Prediction of Gastrointestinal Tract Cancers Using Longitudinal Electronic Health Record Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
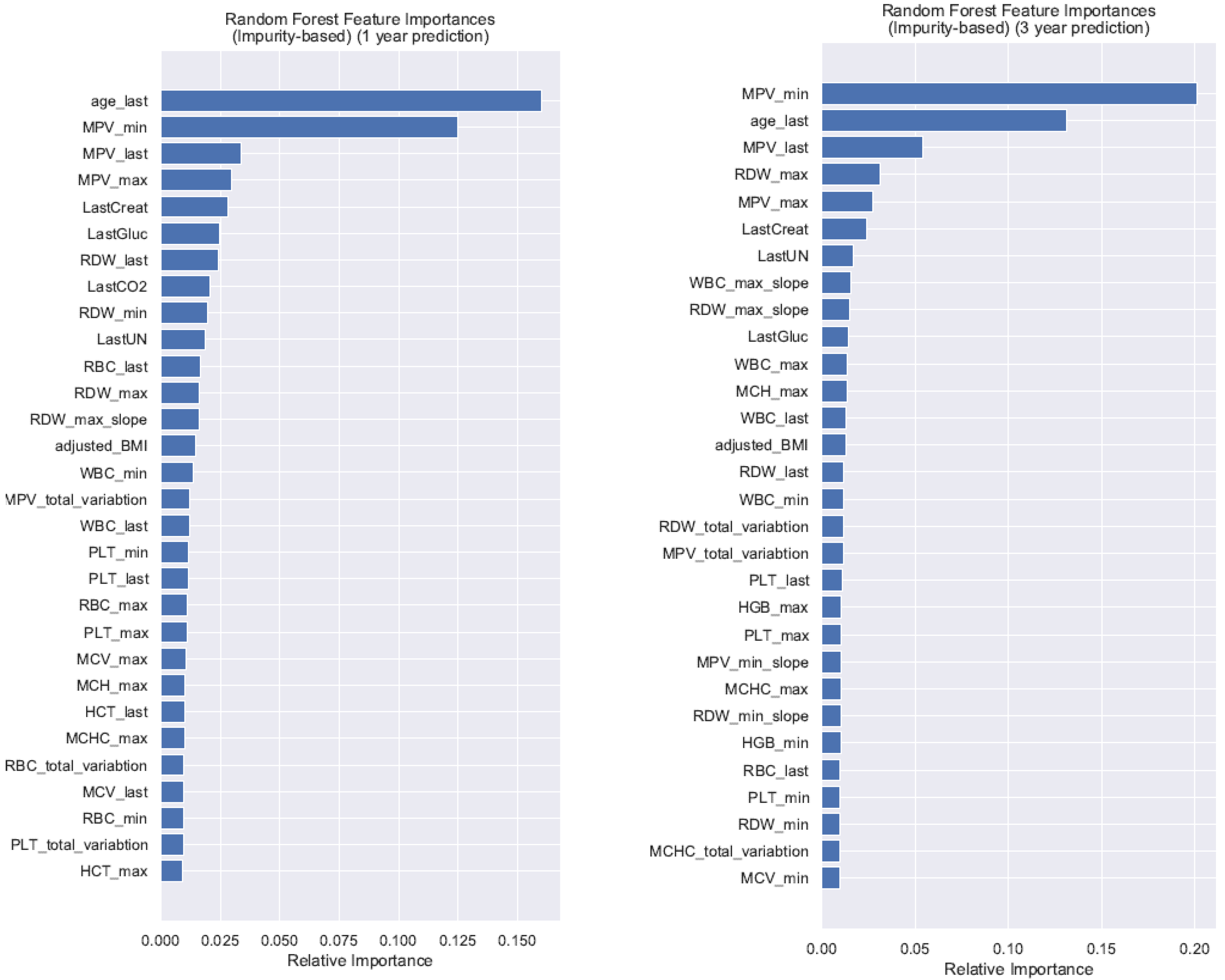
2.1. Predictor Variables
2.2. Primary Outcome
2.3. Model Development
2.4. Statistical Analysis
3. Results
3.1. Baseline Cohort
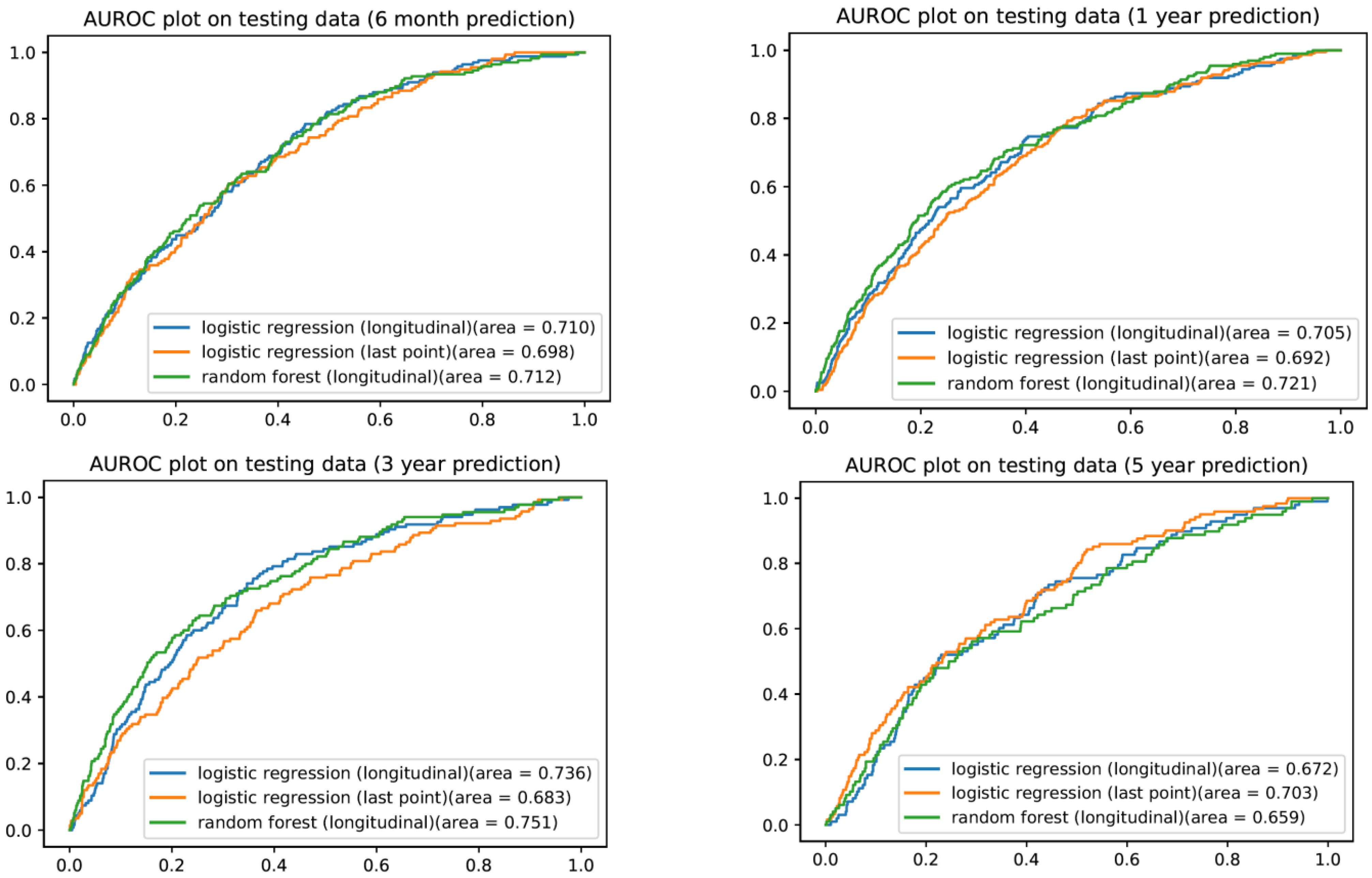
3.2. Single Timepoint Prediction Using Logistic Regression
3.3. Longitudinal Logistic Regression Model
3.4. Longitudinal Random Forest Machine Learning Model
3.5. Subanalysis by Age and Tumor Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Age Group | Time | Imbalance Ratio | Model | AUROC (95% CI) | Sensitivity | Specificity | F-Score | PPV | NPV | Brier Score |
|---|---|---|---|---|---|---|---|---|---|---|
| <50 | 1 year | 29,895:98 | LR | 0.675 (0.632, 0.719) | 0.739 | 0.544 | 0.008 | 0.004 | 0.999 | 0.190 |
| RF | 0.577 (0.524, 0.629) | 0.609 | 0.589 | 0.008 | 0.004 | 0.998 | 0.016 | |||
| 3 years | 25,099:78 | LR | 0.697 (0.666, 0.728) | 0.759 | 0.586 | 0.014 | 0.007 | 0.998 | 0.004 | |
| RF | 0.681 (0.635, 0.727) | 0.655 | 0.580 | 0.012 | 0.006 | 0.998 | 0.155 | |||
| 50 to <75 | 1 year | 42,961:379 | LR | 0.661 (0.643, 0.678) | 0.703 | 0.537 | 0.021 | 0.011 | 0.996 | 0.229 |
| RF | 0.642 (0.619, 0.665) | 0.670 | 0.565 | 0.021 | 0.011 | 0.996 | 0.223 | |||
| 3 years | 34,265:275 | LR | 0.650 (0.628, 0.672) | 0.627 | 0.668 | 0.029 | 0.015 | 0.996 | 0.221 | |
| RF | 0.651 (0.627, 0.675) | 0.590 | 0.686 | 0.029 | 0.015 | 0.995 | 0.207 | |||
| ≥75 | 1 year | 15,040:180 | LR | 0.620 (0.591, 0.650) | 0.525 | 0.685 | 0.041 | 0.021 | 0.991 | 0.234 |
| RF | 0.571 (0.536, 0.606) | 0.644 | 0.488 | 0.032 | 0.016 | 0.991 | 0.218 | |||
| 3 years | 9915:102 | LR | 0.623 (0.586, 0.659) | 0.457 | 0.672 | 0.031 | 0.016 | 0.991 | 0.235 | |
| RF | 0.620 (0.583, 0.657) | 0.543 | 0.643 | 0.034 | 0.018 | 0.992 | 0.084 |
| Cancer Type | Time | Imbalance Ratio | Model | AUROC (95% CI) | Sensitivity | Specificity | F-Score | PPV | NPV | Brier Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Esophagus Cancer | 1 year | 88,470:83 | LR | 0.679 (0.632, 0.725) | 0.600 | 0.649 | 0.003 | 0.002 | 0.999 | 0.179 |
| RF | 0.650 (0.602, 0.699) | 0.640 | 0.582 | 0.003 | 0.001 | 0.999 | 0.150 | |||
| 3 years | 69,672:62 | LR | 0.713 (0.682, 0.745) | 0.704 | 0.616 | 0.005 | 0.002 | 0.999 | 0.169 | |
| RF | 0.616 (0.568, 0.664) | 0.667 | 0.537 | 0.004 | 0.002 | 0.999 | 0.132 | |||
| Stomach Cancer | 1 year | 88,443:110 | LR | 0.664 (0.620, 0.708) | 0.758 | 0.520 | 0.004 | 0.002 | 0.999 | 0.177 |
| RF | 0.614 (0.581, 0.647) | 0.636 | 0.563 | 0.004 | 0.002 | 0.999 | 0.024 | |||
| 3 years | 69,657:77 | LR | 0.727 (0.676, 0.778) | 0.632 | 0.727 | 0.004 | 0.002 | 1.000 | 0.181 | |
| RF | 0.755 (0.709, 0.802) | 0.737 | 0.684 | 0.004 | 0.002 | 1.000 | 0.155 | |||
| Small bowel Cancer | 1 year | 88,503:50 | LR | 0.539 (0.434, 0.644) | 0.500 | 0.622 | 0.001 | 0.001 | 1.000 | 0.155 |
| RF | 0.414 (0.336, 0.492) | 0.286 | 0.640 | 0.001 | 0.000 | 0.999 | 0.001 | |||
| 3 years | 69,704:30 | LR | 0.617 (0.563, 0.671) | 0.500 | 0.621 | 0.001 | 0.000 | 1.000 | 0.155 | |
| RF | 0.566 (0.467, 0.666) | 0.250 | 0.745 | 0.000 | 0.000 | 1.000 | 0.000 | |||
| Colorectal Cancer | 1 year | 88,201:352 | LR | 0.658 (0.633, 0.682) | 0.624 | 0.619 | 0.013 | 0.007 | 0.998 | 0.218 |
| RF | 0.664 (0.644, 0.684) | 0.596 | 0.642 | 0.013 | 0.007 | 0.997 | 0.206 | |||
| 3 years | 69,497:237 | LR | 0.678 (0.652, 0.703) | 0.600 | 0.656 | 0.011 | 0.005 | 0.998 | 0.213 | |
| RF | 0.599 (0.563, 0.634) | 0.508 | 0.675 | 0.010 | 0.005 | 0.998 | 0.003 | |||
| Anal Cancer | 1 year | 88,499:54 | LR | 0.736 (0.683, 0.789) | 0.800 | 0.591 | 0.002 | 0.001 | 1.000 | 0.001 |
| RF | 0.702 (0.645, 0.759) | 0.600 | 0.727 | 0.002 | 0.001 | 1.000 | 0.001 | |||
| 3 years | 69,690:44 | LR | 0.572 (0.486, 0.658) | 0.700 | 0.452 | 0.001 | 0.001 | 1.000 | 0.000 | |
| RF | 0.626 (0.536, 0.716) | 0.700 | 0.520 | 0.001 | 0.001 | 1.000 | 0.000 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef] [PubMed]
- Connell, L.C.; Mota, J.M.; Braghiroli, M.I.; Hoff, P.M. The Rising Incidence of Younger Patients With Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr. Treat. Options Oncol. 2017, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef]
- Low, E.E.; Demb, J.; Liu, L.; Earles, A.; Bustamante, R.; Williams, C.D.; Provenzale, D.; Kaltenbach, T.; Gawron, A.J.; Martinez, M.E.; et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology 2020, 159, 492–501.e7. [Google Scholar] [CrossRef]
- U.S. Preventive Service Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Aparicio, T.; Zaanan, A.; Svrcek, M.; Laurent-Puig, P.; Carrere, N.; Manfredi, S.; Locher, C.; Afchain, P. Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Dig. Liver Dis. 2014, 46, 97–104. [Google Scholar] [CrossRef]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Domper Arnal, M.J.; Ferrandez Arenas, A.; Lanas Arbeloa, A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933–7943. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef]
- Rockey, D.C.; Altayar, O.; Falck-Ytter, Y.; Kalmaz, D. AGA Technical Review on Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020, 159, 1097–1119. [Google Scholar] [CrossRef]
- Read, A.J.; Waljee, A.K.; Sussman, J.B.; Singh, H.; Chen, G.Y.; Vijan, S.; Saini, S.D. Testing Practices, Interpretation, and Diagnostic Evaluation of Iron Deficiency Anemia by US Primary Care Physicians. JAMA Netw. Open 2021, 4, e2127827. [Google Scholar] [CrossRef]
- Chacko, K.M.; Feinberg, L.E. Laboratory screening at preventive health exams: Trend of testing, 1978–2004. Am. J. Prev. Med. 2007, 32, 59–62. [Google Scholar] [CrossRef]
- Ko, C.W.; Siddique, S.M.; Patel, A.; Harris, A.; Sultan, S.; Altayar, O.; Falck-Ytter, Y. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020, 159, 1085–1094. [Google Scholar] [CrossRef]
- Read, A.J.; Waljee, A.K.; Chen, C.S.; Holleman, R.; Kumbier, K.E.; Saini, S.D. Prevalence of Appropriate Testing for Incident Anemia in the US Department of Veterans Affairs. JAMA Netw. Open 2021, 4, e2034406. [Google Scholar] [CrossRef]
- Murphy, D.R.; Laxmisan, A.; Reis, B.A.; Thomas, E.J.; Esquivel, A.; Forjuoh, S.N.; Parikh, R.; Khan, M.M.; Singh, H. Electronic health record-based triggers to detect potential delays in cancer diagnosis. BMJ Qual. Saf. 2014, 23, 8–16. [Google Scholar] [CrossRef]
- Kinar, Y.; Kalkstein, N.; Akiva, P.; Levin, B.; Half, E.E.; Goldshtein, I.; Chodick, G.; Shalev, V. Development and validation of a predictive model for detection of colorectal cancer in primary care by analysis of complete blood counts: A binational retrospective study. J. Am. Med. Inform. Assoc. 2016, 23, 879–890. [Google Scholar] [CrossRef]
- Hornbrook, M.C.; Goshen, R.; Choman, E.; O’Keeffe-Rosetti, M.; Kinar, Y.; Liles, E.G.; Rust, K.C. Early Colorectal Cancer Detected by Machine Learning Model Using Gender, Age, and Complete Blood Count Data. Dig. Dis. Sci. 2017, 62, 2719–2727. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Walter, F.M.; Emery, J.D.; Win, A.K.; Griffin, S.J. Risk Prediction Models for Colorectal Cancer: A Systematic Review. Cancer Prev. Res. 2016, 9, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD). Ann. Intern. Med. 2015, 162, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Sandhaus, L.M.; Meyer, P. How useful are CBC and reticulocyte reports to clinicians? Am. J. Clin. Pathol. 2002, 118, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B.; British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef]
- Komaki, Y.; Komaki, F.; Micic, D.; Ido, A.; Sakuraba, A. Risk of Colorectal Cancer in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2018, 52, 796–804. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Diabetes Increases Risk of Gastric Cancer After Helicobacter pylori Eradication: A Territory-Wide Study With Propensity Score Analysis. Diabetes Care 2019, 42, 1769–1775. [Google Scholar] [CrossRef]
- Miao, Z.F.; Xu, H.; Xu, Y.Y.; Wang, Z.N.; Zhao, T.T.; Song, Y.X.; Xu, H.M. Diabetes mellitus and the risk of gastric cancer: A meta-analysis of cohort studies. Oncotarget 2017, 8, 44881–44892. [Google Scholar] [CrossRef]
- Tseng, C.H.; Tseng, F.H. Diabetes and gastric cancer: The potential links. World J. Gastroenterol. 2014, 20, 1701–1711. [Google Scholar] [CrossRef]
- van der Sommen, F.; de Groof, J.; Struyvenberg, M.; van der Putten, J.; Boers, T.; Fockens, K.; Schoon, E.J.; Curvers, W.; de With, P.; Mori, Y.; et al. Machine learning in GI endoscopy: Practical guidance in how to interpret a novel field. Gut 2020, 69, 2035–2045. [Google Scholar] [CrossRef]
- Waljee, A.K.; Higgins, P.D.; Singal, A.G. A primer on predictive models. Clin. Transl. Gastroenterol. 2014, 5, e44. [Google Scholar] [CrossRef]
- Waljee, A.K.; Wallace, B.I.; Cohen-Mekelburg, S.; Liu, Y.; Liu, B.; Sauder, K.; Stidham, R.W.; Zhu, J.; Higgins, P.D.R. Development and Validation of Machine Learning Models in Prediction of Remission in Patients With Moderate to Severe Crohn Disease. JAMA Netw. Open 2019, 2, e193721. [Google Scholar] [CrossRef]
- Waljee, A.K.; Sauder, K.; Patel, A.; Segar, S.; Liu, B.; Zhang, Y.; Zhu, J.; Stidham, R.W.; Balis, U.; Higgins, P.D. Machine Learning Algorithms for Objective Remission and Clinical Outcomes with Thiopurines. J. Crohn’s Colitis 2017, 11, 801–810. [Google Scholar] [CrossRef]
- Kurlander, J.E.; Waljee, A.K.; Menees, S.B.; Lipson, R.; Kokaly, A.N.; Read, A.J.; Shehadeh, K.S.; Cohn, A.; Saini, S.D. Regression and Random Forest Machine Learning Have Limited Performance in Predicting Bowel Preparation in Veteran Population. Dig. Dis. Sci. 2021, 67, 2827–2841. [Google Scholar] [CrossRef]
- van der Ploeg, T.; Austin, P.C.; Steyerberg, E.W. Modern modelling techniques are data hungry: A simulation study for predicting dichotomous endpoints. BMC Med. Res. Methodol. 2014, 14, 137. [Google Scholar] [CrossRef]
- Frizzell, J.D.; Liang, L.; Schulte, P.J.; Yancy, C.W.; Heidenreich, P.A.; Hernandez, A.F.; Bhatt, D.L.; Fonarow, G.C.; Laskey, W.K. Prediction of 30-Day All-Cause Readmissions in Patients Hospitalized for Heart Failure: Comparison of Machine Learning and Other Statistical Approaches. JAMA Cardiol. 2017, 2, 204–209. [Google Scholar] [CrossRef]
- Pietrzyk, L.; Plewa, Z.; Denisow-Pietrzyk, M.; Zebrowski, R.; Torres, K. Diagnostic Power of Blood Parameters as Screening Markers in Gastric Cancer Patients. Asian Pac. J. Cancer Prev. 2016, 17, 4433–4437. [Google Scholar]
- Copija, A.; Nowakowska-Zajdel, E.; Janion, K.; Walkiewicz, K. Clinical Characteristics of Colorectal Cancer Patients in terms of Selected Platelet Indices. Dis. Markers 2020, 2020, 6145604. [Google Scholar] [CrossRef]
- Kilincalp, S.; Ekiz, F.; Basar, O.; Ayte, M.R.; Coban, S.; Yilmaz, B.; Altinbas, A.; Basar, N.; Aktas, B.; Tuna, Y.; et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets 2014, 25, 592–594. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhang, X.; Qin, Y.Y.; Qin, J.Q.; Lin, F.Q. Mean platelet volume/platelet count ratio in colorectal cancer: A retrospective clinical study. BMC Cancer 2019, 19, 314. [Google Scholar] [CrossRef]
- Stojkovic Lalosevic, M.; Pavlovic Markovic, A.; Stankovic, S.; Stojkovic, M.; Dimitrijevic, I.; Radoman Vujacic, I.; Lalic, D.; Milovanovic, T.; Dumic, I.; Krivokapic, Z. Combined Diagnostic Efficacy of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis. Markers 2019, 2019, 6036979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, Z.; Wang, P.; Hu, X.; Gao, Y.; He, J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol. 2016, 37, 9323–9331. [Google Scholar] [CrossRef] [PubMed]
- Asge Standards Of Practice Comittee; Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359.e2. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Cello, J.P. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N. Engl. J. Med. 1993, 329, 1691–1695. [Google Scholar] [CrossRef]
- Stephens, M.R.; Hopper, A.N.; White, S.R.; Jugool, S.; Stratford, R.; Lewis, W.G.; Allison, M.C. Colonoscopy first for iron-deficiency anaemia: A Numbers Needed to Investigate approach. QJM 2006, 99, 389–395. [Google Scholar] [CrossRef]
- Robertson, D.J.; Lee, J.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Rex, D.K. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1217–1237.e3. [Google Scholar] [CrossRef]
| Total Cohort | n = 148,158 | |
|---|---|---|
| Sex | ||
| Male | 56,163 | 37.9% |
| Female | 91,995 | 62.1% |
| Age | ||
| Mean | 49.4 ± 17.3 | |
| Median (IQR) | 50 (35–62) | |
| Range | 18–104 | |
| Race | Frequency | (%) |
| Caucasian | 120,385 | 81.3 |
| African American | 15,510 | 10.5 |
| Asian | 6795 | 4.6 |
| Native American | 463 | 0.3 |
| Native Hawaiian/Pacific Islander | 76 | 0.1 |
| Other | 3070 | 2.1 |
| Unknown | 1859 | 1.3 |
| Model Tested | Time | AUROC (95% CI) | Optimal Cutoff | PPV | NPV | Sensitivity | Specificity | F-Score | Brier Score |
|---|---|---|---|---|---|---|---|---|---|
| Logistic reg. (Single Timepoint) | 6 month | 0.697 (0.679, 0.715) | 0.009 | 0.014 | 0.996 | 0.603 | 0.690 | 0.027 | 0.007 |
| 1 year | 0.693 (0.675, 0.710) | 0.494 | 0.014 | 0.996 | 0.682 | 0.611 | 0.027 | 0.224 | |
| 3 years | 0.683 (0.665, 0.701) | 0.501 | 0.011 | 0.996 | 0.652 | 0.635 | 0.022 | 0.222 | |
| 5 years | 0.703 (0.686, 0.720) | 0.491 | 0.012 | 0.996 | 0.620 | 0.664 | 0.024 | 0.213 | |
| Logistic reg. (Longitudinal) | 6 month | 0.711 (0.691, 0.731) | 0.008 | 0.014 | 0.996 | 0.665 | 0.634 | 0.027 | 0.008 |
| 1 year | 0.705 (0.689, 0.722) | 0.007 | 0.014 | 0.997 | 0.737 | 0.600 | 0.027 | 0.008 | |
| 3 years | 0.735 (0.713, 0.757) | 0.472 | 0.014 | 0.997 | 0.733 | 0.653 | 0.027 | 0.205 | |
| 5 years | 0.672 (0.653, 0.691) | 0.447 | 0.010 | 0.997 | 0.694 | 0.581 | 0.020 | 0.208 | |
| Random Forest (Longitudinal) | 6 month | 0.713 (0.689, 0.737) | 0.315 | 0.015 | 0.996 | 0.629 | 0.671 | 0.029 | 0.092 |
| 1 year | 0.722 (0.705, 0.739) | 0.381 | 0.015 | 0.996 | 0.677 | 0.660 | 0.029 | 0.134 | |
| 3 years | 0.750 (0.729, 0.771) | 0.368 | 0.015 | 0.997 | 0.689 | 0.695 | 0.029 | 0.116 | |
| 5 years | 0.660 (0.637, 0.682) | 0.323 | 0.011 | 0.996 | 0.561 | 0.697 | 0.022 | 0.097 |
| Model Tested | Time | Test Set Size | Prediction Success | Prediction Failure | True Positive | False Positive | True Negative | False Negative |
|---|---|---|---|---|---|---|---|---|
| Logistic reg. (Single Timepoint) | 6 month | 21,798 | 15,018 | 6780 | 94 | 6718 | 14,924 | 62 |
| 1 year | 27,357 | 16,735 | 10,622 | 152 | 10,551 | 16,583 | 71 | |
| 3 years | 22,065 | 14,018 | 8047 | 92 | 7998 | 13,926 | 49 | |
| 5 years | 18,318 | 12,155 | 6163 | 75 | 6117 | 12,080 | 46 | |
| Logistic reg. (Longitudinal) | 6 month | 21,012 | 13,320 | 7692 | 111 | 7636 | 13,209 | 56 |
| 1 year | 25,536 | 15,342 | 10,194 | 146 | 10,142 | 15,196 | 52 | |
| 3 years | 20,187 | 13,197 | 6990 | 99 | 6954 | 13,098 | 36 | |
| 5 years | 16,100 | 9368 | 6732 | 68 | 6702 | 9300 | 30 | |
| Random Forest (Longitudinal) | 6 month | 21,012 | 14,101 | 6911 | 105 | 6849 | 13,996 | 62 |
| 1 year | 25,536 | 16,859 | 8677 | 134 | 8613 | 16,725 | 64 | |
| 3 years | 20,187 | 14,025 | 6162 | 93 | 6120 | 13,932 | 42 | |
| 5 years | 16,100 | 11,215 | 4885 | 55 | 4842 | 11,160 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Read, A.J.; Zhou, W.; Saini, S.D.; Zhu, J.; Waljee, A.K. Prediction of Gastrointestinal Tract Cancers Using Longitudinal Electronic Health Record Data. Cancers 2023, 15, 1399. https://doi.org/10.3390/cancers15051399
Read AJ, Zhou W, Saini SD, Zhu J, Waljee AK. Prediction of Gastrointestinal Tract Cancers Using Longitudinal Electronic Health Record Data. Cancers. 2023; 15(5):1399. https://doi.org/10.3390/cancers15051399
Chicago/Turabian StyleRead, Andrew J., Wenjing Zhou, Sameer D. Saini, Ji Zhu, and Akbar K. Waljee. 2023. "Prediction of Gastrointestinal Tract Cancers Using Longitudinal Electronic Health Record Data" Cancers 15, no. 5: 1399. https://doi.org/10.3390/cancers15051399
APA StyleRead, A. J., Zhou, W., Saini, S. D., Zhu, J., & Waljee, A. K. (2023). Prediction of Gastrointestinal Tract Cancers Using Longitudinal Electronic Health Record Data. Cancers, 15(5), 1399. https://doi.org/10.3390/cancers15051399





