park, T.; Kim, K.W.; Kim, W.Y. The Impact of Myosteatosis Percentage on Short-Term Mortality in Patients with Septic Shock. J. Clin. Med. 2022, 11, 3031. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship Between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Koie, T.; Ohyama, C.; Yamamoto, H.; Hatakeyama, S.; Kudoh, S.; Yoneyama, T.; Hashimoto, Y.; Kamimura, N. Minimum incision endoscopic radical cystectomy in patients with malignant tumors of the urinary bladder: Clinical and oncological outcomes at a single institution. Eur. J. Surg. Oncol. 2012, 38, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Uchida, K.; Nagano, Y.; Matsushita, K.; Koike, Y.; Okita, Y.; Suzuki, T.; Toiyama, Y. Preoperative myopenia and myosteatosis and their impact on postoperative complications in children with inflammatory bowel disease. Surg. Today 2022. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yagi, S.; Kamo, N.; Okajima, H.; Uemoto, S. Impact of Skeletal Muscle Mass Index, Intramuscular Adipose Tissue Content, and Visceral to Subcutaneous Adipose Tissue Area Ratio on Early Mortality of Living Donor Liver Transplantation. Transplantation 2017, 101, 565–574. [Google Scholar] [CrossRef]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Fujimoto, Y.; Kobayashi, A.; Iida, T.; Yagi, S.; Taura, K.; Hatano, E.; Uemoto, S. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery 2016, 159, 821–833. [Google Scholar] [CrossRef]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Fujimoto, Y.; Masui, T.; Mizumoto, M.; Hammad, A.; Mori, A.; Takaori, K.; Uemoto, S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery 2015, 157, 1088–1098. [Google Scholar] [CrossRef]
- Wang, K.; Gu, Y.; Ni, J.; Zhang, H.; Xie, J.; Xu, T.; Geng, J.; Mao, W.; Peng, B. Combination of Total Psoas Index and Albumin–Globulin Score for the Prognosis Prediction of Bladder Cancer Patients After Radical Cystectomy: A Population-Based Study. Front. Oncol. 2021, 11, 724536. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, P.I.; Afferi, L.; Antonelli, A.; Cerruto, M.A.; Odorizzi, K.; Gozzo, A.; Mordasini, L.; Mattei, A.; Baumeister, P.; Cornelius, J.; et al. The impact of preoperative nutritional status on post-surgical complication and mortality rates in patients undergoing radical cystectomy for bladder cancer: A systematic review of the literature. World J. Urol. 2021, 39, 1045–1081. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Dou, W.-C.; Shao, Y.-X.; Liu, J.-B.; Xiong, S.-C.; Yang, W.-X.; Li, X. The prognostic value of sarcopenia in patients with surgically treated urothelial carcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Iguchi, T.; Koike, H.; Wakamiya, T.; Kikkawa, K.; Kohjimoto, Y.; Hara, I. Impact of preoperative sarcopenia and myosteatosis on prognosis after radical cystectomy in patients with bladder cancer. Int. J. Urol. 2021, 28, 757–762. [Google Scholar] [CrossRef]
- Okugawa, Y.; Kitajima, T.; Yamamoto, A.; Shimura, T.; Kawamura, M.; Fujiwara, T.; Mochiki, I.; Okita, Y.; Tsujiura, M.; Yokoe, T.; et al. Clinical Relevance of Myopenia and Myosteatosis in Colorectal Cancer. J. Clin. Med. 2022, 11, 2617. [Google Scholar] [CrossRef]
- Hou, L.; Deng, Y.; Fan, X.; Zhao, T.; Cui, B.; Lin, L.; Hou, J.; Mao, L.; Zhao, W.; Jiang, K.; et al. A Sex-Stratified Prognostic Nomogram Incorporating Body Compositions for Long-Term Mortality in Cirrhosis. J. Parenter. Enter. Nutr. 2021, 45, 403–413. [Google Scholar] [CrossRef]
- Hopkins, J.J.; Reif, R.L.; Bigam, D.L.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.B. The Impact of Muscle and Adipose Tissue on Long-term Survival in Patients with Stage I to III Colorectal Cancer. Dis. Colon Rectum 2019, 62, 549–560. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; Kwan, M.L.; et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef]
- Kong, J.; Diao, X.; Diao, F.; Fan, X.; Zheng, J.; Yan, D.; Huang, J.; Qin, H.; Lin, T. Causes of death in long-term bladder cancer survivors: A population-based study. Asia-Pac. J. Clin. Oncol. 2019, 15, e167–e174. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, Y.J.; Cartmell, K.B. Sarcopenia in cancer survivors is associated with increased cardiovascular disease risk. Support. Care Cancer 2018, 26, 2313–2321. [Google Scholar] [CrossRef]
| All, n = 166 | Control Group, n = 134 | Severe Sarcopenia Group, n = 32 | p Value | |
|---|---|---|---|---|
| Age, years | 73 (64–77) | 70 (63–77) | 76 (73–81) | <0.001 |
| Male | 124 (75%) | 99 (74%) | 25 (78%) | 0.620 |
| ECOG PS ≥ 2 | 3 (1.8%) | 0 (0.0%) | 3 (9.4%) | 0.007 |
| Hypertension | 71 (43%) | 57 (43%) | 14 (44%) | 0.901 |
| Diabetes mellitus | 19 (11%) | 15 (11%) | 4 (13%) | 0.765 |
| Cardiovascular disease | 40 (24%) | 31 (23%) | 9 (28%) | 0.533 |
| Chronic kidney disease | 78 (47%) | 61 (46%) | 17 (53%) | 0.439 |
| Clinical stage | ||||
| cT3 or cT4 | 74 (45%) | 58 (43%) | 16 (50%) | 0.492 |
| cN1–3 | 16 (9.6%) | 15 (11%) | 1 (3.1%) | 0.313 |
| Neoadjuvant chemotherapy | 85 (51%) | 71 (53%) | 14 (44%) | 0.348 |
| Ileal neobladder | 109 (66%) | 91 (68%) | 18 (56%) | 0.212 |
| Pathological outcomes | ||||
| Pure urothelial carcinoma | 133 (80%) | 110 (82%) | 23 (72%) | 0.193 |
| pT0 | 31 (19%) | 25 (19%) | 6 (19%) | 0.990 |
| pT3 or pT4 | 31 (19%) | 24 (18%) | 7 (22%) | 0.605 |
| pN1–3 | 11 (6.6%) | 9 (6.7%) | 2 (6.3%) | 1.000 |
| Grade 3 | 122 (74%) | 101 (75%) | 21 (66%) | 0.262 |
| Lymphovascular invasion | 50 (30%) | 42 (31%) | 8 (24%) | 0.482 |
| Positive surgical margin | 4 (2.4%) | 4 (3.0%) | 0 (0.0%) | 1.000 |
| Adjuvant chemotherapy | 19 (11%) | 14 (10%) | 5 (16%) | 0.372 |
| Follow-up period, months | 95 (66–135) | 100 (71–137) | 70 (49–125) |
| Factor | p Value | Hazard Ratio | 95% CI | |
|---|---|---|---|---|
| Age | Continuous | <0.001 | 1.110 | 1.069–1.154 |
| Sex | Male | 0.961 | 1.016 | 0.546–1.891 |
| ECOG PS | ≥2 | 0.001 | 8.127 | 2.490–26.52 |
| Hypertension | Present | 0.027 | 1.795 | 1.069–3.011 |
| Diabetes mellitus | Present | 0.891 | 0.942 | 0.404–2.198 |
| History of cardiovascular disease | Positive | 0.289 | 1.376 | 0.763–2.481 |
| Chronic kidney disease | Present | <0.001 | 3.385 | 1.864–6.148 |
| Neoadjuvant chemotherapy | Received | 0.751 | 0.915 | 0.529–1.582 |
| Histology | Pure UC | 0.423 | 0.781 | 0.428–1.428 |
| Pathological T stage | pT0 | 0.613 | 1.188 | 0.610–2.313 |
| Pathological T stage | pT3 or pT4 | 0.619 | 0.840 | 0.424–1.666 |
| Pathological N stage | pN1–3 | 0.952 | 0.969 | 0.348–2.696 |
| Tumor grade | Grade 3 | 0.290 | 0.741 | 0.424–1.292 |
| Lymphovascular invasion | Positive | 0.574 | 0.847 | 0.475–1.511 |
| Adjuvant chemotherapy | Received | 0.113 | 0.470 | 0.185–1.195 |
| Psoas muscle index | Lower than cut-off values | 0.002 | 2.329 | 1.378–3.936 |
| Intramuscular adipose tissue content | Higher than cut-off values | 0.011 | 2.000 | 1.170–3.421 |
| Severe sarcopenia | Present | <0.001 | 3.106 | 1.815–5.316 |
| Model 1 | Factor | pValue | Hazard Ratio | 95% CI |
| Age | Continuous | <0.001 | 1.084 | 1.037–1.133 |
| ECOG PS | ≥2 | 0.066 | 3.266 | 0.923–11.56 |
| Hypertension | Present | 0.269 | 1.380 | 0.779–2.443 |
| Chronic kidney disease | Present | 0.017 | 2.156 | 1.148–4.049 |
| Psoas muscle index | Lower than cut-off values | 0.439 | 1.267 | 0.696–2.308 |
| Model 2 | Factor | pValue | Hazard Ratio | 95% CI |
| Age | Continuous | 0.001 | 1.082 | 1.035–1.132 |
| ECOG PS | ≥2 | 0.055 | 3.415 | 0.973–11.99 |
| Hypertension | Present | 0.257 | 1.395 | 0.785–2.480 |
| Chronic kidney disease | Present | 0.016 | 2.178 | 1.156–4.103 |
| Intramuscular adipose tissue content | Higher than cut-off values | 0.367 | 1.377 | 0.688–2.757 |
| Model 3 | Factor | pValue | Hazard Ratio | 95% CI |
| Age | Continuous | <0.001 | 1.080 | 1.035–1.127 |
| ECOG PS | ≥2 | 0.193 | 2.365 | 0.647–8.643 |
| Hypertension | Present | 0.184 | 1.475 | 0.831–2.619 |
| Chronic kidney disease | Present | 0.014 | 2.195 | 1.175–4.100 |
| Severe sarcopenia | Present | 0.047 | 1.909 | 1.007–3.619 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, N.; Momota, M.; Horiguchi, H.; Hamano, I.; Mikami, J.; Hatakeyama, S.; Ito, H.; Yoneyama, T.; Hashimoto, Y.; Nishimura, S.; et al. Combination of Muscle Quantity and Quality Is Useful to Assess the Necessity of Surveillance after a 5-Year Cancer-Free Period in Patients Who Undergo Radical Cystectomy: A Multi-Institutional Retrospective Study. Cancers 2023, 15, 1489. https://doi.org/10.3390/cancers15051489
Fujita N, Momota M, Horiguchi H, Hamano I, Mikami J, Hatakeyama S, Ito H, Yoneyama T, Hashimoto Y, Nishimura S, et al. Combination of Muscle Quantity and Quality Is Useful to Assess the Necessity of Surveillance after a 5-Year Cancer-Free Period in Patients Who Undergo Radical Cystectomy: A Multi-Institutional Retrospective Study. Cancers. 2023; 15(5):1489. https://doi.org/10.3390/cancers15051489
Chicago/Turabian StyleFujita, Naoki, Masaki Momota, Hirotaka Horiguchi, Itsuto Hamano, Jotaro Mikami, Shingo Hatakeyama, Hiroyuki Ito, Takahiro Yoneyama, Yasuhiro Hashimoto, Shoji Nishimura, and et al. 2023. "Combination of Muscle Quantity and Quality Is Useful to Assess the Necessity of Surveillance after a 5-Year Cancer-Free Period in Patients Who Undergo Radical Cystectomy: A Multi-Institutional Retrospective Study" Cancers 15, no. 5: 1489. https://doi.org/10.3390/cancers15051489
APA StyleFujita, N., Momota, M., Horiguchi, H., Hamano, I., Mikami, J., Hatakeyama, S., Ito, H., Yoneyama, T., Hashimoto, Y., Nishimura, S., Yoshikawa, K., & Ohyama, C. (2023). Combination of Muscle Quantity and Quality Is Useful to Assess the Necessity of Surveillance after a 5-Year Cancer-Free Period in Patients Who Undergo Radical Cystectomy: A Multi-Institutional Retrospective Study. Cancers, 15(5), 1489. https://doi.org/10.3390/cancers15051489






