Is Segmental Ureterectomy Associated with Inferior Survival for Localized Upper-Tract Urothelial Carcinoma of the Ureter Compared to Radical Nephroureterectomy?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
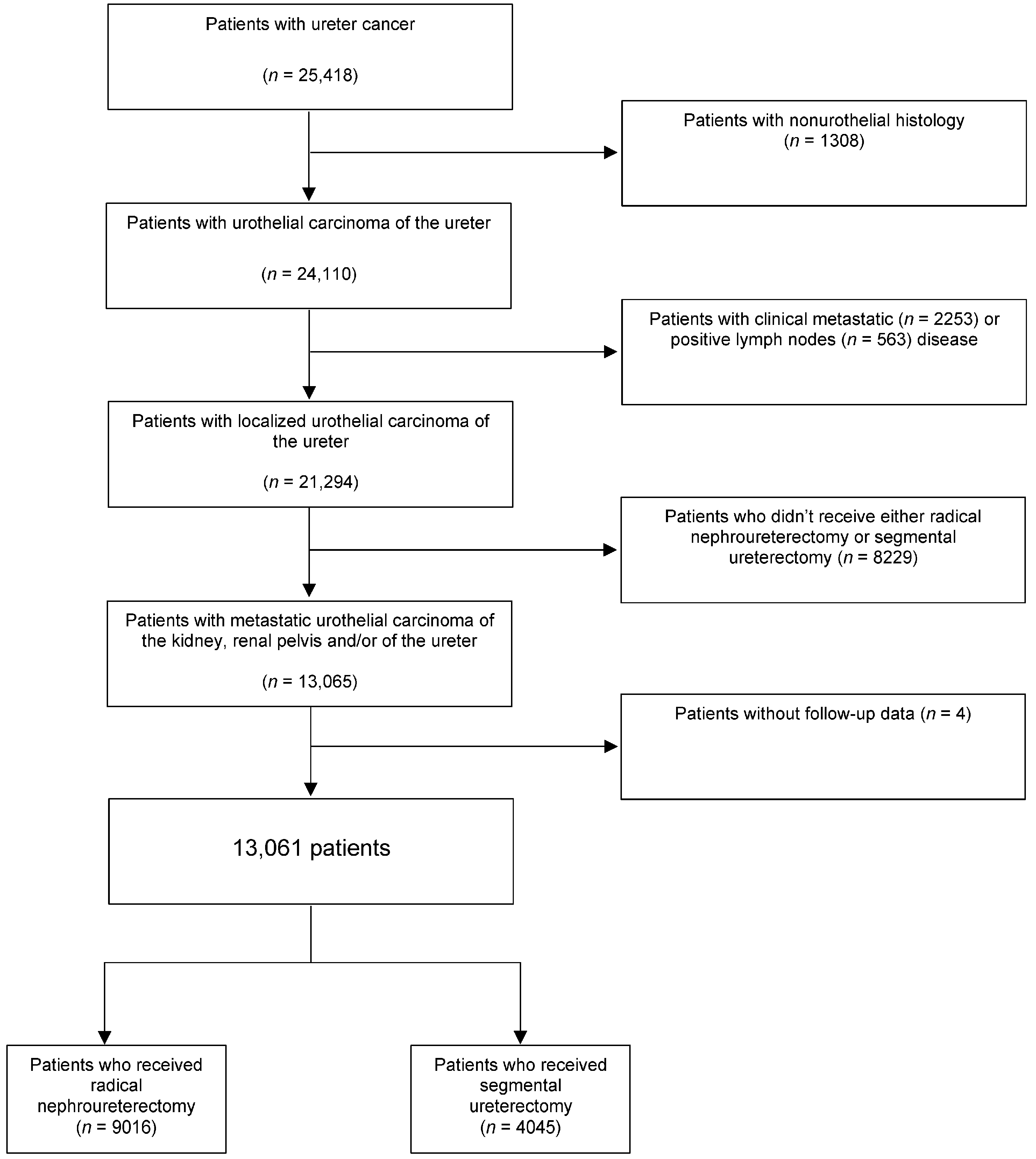
2.1. Data Source and Study Population
2.2. Exposure of Interest
2.3. Definition of the Variable of Interest
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Baseline Patient Characteristics and Predictors of Receiving Segmental Ureterectomy
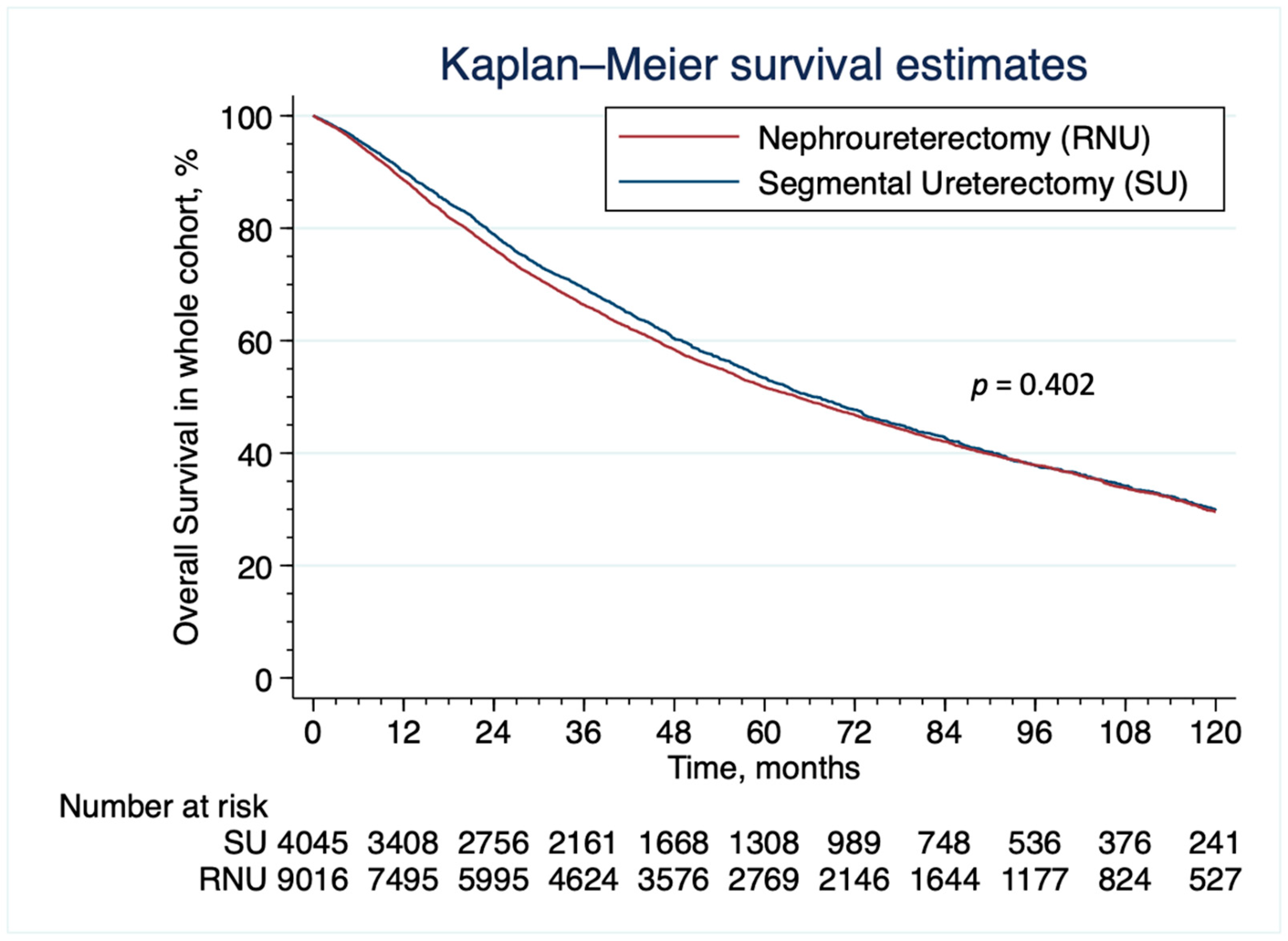
3.2. Patient Characteristics and Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Gontero, P.; Van Rhijn, B.W.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur. Urol. 2018, 73, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.J.; Ellison, L.M. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J. Urol. 2000, 164, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, C.C.; Nocera, L.; Stolzenbach, L.F.; Wenzel, M.; Cucchiara, V.; Tian, Z.; Shariat, S.F.; Saad, F.; Longo, N.; Montorsi, F.; et al. Incidence and Survival Rates of Contemporary Patients with Invasive Upper Tract Urothelial Carcinoma. Eur. Urol. Oncol. 2020, 4, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; Smith, A.K.; Larson, B.T.; Gong, M.C.; Campbell, S.C.; Raghavan, D.; Dreicer, R.; Hansel, D.E.; Stephenson, A.J. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer 2010, 116, 2967–2973. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in Renal Function Following Nephroureterectomy May Affect the Use of Perioperative Chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef]
- Kaag, M.; Trost, L.; Thompson, R.H.; Favaretto, R.; Elliott, V.; Shariat, S.F.; Maschino, A.; Vertosick, E.; Raman, J.D.; Dalbagni, G. Preoperative predictors of renal function decline after radical nephroureterectomy for upper tract urothelial carcinoma. BJU Int. 2013, 114, 674–679. [Google Scholar] [CrossRef]
- Vaughn, D.J. Chemotherapeutic options for cisplatin-ineligible patients with advanced carcinoma of the urothelium. Cancer Treat. Rev. 2008, 34, 328–338. [Google Scholar] [CrossRef]
- Jeldres, C.; Lughezzani, G.; Sun, M.; Isbarn, H.; Shariat, S.F.; Budaus, L.; Lattouf, J.-B.; Widmer, H.; Graefen, M.; Montorsi, F.; et al. Segmental Ureterectomy Can Safely be Performed in Patients with Transitional Cell Carcinoma of the Ureter. J. Urol. 2010, 183, 1324–1329. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, X.; Zhang, X.; Li, Q.; Liu, S.; Yu, L.; Xu, T. Segmental Ureterectomy is Acceptable for High-risk Ureteral Carcinoma Comparing to Radical Nephroureterectomy. J. Investig. Surg. 2019, 32, 746–753. [Google Scholar] [CrossRef]
- Lucca, I.; Klatte, T.; Rouprêt, M.; Shariat, S.F. Kidney-sparing surgery for upper tract urothelial cancer. Curr. Opin. Urol. 2015, 25, 100–104. [Google Scholar] [CrossRef]
- Seisen, T.; Colin, P.; Rouprêt, M. Risk-adapted strategy for the kidney-sparing management of upper tract tumours. Nat. Rev. Urol. 2015, 12, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.P.; Friedlander, D.F.; Trinh, Q.-D. Secondary data sources for health services research in urologic oncology. Urol. Oncol. Semin. Orig. Investig. 2017, 36, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.P.; Trinh, Q.-D. Secondary data analysis: Techniques for comparing interventions and their limitations. Curr. Opin. Urol. 2017, 27, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B. American Joint Committee on, C.: AJCC Cancer Staging Manual, 18th ed.; Stephen, B., Edge, M.D., FACS, Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Li, F.; Thomas, L.E. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2018, 188, 250–257. [Google Scholar] [CrossRef]
- Cole, S.R.; Hernán, M.A. Adjusted survival curves with inverse probability weights. Comput. Methods Programs Biomed. 2004, 75, 45–49. [Google Scholar] [CrossRef]
- Veccia, A.; Antonelli, A.; Checcucci, E.; Falagario, U.; Carrieri, G.; Guruli, G.; De Sio, M.; Simeone, C.; Porpiglia, F.; Autorino, R. Segmental Ureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review and Meta-analysis of Comparative Studies. Clin. Genitourin. Cancer 2020, 18, e10–e20. [Google Scholar] [CrossRef]
- Piraino, J.A.; Snow, Z.A.; Edwards, D.C.; Hager, S.; McGreen, B.H.; Diorio, G.J. Nephroureterectomy vs. segmental ureterectomy of clinically localized, high-grade, urothelial carcinoma of the ureter: Practice patterns and outcomes. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 851.e1–851.e10. [Google Scholar]
- Huang, W.; Donin, N.M.; Levey, A.S.; Campbell, S.C. Chronic Kidney Disease and Kidney Cancer Surgery: New Perspectives. J. Urol. 2020, 203, 475–485. [Google Scholar] [CrossRef]
- Kato, T.; Nakayama, R.; Haba, T.; Kawaguchi, M.; Komiya, A.; Koike, H. Oncological and renal outcomes of segmental ureterectomy vs. radical nephroureterectomy for upper tract urothelial carcinoma. Oncol. Lett. 2018, 16, 6861–6867. [Google Scholar] [CrossRef]
- Gakis, G.; Schubert, T.; Alemozaffar, M.; Bellmunt, J.; Bochner, B.H.; Boorjian, S.A.; Daneshmand, S.; Huang, W.C.; Kondo, T.; Konety, B.R.; et al. Update of the ICUD-SIU consultation on upper tract urothelial carcinoma 2016: Treatment of localized high-risk disease. World J. Urol. 2016, 35, 327–335. [Google Scholar] [CrossRef]
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.T.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W.F.; et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277. [Google Scholar] [CrossRef]
- Tully, K.H.; Krimphove, M.J.; Huynh, M.J.; Marchese, M.; Kibel, A.S.; Noldus, J.; Kluth, L.A.; McGregor, B.; Chang, S.L.; Trinh, Q.-D.; et al. Differences in survival and impact of adjuvant chemotherapy in patients with variant histology of tumors of the renal pelvis. World J. Urol. 2019, 38, 2227–2236. [Google Scholar] [CrossRef]
- Thouvenin, J.; Chanzá, N.M.; Alhalabi, O.; Lang, H.; Tannir, N.M.; Barthélémy, P.; Malouf, G.G. Efficacy of Immune Checkpoint Inhibitors in Upper Tract Urothelial Carcinomas: Current Knowledge and Future Directions. Cancers 2021, 13, 4341. [Google Scholar] [CrossRef] [PubMed]
- Oswald, D.; Pallauf, M.; Deininger, S.; Törzsök, P.; Sieberer, M.; Eiben, C. Neoadjuvant Chemotherapy before Nephroureterectomy in High-Risk Upper Tract Urothelial Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4841. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, M.; Nguyen, D.-D.; Modonutti, D.; Haeuser, L.; Lipsitz, S.; Mossanen, M.; Kibel, A.S.; Lughezzani, G.; Trinh, Q.-D.; Cole, A.P. Impact of high-intensity local treatment on overall survival in stage IV upper tract urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 436.e1–436.e10. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Unweighted Population, No. (%) | Weighted Population, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall n = 13,061 | RNU n = 9016 (69%) | SU n = 4045 (31.0%) | Standardized Difference (%) | Overall | RNU | SU | Standardized Difference (%) | ||
| Mean (SD) Age, Years | 71.9 (10.0) | 71.8 (10.0) | 71.9 (10.0) | 1.1 | 71.9 | 71.9 | 72.0 | 0.8 | |
| Age categories, years | <60 | 1559 (11.9) | 1078 (12) | 481 (11.9) | −0.2 | 11.8 | 11.8 | 11.8 | 0 |
| 60–69 | 3441 (26.4) | 2371 (26.3) | 1070 (26.5) | 0.4 | 26.4 | 26.4 | 26.4 | 0 | |
| 70–79 | 4852 (37.2) | 3379 (37.5) | 1473 (36.4) | −2.2 | 36.8 | 36.8 | 36.8 | 0 | |
| >80 | 3209 (24.6) | 2188 (24.3) | 1021 (25.2) | 2.3 | 25.0 | 25.0 | 25.0 | 0 | |
| Gender, n (%) | Male | 8239 (63.1) | 5544 (61.5) | 2695 (66.6) | 10.7 | 65.0 | 65.0 | 65.0 | 0 |
| Female | 4822 (36.9) | 3472 (38.5) | 1350 (33.4) | −10.7 | 35.0 | 35.0 | 35.0 | 0 | |
| Race, n (%) | White | 12,104 (92.7) | 8345 (92.6) | 3759 (92.9) | 1.4 | 92.8 | 92.8 | 92.8 | 0 |
| Black | 443 (3.4) | 314 (3.5) | 129 (3.2) | −1.6 | 3.3 | 3.3 | 3.3 | 0 | |
| Other | 399 (3.1) | 271 (3.0) | 128 (3.2) | 0.9 | 3.1 | 3.1 | 3.1 | 0 | |
| Unknown | 115 (0.9) | 86 (1.0) | 29 (0.7) | −2.6 | 0.8 | 0.8 | 0.8 | 0 | |
| Comorbidity index, n (%) | 0 | 8377 (64.1) | 5791 (64.2) | 2586 (63.9) | −0.6 | 64.0 | 64.0 | 64.0 | 0 |
| 1 | 3281 (25.1) | 2253 (25.0) | 1028 (25.4) | 1.0 | 25.2 | 25.2 | 25.2 | 0 | |
| 2 | 1028 (7.9) | 714 (7.9) | 314 (7.8) | −0.6 | 7.9 | 7.9 | 7.9 | 0 | |
| ≥3 | 375 (2.9) | 258 (2.9) | 117 (2.9) | 0.2 | 2.9 | 2.9 | 2.9 | 0 | |
| Insurance status, n (%) | Private | 3218 (24.6) | 2180 (24.2) | 1038 (25.7) | 3.4 | 25.1 | 25.1 | 25.1 | 0 |
| Medicaid | 364 (2.8) | 256 (2.8) | 108 (2.7) | −1.0 | 2.7 | 2.7 | 2.7 | 0 | |
| Medicare | 9170 (70.2) | 6351 (70.4) | 2819 (69.7) | −1.6 | 70.1 | 70.1 | 70.1 | 0 | |
| Uninsured | 146 (1.1) | 110 (1.2) | 36 (0.9) | −3.2 | 1.0 | 1.0 | 1.0 | 0 | |
| Unknown | 163 (1.3) | 119 (1.3) | 44 (1.1) | −2.1 | 1.2 | 1.2 | 1.2 | 0 | |
| Income, n (%) | High | 8054 (61.7) | 5476 (60.7) | 2578 (63.7) | 6.2 | 62.6 | 62.6 | 62.6 | 0 |
| Low | 4932 (37.8) | 3494 (38.8) | 1438 (35.6) | −6.6 | 36.8 | 36.8 | 36.8 | 0 | |
| Unknown | 75 (0.6) | 46 (0.5) | 29 (0.7) | 2.6 | 0.6 | 0.6 | 0.6 | 0 | |
| Education, n (%) | High | 8103 (62.0) | 5491 (60.9) | 2612 (64.6) | 7.6 | 63.2 | 63.2 | 63.2 | 0 |
| Low | 4891 (37.5) | 3483 (38.6) | 1408 (34.8) | −7.9 | 36.2 | 36.2 | 36.2 | 0 | |
| Unknown | 67 (0.5) | 42 (0.5) | 25 (0.6) | 2.1 | 0.5 | 0.5 | 0.5 | 0 | |
| Facility type, n (%) | Academic | 4563 (34.9) | 2962 (32.9) | 1601 (39.6) | 14.0 | 37.1 | 37.1 | 37.1 | 0 |
| Non-academic | 8467 (64.8) | 6035 (64.9) | 2432 (60.1) | −14.2 | 62.6 | 62.6 | 62.6 | 0 | |
| Unknown | 31 (0.2) | 19 (0.2) | 12 (0.3) | 1.7 | 0.3 | 0.3 | 0.3 | 0 | |
| Facility location, n (%) | East | 5766 (44.2) | 4018 (44.6) | 1748 (43.2) | −2.7 | 43.7 | 43.7 | 43.7 | 0 |
| Central | 5546 (42.5) | 3877 (43.0) | 1669 (41.3) | −3.5 | 41.8 | 41.8 | 41.8 | 0 | |
| West | 1718 (13.2) | 1102 (12.2) | 616 (15.2) | 8.7 | 14.3 | 14.3 | 14.3 | 0 | |
| Unknown | 31 (0.2) | 19 (0.2) | 12 (0.3) | 1.7 | 0.3 | 0.3 | 0.3 | 0 | |
| Facility county (%) | Metro | 10,531 (80.6) | 7239 (80.3) | 3292 (81.4) | 2.8 | 80.9 | 80.9 | 80.9 | 0 |
| Urban | 1928 (14.8) | 1348 (15.0) | 580 (14.3) | −1.7 | 14.7 | 14.7 | 14.7 | 0 | |
| Rural | 255 (2.0) | 190 (2.1) | 65 (1.6) | −3.7 | 1.7 | 1.7 | 1.7 | 0 | |
| Unknown | 347 (2.7) | 239 (2.7) | 108 (2.7) | 0.1 | 2.7 | 2.7 | 2.7 | 0 | |
| Facility distance (%) | First | 7158 (54.8) | 5011 (55.6) | 2147 (53.1) | −5.0 | 54.1 | 54.1 | 54.1 | 0 |
| Second | 4194 (32.1) | 2878 (31.9) | 1316 (32.5) | 1.3 | 32.2 | 32.2 | 32.2 | 0 | |
| Third | 1653 (12.7) | 1094 (12.1) | 559 (13.8) | 5.0 | 13.1 | 13.1 | 13.1 | 0 | |
| Unknown | 56 (0.4) | 33 (0.4) | 23 (0.6) | 3.0 | 0.5 | 0.5 | 0.5 | 0 | |
| Clinical T stage (%) | ≤T1 | 5158 (39.5) | 3454 (38.3) | 1704 (42.1) | 7.8 | 40.9 | 40.9 | 40.9 | 0 |
| T2 | 1079 (8.3) | 741 (8.2) | 338 (8.4) | 0.5 | 8.3 | 8.3 | 8.3 | 0 | |
| T3 | 827 (6.3) | 640 (7.1) | 338 (4.6) | −10.6 | 5.3 | 5.3 | 5.3 | 0 | |
| T4 | 92 (0.7) | 74 (0.8) | 18 (0.4) | −4.7 | 0.5 | 0.5 | 0.5 | 0 | |
| Unknown | 5905 (45.2) | 4107 (45.6) | 1798 (44.5) | −2.2 | 45.0 | 45.0 | 45.0 | 0 | |
| Tumor size (%) | ≤2 cm | 3675 (28.1) | 2258 (25.0) | 1417 (35.0) | 21.9 | 32.4 | 32.4 | 32.4 | 0 |
| >2 cm | 6505 (49.8) | 5065 (56.2) | 1440 (35.6) | −42.2 | 41.8 | 41.8 | 41.8 | 0 | |
| Unknown | 2881 (22.1) | 1693 (18.8) | 1188 (29.4) | 25.0 | 25.7 | 25.7 | 25.7 | 0 | |
| Tumor grade (%) | Low grade | 1975 (15.1) | 1291 (14.3) | 684 (16.9) | 7.1 | 15.8 | 15.8 | 15.8 | 0 |
| High grade | 4392 (33.6) | 3138 (34.8) | 1254 (31.0) | −8.1 | 32.3 | 32.3 | 32.3 | 0 | |
| Unknown | 6694 (51.3) | 4587 (50.9) | 2107 (52.1) | 2.4 | 51.8 | 51.8 | 51.8 | 0 | |
| Lymph vascular invasion (%) | Not present | 4488 (34.4) | 3160 (35.1) | 1328 (32.8) | −4.7 | 33.6 | 33.6 | 33.6 | 0 |
| Present | 954 (7.3) | 693 (7.7) | 261 (6.5) | −4.8 | 6.8 | 6.8 | 6.8 | 0 | |
| Unknown | 7619 (58.3) | 5163 (57.3) | 2456 (60.7) | 7.0 | 59.5 | 59.5 | 59.5 | 0 | |
| Chemotherapy (%) | Not received | 10,997 (84.2) | 7530 (83.5) | 3467 (85.7) | 6.1 | 85.1 | 85.1 | 85.1 | 0 |
| Received | 1568 (12.1) | 1142 (12.7) | 426 (10.5) | −6.7 | 11.1 | 11.1 | 11.1 | 0 | |
| Unknown | 496 (3.8) | 344 (3.8) | 152 (3.8) | −0.3 | 3.8 | 3.8 | 3.8 | 0 | |
| Variable | Hazard Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Age | <60 | Ref | ||
| 60–69 | 1.06 | 0.92–1.22 | 0.433 | |
| 70–79 | 1.04 | 0.89–1.21 | 0.637 | |
| >79 | 1.18 | 1.00–1.38 | 0.047 | |
| Gender | Male | Ref | ||
| Female | 0.81 | 0.75–0.88 | <0.001 | |
| Race | White | Ref | ||
| Black | 1.00 | 0.81–1.25 | 0.970 | |
| Other | 0.990 | 0.79–1.24 | 0.922 | |
| Unknown | 0.76 | 0.49–1.17 | 0.209 | |
| Comorbidity index | 0 | Ref | ||
| 1 | 1.09 | 1.00–1.19 | 0.061 | |
| 2 | 1.03 | 0.89–1.19 | 0.656 | |
| ≥3 | 1.06 | 0.84–1.33 | 0.632 | |
| Insurance status | Private | Ref | ||
| Medicaid | 0.88 | 0.69–1.13 | 0.315 | |
| Medicare | 0.95 | 0.86–1.06 | 0.364 | |
| Uninsured | 0.76 | 0.51–1.13 | 0.171 | |
| Unknown | 0.79 | 0.55–1.15 | 0.217 | |
| Annual income | High | Ref | ||
| Low | 0.95 | 0.86–1.05 | 0.297 | |
| Unknown | 2.87 | 0.68–1.21 | 0.150 | |
| Education | High | Ref | ||
| Low | 0.89 | 0.81–0.98 | 0.014 | |
| Unknown | 0.15 | 0.02–1.28 | 0.083 | |
| Facility type | Academic | Ref | ||
| Non-academic | 0.75 | 0.69–0.82 | <0.001 | |
| Unknown | 1.39 | 0.66–2.95 | 0.386 | |
| Facility location | East | Ref | ||
| Central | 1.02 | 0.94–1.12 | 0.570 | |
| West | 1.37 | 1.22–1.54 | <0.001 | |
| Facility county | Metro | Ref | ||
| Urban | 0.96 | 0.84–1.09 | 0.488 | |
| Rural | 0.77 | 0.57–1.05 | 0.098 | |
| Unknown | 0.97 | 0.75–1.25 | 0.807 | |
| Distance | First | Ref | ||
| Second | 1.08 | 0.99–1.19 | 0.077 | |
| Third | 1.16 | 1.01–1.33 | 0.040 | |
| Unknown | 3.51 | 0.66–1.85 | 0.139 | |
| Year of diagnosis | 2004/6 | Ref | ||
| 2007/9 | 1.08 | 0.97–1.21 | 0.157 | |
| 2010/12 | 1.27 | 0.99–1.61 | 0.056 | |
| 2013/15 | 1.18 | 0.92–1.51 | 0.190 | |
| Clinical T stage | ≤ T1 | Ref | ||
| T2 | 0.99 | 0.85–1.15 | 0.885 | |
| T3 | 0.67 | 0.56–0.80 | <0.001 | |
| T4 | 0.51 | 0.30–0.88 | 0.015 | |
| Unknown | 0.93 | 0.86–1.02 | 0.120 | |
| Tumor grade | Low grade | Ref | ||
| High grade | 0.76 | 0.67–0.86 | <0.001 | |
| Unknown | 0.76 | 0.61–0.96 | 0.023 | |
| Tumor size | <2 cm | Ref | ||
| >2 cm | 0.45 | 0.42–0.50 | <0.001 | |
| Unknown | 1.12 | 1.01–1.24 | 0.027 | |
| Lymph vascular invasion | Not present | Ref | ||
| Present | 1.11 | 0.94–1.31 | 0.239 | |
| Unknown | 1.32 | 1.15–1.51 | <0.001 |
| Heading | Hazard Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Type of Surgery | Radical Nephroureterectomy | 1 [REF] | --- | --- |
| Segmental Ureterectomy | 0.98 | 0.93–1.04 | 0.538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paciotti, M.; Alkhatib, K.Y.; Nguyen, D.-D.; Yim, K.; Lipsitz, S.R.; Mossanen, M.; Casale, P.; Pierorazio, P.M.; Kibel, A.S.; Trinh, Q.-D.; et al. Is Segmental Ureterectomy Associated with Inferior Survival for Localized Upper-Tract Urothelial Carcinoma of the Ureter Compared to Radical Nephroureterectomy? Cancers 2023, 15, 1373. https://doi.org/10.3390/cancers15051373
Paciotti M, Alkhatib KY, Nguyen D-D, Yim K, Lipsitz SR, Mossanen M, Casale P, Pierorazio PM, Kibel AS, Trinh Q-D, et al. Is Segmental Ureterectomy Associated with Inferior Survival for Localized Upper-Tract Urothelial Carcinoma of the Ureter Compared to Radical Nephroureterectomy? Cancers. 2023; 15(5):1373. https://doi.org/10.3390/cancers15051373
Chicago/Turabian StylePaciotti, Marco, Khalid Y. Alkhatib, David-Dan Nguyen, Kendrick Yim, Stuart R. Lipsitz, Matthew Mossanen, Paolo Casale, Phillip M. Pierorazio, Adam S. Kibel, Quoc-Dien Trinh, and et al. 2023. "Is Segmental Ureterectomy Associated with Inferior Survival for Localized Upper-Tract Urothelial Carcinoma of the Ureter Compared to Radical Nephroureterectomy?" Cancers 15, no. 5: 1373. https://doi.org/10.3390/cancers15051373
APA StylePaciotti, M., Alkhatib, K. Y., Nguyen, D.-D., Yim, K., Lipsitz, S. R., Mossanen, M., Casale, P., Pierorazio, P. M., Kibel, A. S., Trinh, Q.-D., Buffi, N. M., Lughezzani, G., & Cole, A. P. (2023). Is Segmental Ureterectomy Associated with Inferior Survival for Localized Upper-Tract Urothelial Carcinoma of the Ureter Compared to Radical Nephroureterectomy? Cancers, 15(5), 1373. https://doi.org/10.3390/cancers15051373







