Predictive and Prognostic Value of Oncogene Mutations and Microsatellite Instability in Locally-Advanced Rectal Cancer Treated with Neoadjuvant Radiation-Based Therapy: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Outcomes
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Eligible Studies
3.2. KRAS
3.2.1. Pathological Complete Response
3.2.2. Downstaging
3.2.3. Recurrence Risk
3.2.4. Overall Survival
3.3. MSI Status
3.3.1. Pathological Complete Response
3.3.2. Downstaging
3.3.3. Recurrence Risk
3.3.4. Overall Survival
3.4. TP53, BRAF, PIK3CA, and SMAD4
3.4.1. Pathological Complete Response
3.4.2. Downstaging
3.4.3. Recurrence Risk
3.4.4. Overall Survival
3.5. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.P.; Ngan, S.Y.; Leong, T. Current State of Neoadjuvant Radiotherapy for Rectal Cancer. Clin. Color. Cancer 2022, 21, 63–70. [Google Scholar] [CrossRef]
- Roeder, F.; Meldolesi, E.; Gerum, S.; Valentini, V.; Rodel, C. Recent advances in (chemo-)radiation therapy for rectal cancer: A comprehensive review. Radiat. Oncol. 2020, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.N.; Kim, H.J. Total neoadjuvant therapy in locally advanced rectal cancer: Role of systemic chemotherapy. Ann. Gastroenterol. Surg. 2019, 3, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Belluco, C.; De Paoli, A.; Canzonieri, V.; Sigon, R.; Fornasarig, M.; Buonadonna, A.; Boz, G.; Innocente, R.; Perin, T.; Cossaro, M.; et al. Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: Implications for local excision surgical strategies. Ann. Surg. Oncol. 2011, 18, 3686–3693. [Google Scholar] [CrossRef]
- Valentini, V.; van Stiphout, R.G.; Lammering, G.; Gambacorta, M.A.; Barba, M.C.; Bebenek, M.; Bonnetain, F.; Bosset, J.F.; Bujko, K.; Cionini, L.; et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J. Clin. Oncol. 2011, 29, 3163–3172. [Google Scholar] [CrossRef]
- Barina, A.; De Paoli, A.; Delrio, P.; Guerrieri, M.; Muratore, A.; Bianco, F.; Vespa, D.; Asteria, C.; Morpurgo, E.; Restivo, A.; et al. Rectal sparing approach after preoperative radio- and/or chemotherapy (RESARCH) in patients with rectal cancer: A multicentre observational study. Technol. Coloproctology 2017, 21, 633–640. [Google Scholar] [CrossRef]
- Capelli, G.; De Simone, I.; Spolverato, G.; Cinquini, M.; Moschetti, I.; Lonardi, S.; Masi, G.; Carlomagno, C.; Corsi, D.; Luppi, G.; et al. Non-Operative Management Versus Total Mesorectal Excision for Locally Advanced Rectal Cancer with Clinical Complete Response After Neoadjuvant Chemoradiotherapy: A GRADE Approach by the Rectal Cancer Guidelines Writing Group of the Italian Association of Medical Oncology (AIOM). J. Gastrointest. Surg. 2020, 24, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, T.; Xiao, L.; Yang, S.; Liu, Q.; Gao, Y.; Chen, G.; Xiao, W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1555–e1566. [Google Scholar] [CrossRef]
- Bitterman, D.S.; Resende Salgado, L.; Moore, H.G.; Sanfilippo, N.J.; Gu, P.; Hatzaras, I.; Du, K.L. Predictors of Complete Response and Disease Recurrence Following Chemoradiation for Rectal Cancer. Front. Oncol. 2015, 5, 286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, M.; Chen, D.; Li, P.; Tang, B.; Li, J. Role of MRIbased radiomics in locally advanced rectal cancer (Review). Oncol. Rep. 2022, 47, 34. [Google Scholar] [CrossRef] [PubMed]
- Boukouris, A.E.; Theochari, M.; Stefanou, D.; Papalambros, A.; Felekouras, E.; Gogas, H.; Ziogas, D.C. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit. Rev. Oncol. Hematol. 2022, 173, 103663. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Vitiello, P.P.; Marsoni, S.; Siena, S.; Tabernero, J.; Trusolino, L.; Bernards, R.; Bardelli, A. Precision oncology in metastatic colorectal cancer—From biology to medicine. Nat. Rev. Clin. Oncol. 2021, 18, 506–525. [Google Scholar] [CrossRef]
- Chatila, W.K.; Kim, J.K.; Walch, H.; Marco, M.R.; Chen, C.T.; Wu, F.; Omer, D.M.; Khalil, D.N.; Ganesh, K.; Qu, X.; et al. Genomic and transcriptomic determinants of response to neoadjuvant therapy in rectal cancer. Nat. Med. 2022, 28, 1646–1655. [Google Scholar] [CrossRef]
- Frattini, M.; Balestra, D.; Suardi, S.; Oggionni, M.; Alberici, P.; Radice, P.; Costa, A.; Daidone, M.G.; Leo, E.; Pilotti, S.; et al. Different genetic features associated with colon and rectal carcinogenesis. Clin. Cancer Res. 2004, 10, 4015–4021. [Google Scholar] [CrossRef] [PubMed]
- Kapiteijn, E.; Liefers, G.J.; Los, L.C.; Kranenbarg, E.K.; Hermans, J.; Tollenaar, R.A.; Moriya, Y.; van de Velde, C.J.; van Krieken, J.H. Mechanisms of oncogenesis in colon versus rectal cancer. J. Pathol. 2001, 195, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Iseas, S.; Sendoya, J.M.; Robbio, J.; Coraglio, M.; Kujaruk, M.; Mikolaitis, V.; Rizzolo, M.; Cabanne, A.; Ruiz, G.; Salanova, R.; et al. Prognostic Impact of An Integrative Landscape of Clinical, Immune, and Molecular Features in Non-Metastatic Rectal Cancer. Front. Oncol. 2021, 11, 801880. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Roncato, R.; Palazzari, E.; Toffoli, G.; Cecchin, E. Germline and Somatic Pharmacogenomics to Refine Rectal Cancer Patients Selection for Neo-Adjuvant Chemoradiotherapy. Front. Pharmacol. 2020, 11, 897. [Google Scholar] [CrossRef]
- O’Connell, E.; Reynolds, I.S.; McNamara, D.A.; Prehn, J.H.M.; Burke, J.P. Microsatellite instability and response to neoadjuvant chemoradiotherapy in rectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2020, 34, 57–62. [Google Scholar] [CrossRef]
- Mace, A.G.; Pai, R.K.; Stocchi, L.; Kalady, M.F. American Joint Committee on Cancer and College of American Pathologists regression grade: A new prognostic factor in rectal cancer. Dis. Colon Rectum 2015, 58, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Yasutomi, M.; Baba, S.; Hojo, K.; Kato, Y.; Kodaira, S.; Koyama, Y. Japanese Classification of Colorectal Carcinoma, 1st ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 1997. [Google Scholar]
- Gavioli, M.; Bagni, A.; Piccagli, I.; Fundaro, S.; Natalini, G. Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer: Comparison between sonographic and histopathologic changes. Dis. Colon Rectum 2000, 43, 1075–1083. [Google Scholar] [CrossRef]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Color. Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.M.; Dodds, E.; Warren, B.F.; Cunningham, C.; George, B.D.; Jones, A.C.; Mortensen, N.J. Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: Correlation with rectal cancer regression grade. Dis. Colon Rectum 2004, 47, 2025–2031. [Google Scholar] [CrossRef]
- Ryan, R.; Gibbons, D.; Hyland, J.M.; Treanor, D.; White, A.; Mulcahy, H.E.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef]
- The Newcastle-Ottawa Scale (NOS). Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 October 2022).
- Normand, S.L. Meta-analysis: Formulating, evaluating, combining, and reporting. Stat. Med. 1999, 18, 321–359. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- El Otmani, I.; El Agy, F.; El Baradai, S.; Bouguenouch, L.; Lahmidani, N.; El Abkari, M.; Benajah, D.A.; Toughrai, I.; El Bouhaddouti, H.; Mouaqit, O.; et al. Analysis of Molecular Pretreated Tumor Profiles as Predictive Biomarkers of Therapeutic Response and Survival Outcomes after Neoadjuvant Therapy for Rectal Cancer in Moroccan Population. Dis. Markers 2020, 2020, 8459303. [Google Scholar] [CrossRef]
- Peng, J.; Lin, J.; Qiu, M.; Zhao, Y.; Deng, Y.; Shao, J.; Ding, P.; Zhang, H.; Wan, D.; Lu, Z.; et al. Oncogene mutation profile predicts tumor regression and survival in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy and radical surgery. Tumor Biol. 2017, 39, 1010428317709638. [Google Scholar] [CrossRef]
- Bengala, C.; Bettelli, S.; Bertolini, F.; Sartori, G.; Fontana, A.; Malavasi, N.; Depenni, R.; Zironi, S.; Del Giovane, C.; Luppi, G.; et al. Prognostic role of EGFR gene copy number and KRAS mutation in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy. Br. J. Cancer 2010, 103, 1019–1024. [Google Scholar] [CrossRef]
- Chow, O.S.; Kuk, D.; Keskin, M.; Smith, J.J.; Camacho, N.; Pelossof, R.; Chen, C.T.; Chen, Z.; Avila, K.; Weiser, M.R.; et al. KRAS and Combined KRAS/TP53 Mutations in Locally Advanced Rectal Cancer are Independently Associated with Decreased Response to Neoadjuvant Therapy. Ann. Surg. Oncol. 2016, 23, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Duldulao, M.P.; Lee, W.; Nelson, R.A.; Li, W.; Chen, Z.; Kim, J.; Garcia-Aguilar, J. Mutations in specific codons of the KRAS oncogene are associated with variable resistance to neoadjuvant chemoradiation therapy in patients with rectal adenocarcinoma. Ann. Surg. Oncol. 2013, 20, 2166–2171. [Google Scholar] [CrossRef]
- Erben, P.; Strobel, P.; Horisberger, K.; Popa, J.; Bohn, B.; Hanfstein, B.; Kahler, G.; Kienle, P.; Post, S.; Wenz, F.; et al. KRAS and BRAF mutations and PTEN expression do not predict efficacy of cetuximab-based chemoradiotherapy in locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1032–1038. [Google Scholar] [CrossRef]
- Gaedcke, J.; Grade, M.; Jung, K.; Schirmer, M.; Jo, P.; Obermeyer, C.; Wolff, H.A.; Herrmann, M.K.; Beissbarth, T.; Becker, H.; et al. KRAS and BRAF mutations in patients with rectal cancer treated with preoperative chemoradiotherapy. Radiother. Oncol. 2010, 94, 76–81. [Google Scholar] [CrossRef]
- Hu-Lieskovan, S.; Vallbohmer, D.; Zhang, W.; Yang, D.; Pohl, A.; Labonte, M.J.; Grimminger, P.P.; Holscher, A.H.; Semrau, R.; Arnold, D.; et al. EGF61 polymorphism predicts complete pathologic response to cetuximab-based chemoradiation independent of KRAS status in locally advanced rectal cancer patients. Clin. Cancer Res. 2011, 17, 5161–5169. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hong, Y.S.; Kim, D.Y.; Kim, T.W.; Kim, J.H.; Im, S.A.; Lee, K.S.; Yun, T.; Jeong, S.Y.; Choi, H.S.; et al. Preoperative chemoradiation with cetuximab, irinotecan, and capecitabine in patients with locally advanced resectable rectal cancer: A multicenter Phase II study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.L.; Li, B.; Ye, Q.F. Effect of neoadjuvant cetuximab, capecitabine, and radiotherapy for locally advanced rectal cancer: Results of a phase II study. Int. J. Color. Dis. 2012, 27, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Zauber, N.P.; Marotta, S.P.; Berman, E.; Grann, A.; Rao, M.; Komati, N.; Ribiero, K.; Bishop, D.T. Molecular genetic changes associated with colorectal carcinogenesis are not prognostic for tumor regression following preoperative chemoradiation of rectal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 472–476. [Google Scholar] [CrossRef]
- Zhou, P.; Goffredo, P.; Ginader, T.; Thompson, D.; Hrabe, J.; Gribovskaja-Rupp, I.; Kapadia, M.; Hassan, I. Impact of KRAS status on tumor response and survival after neoadjuvant treatment of locally advanced rectal cancer. J. Surg. Oncol. 2021, 123, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, J.H.; Shim, B.Y.; Kim, S.H.; Chung, M.J.; Kye, B.H.; Kim, H.J.; Cho, H.M.; Jang, H.S. KRAS Mutation Status Is Not a Predictor for Tumor Response and Survival in Rectal Cancer Patients Who Received Preoperative Radiotherapy With 5-Fluoropyrimidine Followed by Curative Surgery. Medicine 2015, 94, e1284. [Google Scholar] [CrossRef]
- Krajnovic, M.; Markovic, B.; Knezevic-Usaj, S.; Nikolic, I.; Stanojevic, M.; Nikolic, V.; Siljic, M.; Jovanovic Cupic, S.; Dimitrijevic, B. Locally advanced rectal cancers with simultaneous occurrence of KRAS mutation and high VEGF expression show invasive characteristics. Pathol. Res. Pract. 2016, 212, 598–603. [Google Scholar] [CrossRef]
- Velenik, V.; Ocvirk, J.; Oblak, I.; Anderluh, F. Cetuximab in preoperative treatment of rectal cancer—Term outcome of the XERT trial. Radiol. Oncol. 2012, 46, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Asawa, P.; Bakalov, V.; Kancharla, P.; Abel, S.; Chahine, Z.; Monga, D.K.; Kirichenko, A.V.; Wegner, R.E. The prognostic value of KRAS mutation in locally advanced rectal cancer. Int. J. Color. Dis. 2022, 37, 1199–1207. [Google Scholar] [CrossRef]
- Du, C.; Zhao, J.; Xue, W.; Dou, F.; Gu, J. Prognostic value of microsatellite instability in sporadic locally advanced rectal cancer following neoadjuvant radiotherapy. Histopathology 2013, 62, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, H.; Wang, C.; Zhang, J.; Cai, Y.; Xie, X.; Huang, Y.; Deng, Y. The prognostic and predictive value of mismatch repair status in patients with locally advanced rectal cancer following neoadjuvant therapy. Ann. Transl. Med. 2022, 10, 491. [Google Scholar] [CrossRef]
- Yilmaz Rakici, S.; Bedir, R.; Hatipoglu, C. Are there predictors that can determine neoadjuvant treatment responses in rectal cancer? Turk. J. Gastroenterol. 2019, 30, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Zhou, Y.; Ye, Z.; Xiong, J.; Lan, H.; Wang, F. Tumor-Infiltrating Lymphocytes in Colorectal Cancer: The Fundamental Indication and Application on Immunotherapy. Front. Immunol. 2021, 12, 808964. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Renz, P.; Wegner, R.E.; Finley, G.; Raj, M.; Monga, D.; McCormick, J.; Kirichenko, A. Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: A National Cancer Database (NCDB) Analysis. Ann. Surg. 2020, 271, 716–723. [Google Scholar] [CrossRef]
- Bando, H.; Tsukada, Y.; Inamori, K.; Togashi, Y.; Koyama, S.; Kotani, D.; Fukuoka, S.; Yuki, S.; Komatsu, Y.; Homma, S.; et al. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin. Cancer Res. 2022, 28, 1136–1146. [Google Scholar] [CrossRef]
- Lopez-Crapez, E.; Bibeau, F.; Thezenas, S.; Ychou, M.; Simony-Lafontaine, J.; Thirion, A.; Azria, D.; Grenier, J.; Senesse, P. p53 status and response to radiotherapy in rectal cancer: A prospective multilevel analysis. Br. J. Cancer 2005, 92, 2114–2121. [Google Scholar] [CrossRef]
- Abdul-Jalil, K.I.; Sheehan, K.M.; Toomey, S.; Schmid, J.; Prehn, J.; O’Grady, A.; Cummins, R.; O’Neill, B.; McNamara, D.A.; Deasy, J.; et al. The frequencies and clinical implications of mutations in 33 kinase-related genes in locally advanced rectal cancer: A pilot study. Ann. Surg. Oncol. 2014, 21, 2642–2649. [Google Scholar] [CrossRef]
- Russo, A.L.; Ryan, D.P.; Borger, D.R.; Wo, J.Y.; Szymonifka, J.; Liang, W.Y.; Kwak, E.L.; Blaszkowsky, L.S.; Clark, J.W.; Allen, J.N.; et al. Mutational and clinical predictors of pathologic complete response in the treatment of locally advanced rectal cancer. J. Gastrointest. Cancer 2014, 45, 34–39. [Google Scholar] [CrossRef]
- Kandioler, D.; Zwrtek, R.; Ludwig, C.; Janschek, E.; Ploner, M.; Hofbauer, F.; Kuhrer, I.; Kappel, S.; Wrba, F.; Horvath, M.; et al. TP53 genotype but not p53 immunohistochemical result predicts response to preoperative short-term radiotherapy in rectal cancer. Ann. Surg. 2002, 235, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Bignucolo, A.; Scarabel, L.; Toffoli, G.; Cecchin, E.; De Mattia, E. Predicting drug response and toxicity in metastatic colorectal cancer: The role of germline markers. Expert Rev. Clin. Pharmacol. 2022, 15, 689–713. [Google Scholar] [CrossRef]
- Bignucolo, A.; De Mattia, E.; Cecchin, E.; Roncato, R.; Toffoli, G. Pharmacogenomics of Targeted Agents for Personalization of Colorectal Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 1522. [Google Scholar] [CrossRef]
- Bernhard, E.J.; Stanbridge, E.J.; Gupta, S.; Gupta, A.K.; Soto, D.; Bakanauskas, V.J.; Cerniglia, G.J.; Muschel, R.J.; McKenna, W.G. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000, 60, 6597–6600. [Google Scholar]
- Jones, H.A.; Hahn, S.M.; Bernhard, E.; McKenna, W.G. Ras inhibitors and radiation therapy. Semin. Radiat. Oncol. 2001, 11, 328–337. [Google Scholar] [CrossRef]
- McKenna, W.G.; Weiss, M.C.; Bakanauskas, V.J.; Sandler, H.; Kelsten, M.L.; Biaglow, J.; Tuttle, S.W.; Endlich, B.; Ling, C.C.; Muschel, R.J. The role of the H-ras oncogene in radiation resistance and metastasis. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.; Burke, J.P.; Coffey, J.C. KRAS mutation does not predict the efficacy of neo-adjuvant chemoradiotherapy in rectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2013, 22, 105–111. [Google Scholar] [CrossRef]
- Peng, J.; Lv, J.; Peng, J. KRAS mutation is predictive for poor prognosis in rectal cancer patients with neoadjuvant chemoradiotherapy: A systemic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Swets, M.; Graham Martinez, C.; van Vliet, S.; van Tilburg, A.; Gelderblom, H.; Marijnen, C.A.M.; van de Velde, C.J.H.; Nagtegaal, I.D. Microsatellite instability in rectal cancer: What does it mean? A study of two randomized trials and a systematic review of the literature. Histopathology 2022, 81, 352–362. [Google Scholar] [CrossRef]
- Cercek, A.; Dos Santos Fernandes, G.; Roxburgh, C.S.; Ganesh, K.; Ng, S.; Sanchez-Vega, F.; Yaeger, R.; Segal, N.H.; Reidy-Lagunes, D.L.; Varghese, A.M.; et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin. Cancer Res. 2020, 26, 3271–3279. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X. Effects of molecular markers on the treatment decision and prognosis of colorectal cancer: A narrative review. J. Gastrointest. Oncol. 2021, 12, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Min, B.S.; Kim, T.I.; Cheon, J.H.; Kim, N.K.; Kim, H.; Kim, W.H. The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. Eur. J. Cancer 2012, 48, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Canzonieri, V.; Polesel, J.; Mezzalira, S.; Dalle Fratte, C.; Dreussi, E.; Roncato, R.; Bignucolo, A.; Innocente, R.; Belluco, C.; et al. SMAD3 Host and Tumor Profiling to Identify Locally Advanced Rectal Cancer Patients at High Risk of Poor Response to Neoadjuvant Chemoradiotherapy. Front. Pharmacol. 2021, 12, 778781. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Polesel, J.; Roncato, R.; Labriet, A.; Bignucolo, A.; Gagno, S.; Buonadonna, A.; D’Andrea, M.; Levesque, E.; Jonker, D.; et al. IL15RA and SMAD3 Genetic Variants Predict Overall Survival in Metastatic Colorectal Cancer Patients Treated with FOLFIRI Therapy: A New Paradigm. Cancers 2021, 13, 1705. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.W.; Wilson-Van Patten, C.; Meyers, M.; Kunugi, K.A.; Cuthill, S.; Reznikoff, C.; Garces, C.; Boland, C.R.; Kinsella, T.J.; Fishel, R.; et al. Defective expression of the DNA mismatch repair protein, MLH1, alters G2-M cell cycle checkpoint arrest following ionizing radiation. Cancer Res. 1998, 58, 767–778. [Google Scholar]
- Franchitto, A.; Pichierri, P.; Piergentili, R.; Crescenzi, M.; Bignami, M.; Palitti, F. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene 2003, 22, 2110–2120. [Google Scholar] [CrossRef]
- Meillan, N.; Vernerey, D.; Lefevre, J.H.; Manceau, G.; Svrcek, M.; Augustin, J.; Flejou, J.F.; Lascols, O.; Simon, J.M.; Cohen, R.; et al. Mismatch Repair System Deficiency Is Associated With Response to Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 824–833. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, B.H.; Song, C.; Kang, S.B.; Lee, H.S.; Lee, K.W.; Chie, E.K.; Kim, J.S. Microsatellite Instability Correlated Inflammatory Markers and their Prognostic Value in the Rectal Cancer Following Neoadjuvant Chemoradiotherapy: A Hypothesis-generating Study. In Vivo 2020, 34, 2119–2126. [Google Scholar] [CrossRef]
- Ni, K.; Zhan, Y.; Liu, Z.; Zhao, X.Z.; Wang, W.; Wang, G.; Zhang, Z.; Li, G.; Zhang, X.; Zhang, C. Mismatch repair system deficiency is associated with chemoradiotherapy resistance in locally advanced rectal adenocarcinoma patients. J. Surg. Oncol. 2022, 125, 692–702. [Google Scholar] [CrossRef]
- Sclafani, F.; Wilson, S.H.; Cunningham, D.; Gonzalez De Castro, D.; Kalaitzaki, E.; Begum, R.; Wotherspoon, A.; Capdevila, J.; Glimelius, B.; Rosello, S.; et al. Analysis of KRAS, NRAS, BRAF, PIK3CA and TP53 mutations in a large prospective series of locally advanced rectal cancer patients. Int. J. Cancer 2020, 146, 94–102. [Google Scholar] [CrossRef] [PubMed]
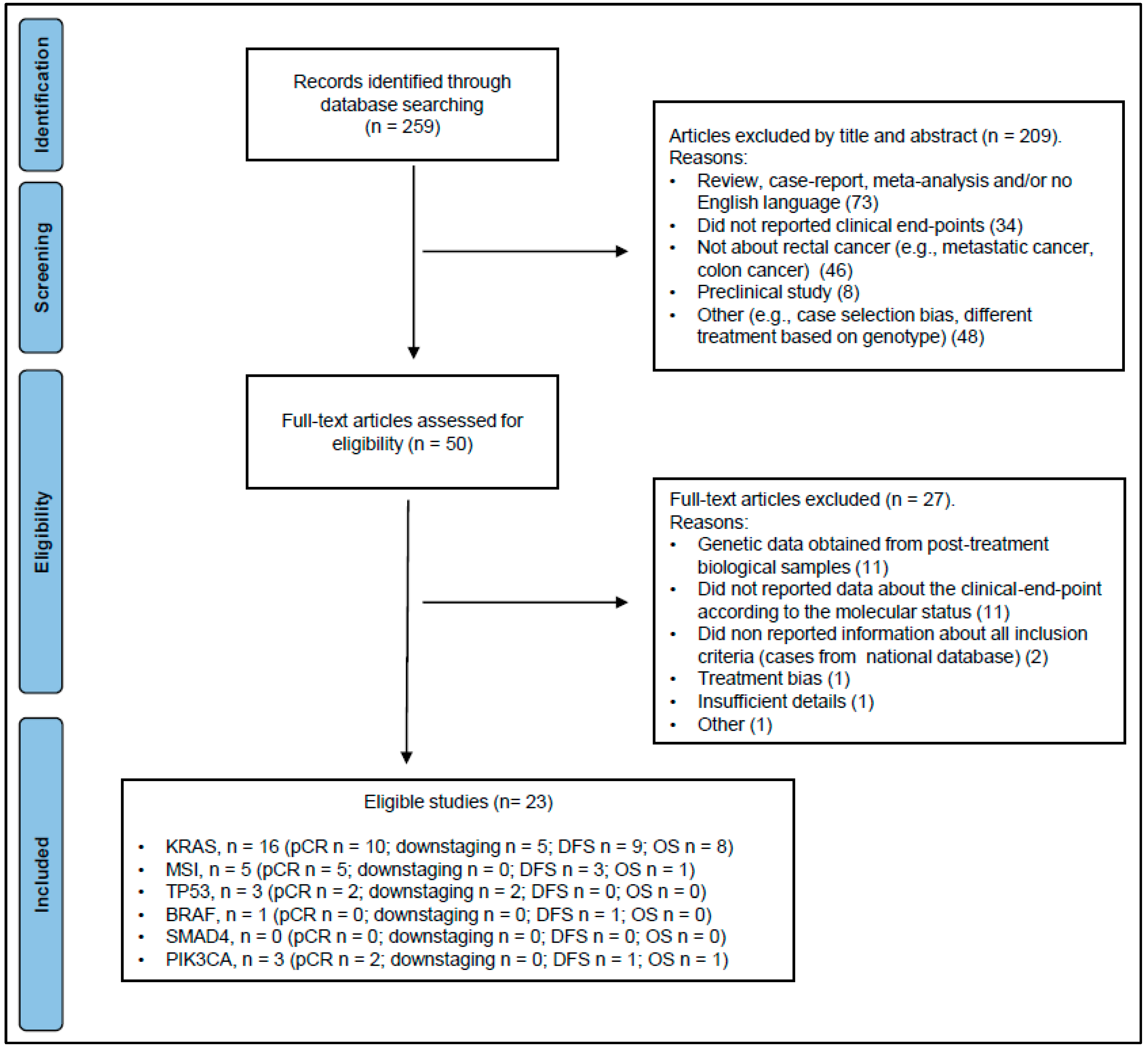
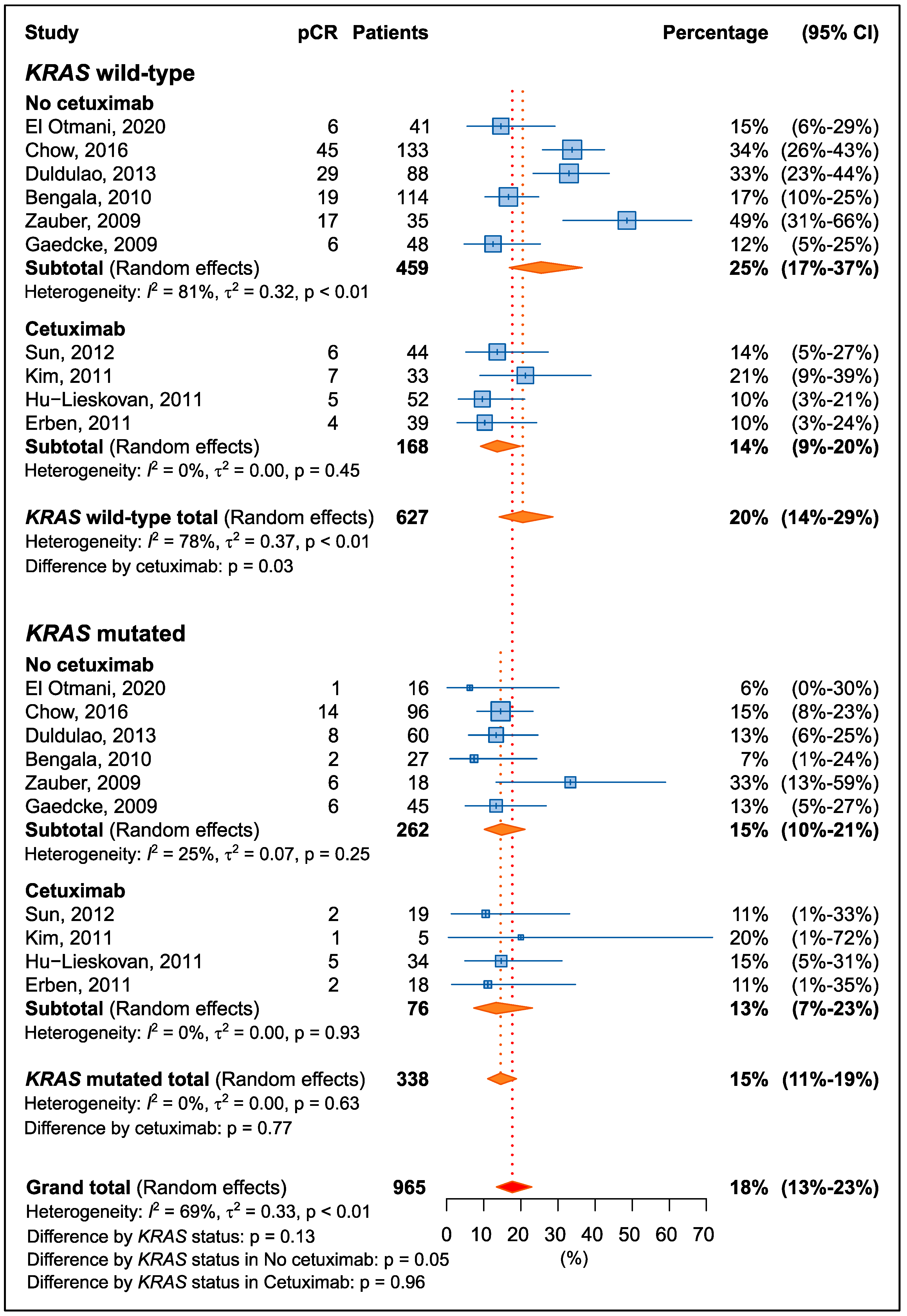
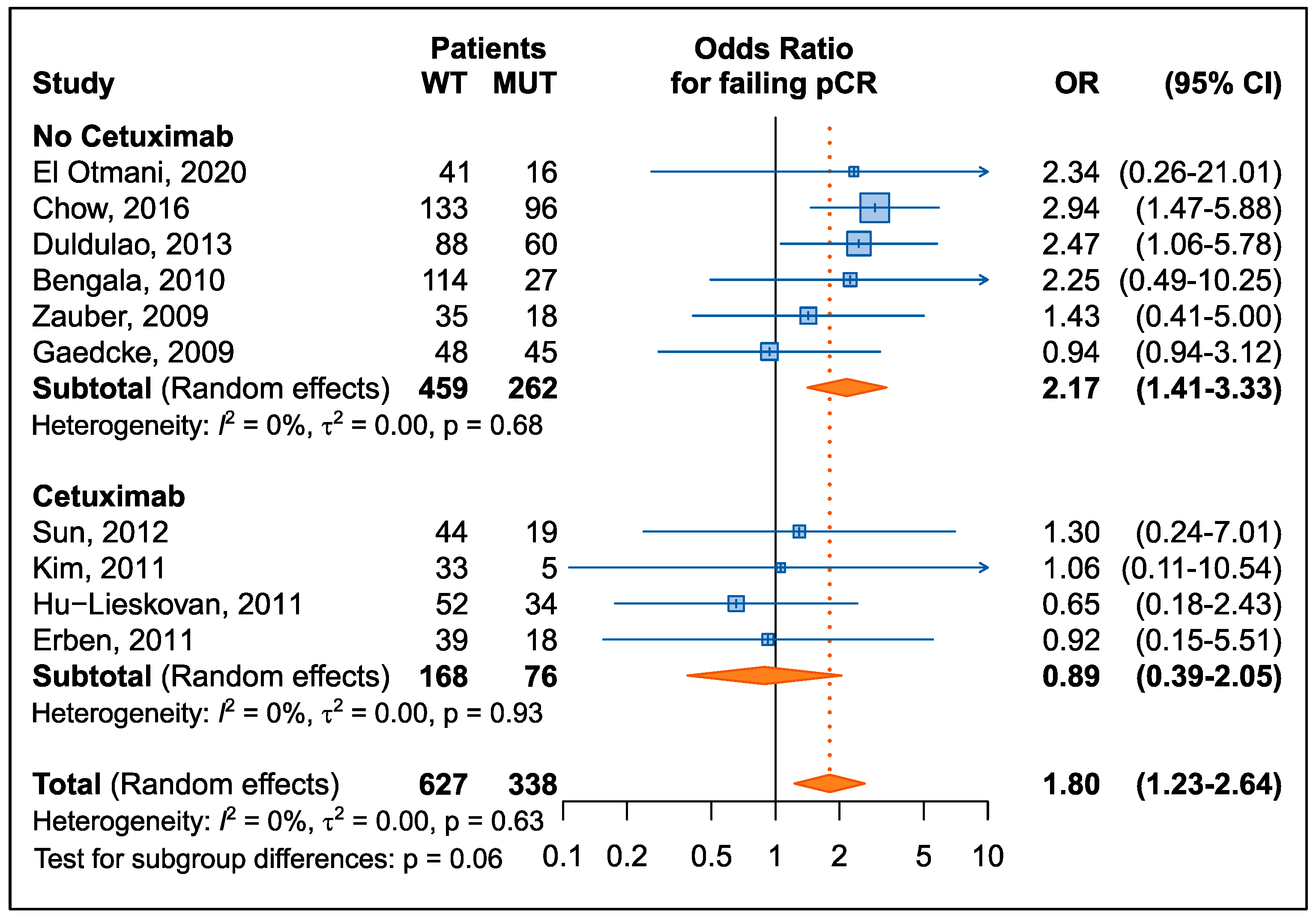
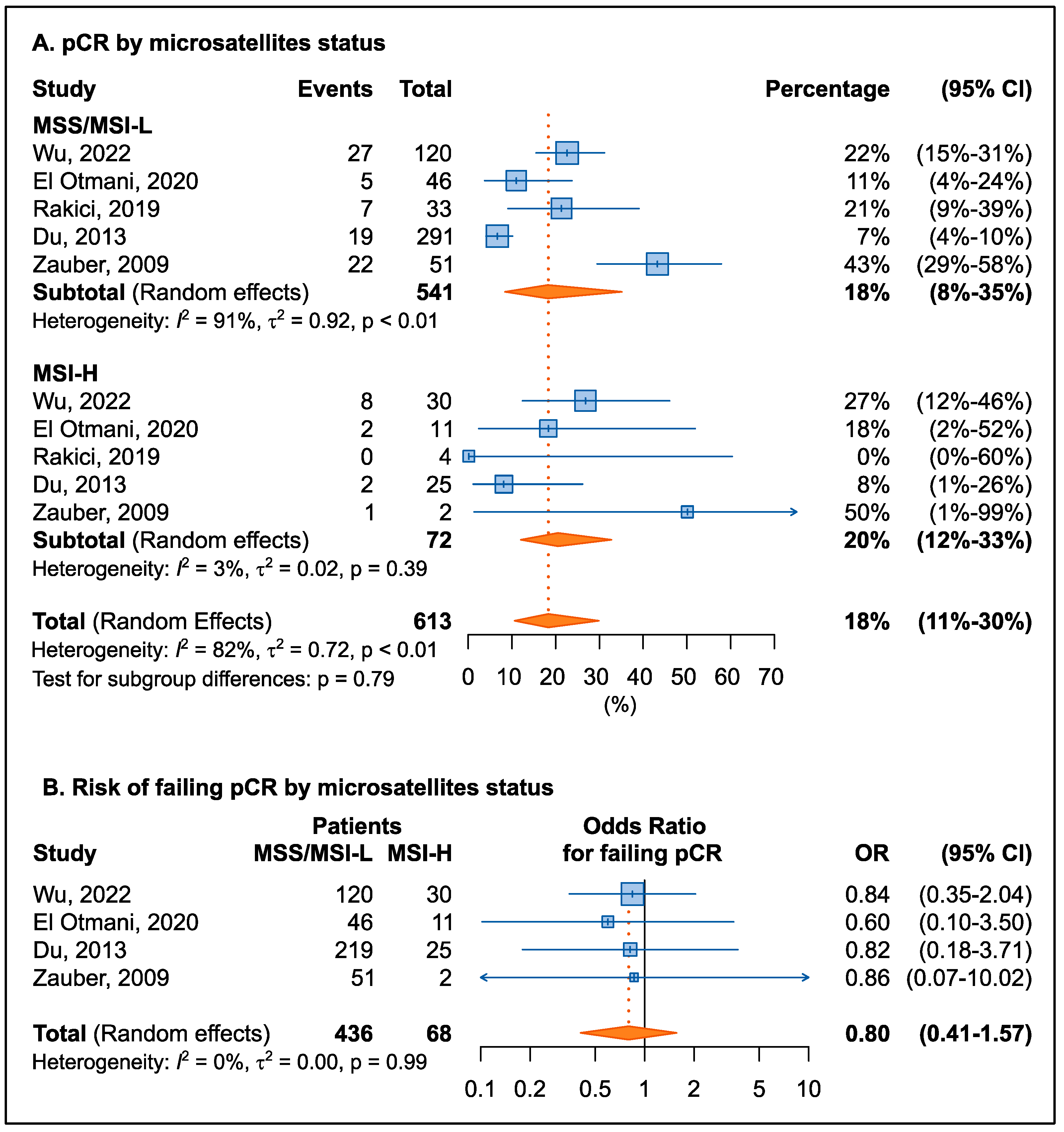
| First Author, Year | Country | N | Therapy Strategy | FLs | Other Drug | KRAS Mut (%) | pCR (%) | NOS Score |
|---|---|---|---|---|---|---|---|---|
| El Otmani, 2020 [29] | Morocco | 57 | CRT/RT + surgery | 5-FU | - | 28% | 12% | 7 |
| Chow, 2016 [32] | USA | 229 | CRT/intensified CRT + surgery | 5-FU | OXA | 42% | 26% | 7 |
| Duldulao, 2013 [33] | USA | 148 | CRT/intensified CRT + surgery | 5-FU | OXA | 41% | 25% | 7 |
| Sun, 2012 [38] | China | 63 | CRT + surgery | CAPE | CTX | 30% | 13% | 7 |
| Kim, 2011 [37] | Korea | 38 | CRT + surgery | CAPE | CTX, IRI | 13% | 21% | 7 |
| Hu-Lieskovan, 2011 [36] | Europe | 86 | CRT + surgery | 5-FU, CAPE | CTX, OXA | 40% | 12% | 7 |
| Erben, 2011 [34] | Europe | 57 | intensified CRT + surgery | CAPE | CTX, IRI | 32% | 11% | 7 |
| Bengala, 2010 [31] | Europe | 141 | CRT + surgery | 5-FU, CAPE | OXA | 19% | 15% | 7 |
| Zauber, 2009 [39] | Europe | 53 | CRT/RT + surgery | 5-FU | -- | 34% | 43% | 7 |
| Gaedcke, 2010 [35] | Europe | 93 | CRT + surgery | 5-FU | OXA | 48% | 13% | 7 |
| Downstaging | T Downstaging | N Downstaging | ||||
|---|---|---|---|---|---|---|
| Rate (95% CI) | PHet | Rate (95% CI) | PHet | Rate (95% CI) | PHet | |
| Studies (n) | 4 | 3 | 1 | |||
| KRAS | ||||||
| Wild-type | 0.52 (0.27–0.77) | p < 0.01 | 0.54 (0.46–0.61) | p = 0.48 | 0.61 (0.49–0.72) | - |
| Mutated | 0.55 (0.37–0.71) | p = 0.02 | 0.44 (0.34–0.54) | p = 0.62 | 0.62 (0.41–0.80) | - |
| p = 0.87 | p = 0.14 | p = 1.00 | ||||
| First Author, Year | Country | N | Therapy Strategy | FLs | Other Drug | MSI-H (%) | pCR (%) | NOS Score |
|---|---|---|---|---|---|---|---|---|
| Wu, 2022 [46] | China | 150 | CRT + surgery | 5-FU | OXA | 20% | 23% | 7 |
| El Otmani, 2020 [29] | Morocco | 57 | CRT/RT + surgery | 5-FU | -- | 19% | 12% | 7 |
| Yilmaz Rakici, 2019 [47] | Turkey | 37 | CRT/RT + surgery | 5-FU, CAPE | -- | 11% | 19% | 9 |
| Du, 2013 [45] | China | 316 | RT + surgery | -- | -- | 8% | 7% | 7 |
| Zauber, 2009 [39] | USA | 53 | CRT/RT + surgery | 5-FU | -- | 4% | 43% | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mattia, E.; Polesel, J.; Mezzalira, S.; Palazzari, E.; Pollesel, S.; Toffoli, G.; Cecchin, E. Predictive and Prognostic Value of Oncogene Mutations and Microsatellite Instability in Locally-Advanced Rectal Cancer Treated with Neoadjuvant Radiation-Based Therapy: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1469. https://doi.org/10.3390/cancers15051469
De Mattia E, Polesel J, Mezzalira S, Palazzari E, Pollesel S, Toffoli G, Cecchin E. Predictive and Prognostic Value of Oncogene Mutations and Microsatellite Instability in Locally-Advanced Rectal Cancer Treated with Neoadjuvant Radiation-Based Therapy: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(5):1469. https://doi.org/10.3390/cancers15051469
Chicago/Turabian StyleDe Mattia, Elena, Jerry Polesel, Silvia Mezzalira, Elisa Palazzari, Sara Pollesel, Giuseppe Toffoli, and Erika Cecchin. 2023. "Predictive and Prognostic Value of Oncogene Mutations and Microsatellite Instability in Locally-Advanced Rectal Cancer Treated with Neoadjuvant Radiation-Based Therapy: A Systematic Review and Meta-Analysis" Cancers 15, no. 5: 1469. https://doi.org/10.3390/cancers15051469
APA StyleDe Mattia, E., Polesel, J., Mezzalira, S., Palazzari, E., Pollesel, S., Toffoli, G., & Cecchin, E. (2023). Predictive and Prognostic Value of Oncogene Mutations and Microsatellite Instability in Locally-Advanced Rectal Cancer Treated with Neoadjuvant Radiation-Based Therapy: A Systematic Review and Meta-Analysis. Cancers, 15(5), 1469. https://doi.org/10.3390/cancers15051469










