Blood–Brain Barrier Disruption (BBBD)-Based Immunochemotherapy for Primary Central Nervous System Lymphoma (PCNSL), Early Results of a Phase II Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcomes
2.3. Procedures
2.4. Local Treatment of Intraocular Lymphoma
2.5. Side Effects
2.6. Response Assessment
2.7. Statistics
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haldorsen, I.S.; Krossnes, B.K.; Aarseth, J.H.; Scheie, D.; Johannesen, T.B.; Mella, O.; Espeland, A. Increasing incidence and continued dismal outcome of primary central nervous system lymphoma in Norway 1989–2003: Time trends in a 15-year national survey. Cancer 2007, 110, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.E.; Janney, C.A.; Rao, R.D.; Cerhan, J.R.; Kurtin, P.J.; Schiff, D.; Kaplan, R.S.; O’Neil, B.P. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: A surveillance, epidemiology, and end results analysis. Cancer 2002, 95, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Camilleri-Broët, S.; Martin, A.; Moreau, A.; Angonin, R.; Hénin, D.; Gontier, M.F.; Rousselet, M.C.; Caulet-Maugendre, S.; Cuillière, P.; Lefrancq, T.; et al. Primary Central Nervous System Lymphomas in 72 Immunocompetent Patients: Pathologic Findings and Clinical Correlations. Am. J. Clin. Pathol. 1998, 110, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T. Primary central nervous system lymphoma: A curable disease. Hematol. Oncol. 2019, 37 (Suppl. S1), 15–18. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Holdhoff, M.; Nayak, L.; Rubenstein, J.L. Evolving Treatments for Primary Central Nervous System Lymphoma. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 454–466. [Google Scholar] [CrossRef]
- Garcilazo-Reyes, Y.; Alentorn, A.; Duran-Pena, A.; Hoang-Xuan, K.; Houillier, C. Treatment of Primary Central Nervous System Lymphoma in Immunocompetent Patients. Curr. Treat. Options Neurol. 2019, 21, 39. [Google Scholar] [CrossRef]
- Gaut, D.; Schiller, G.J. Hematopoietic stem cell transplantation in primary central nervous system lymphoma: A review of the literature. Int. J. Hematol. 2019, 109, 260–277. [Google Scholar] [CrossRef]
- Grommes, C.; Rubenstein, J.L.; DeAngelis, L.M.; Ferreri, A.J.M.; Batchelor, T.T. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro-Oncology 2019, 21, 296–305. [Google Scholar] [CrossRef]
- Han, C.H.; Batchelor, T.T. Diagnosis and management of primary central nervous system lymphoma. Cancer 2017, 123, 4314–4324. [Google Scholar] [CrossRef]
- Grommes, C.; DeAngelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef]
- Löw, S.; Han, C.H.; Batchelor, T.T. Primary central nervous system lymphoma. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418793562. [Google Scholar] [CrossRef] [PubMed]
- Hoang-Xuan, K.; Deckert, M.; Ferreri, A.J.M.; Furtner, J.; Perez-Larraya, J.G.; Henriksson, R.; Hottinger, A.F.; Kasenda, B.; Lefranc, F.; Lossos, A.; et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro-Oncology 2023, 25, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Grommes, C. Primary central nervous system lymphoma. Blood 2022, 140, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; La Rosée, P.; Schorb, E.; et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; La Rosée, P.; Binder, M.; Fabbri, A.; Torri, V.; Minacapelli, E.; et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017, 4, e510–e523. [Google Scholar] [CrossRef] [PubMed]
- Harjama, L.; Kuitunen, H.; Turpeenniemi-Hujanen, T.; Haapasaari, K.M.; Leppä, S.; Mannisto, S.; Karjalainen-Lindsberg, M.-L.; Lehtinen, T.; Eray, M.; Vornanen, M.; et al. Constant pattern of relapse in primary central nervous lymphoma patients treated with high-dose methotrexate combinations. A Finnish retrospective study. Acta Oncol. 2015, 54, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Goldman, D.L.; Dahlborg, S.A.; Crossen, J.; Ramsey, F.; Roman-Goldstein, S.; Braziel, R.; Dana, B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: Prolonged survival and preservation of cognitive function. J. Clin. Oncol. 1991, 9, 1580–1590. [Google Scholar] [CrossRef]
- Angelov, L.; Doolittle, N.D.; Kraemer, D.F.; Siegal, T.; Barnett, G.H.; Peereboom, D.M.; Stevens, G.; McGregor, J.; Jahnke, K.; Lacy, C.A.; et al. Blood-Brain Barrier Disruption and Intra-Arterial Methotrexate-Based Therapy for Newly Diagnosed Primary CNS Lymphoma: A Multi-Institutional Experience. J. Clin. Oncol. 2009, 27, 3503–3509. [Google Scholar] [CrossRef]
- Kuitunen, H.; Tokola, S.; Siniluoto, T.; Isokangas, M.; Sonkajärvi, E.; Alahuhta, S.; Turpeenniemi-Hujanen, T.; Jantunen, E.; Nousiainen, T.; Vasala, K.; et al. Promising treatment results with blood brain barrier disruption (BBBD) based immunochemotherapy combined with autologous stem cell transplantation (ASCT) in patients with primary central nervous system lymphoma (PCNSL). J. Neuro-Oncol. 2017, 131, 293–300. [Google Scholar] [CrossRef]
- Abrey, L.E.; Batchelor, T.T.; Ferreri, A.J.; Gospodarowicz, M.; Pulczynski, E.J.; Zucca, E.; Smith, J.; Korfel, A.; Soussain, C.; DeAngelis, L.; et al. Report of an International Workshop to Standardize Baseline Evaluation and Response Criteria for Primary CNS Lymphoma. J. Clin. Oncol. 2005, 23, 5034–5043. [Google Scholar] [CrossRef]
- Schorb, E.; Finke, J.; Ferreri, A.J.M.; Ihorst, G.; Mikesch, K.; Kasenda, B.; Fritsch, K.; Fricker, H.; Burger, E.; Grishina, O.; et al. High-dose chemotherapy and autologous stem cell transplant compared with conventional chemotherapy for consolidation in newly diagnosed primary CNS lymphoma—A randomized phase III trial (MATRix). BMC Cancer 2016, 16, 282. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Wilson, D.J. Combination intravitreal rituximab and methotrexate for massive subretinal lymphoma. Eye 2010, 24, 1625–1627. [Google Scholar] [CrossRef] [PubMed]
- Abrey, L.E.; Ben-Porat, L.; Panageas, K.S.; Yahalom, J.; Berkey, B.; Curran, W.; Schultz, C.; Leibel, S.; Nelson, D.; Mehta, M.; et al. Primary Central Nervous System Lymphoma: The Memorial Sloan-Kettering Cancer Center Prognostic Model. J. Clin. Oncol. 2006, 24, 5711–5715. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Reni, M.; Foppoli, M.; Martelli, M.; Pangalis, G.A.; Frezzato, M.; Cabras, M.G.; Fabbri, A.; Corazzelli, G.; Ilariucci, F.; et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet 2009, 374, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Bendszus, M.; Koltzenburg, M.; Burger, R.; Warmuth-Metz, M.; Hofmann, E.; Solymosi, L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: A prospective study. Lancet 1999, 354, 1594–1597. [Google Scholar] [CrossRef]
- Shibazaki, K.; Iguchi, Y.; Kimura, K.; Ueno, Y.; Inoue, T. New asymptomatic ischemic lesions on diffusion-weighted imaging after cerebral angiography. J. Neurol. Sci. 2008, 266, 150–155. [Google Scholar] [CrossRef]
- McAllister, L.D.; Doolittle, N.D.; Guastadisegni, P.E.; Kraemer, D.F.; Lacy, C.A.; Crossen, J.R.; Neuwelt, E.A. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery 2000, 46, 51–60. [Google Scholar] [CrossRef]
- Yuen, H.L.A.; Slocombe, A.; Heron, V.; Chunilal, S.; Shortt, J.; Tatarczuch, M.; Grigoriadis, G.; Patil, S.; Gregory, G.P.; Opat, S.; et al. Venous thromboembolism in primary central nervous system lymphoma during frontline chemoimmunotherapy. Res. Pract. Thromb. Haemost. 2020, 4, 997–1003. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Chakravarti, A.; Finkelstein, D.M.; Hochberg, F.H.; Batchelor, T.T.; Loeffler, J.S. Results of Whole-Brain Radiation as Salvage of Methotrexate Failure for Immunocompetent Patients with Primary CNS Lymphoma. J. Clin. Oncol. 2005, 23, 1507–1513. [Google Scholar] [CrossRef]
- Hottinger, A.F.; DeAngelis, L.M.; Yahalom, J.; Abrey, L.E. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology 2007, 69, 1178–1182. [Google Scholar] [CrossRef]
- Herrlinger, U.; Schäfer, N.; Fimmers, R.; Griesinger, F.; Rauch, M.; Kirchen, H.; Roth, P.; Glas, M.; Bamberg, M.; Martus, P.; et al. Early whole brain radiotherapy in primary CNS lymphoma: Negative impact on quality of life in the randomized G-PCNSL-SG1 trial. J. Cancer Res. Clin. Oncol. 2017, 143, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Houillier, C.; Taillandier, L.; Dureau, S.; Lamy, T.; Laadhari, M.; Chinot, O.; Moluçon-Chabrot, C.; Soubeyran, P.; Gressin, R.; Choquet, S.; et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J. Clin. Oncol. 2019, 37, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kepka, L.; Tyc-Szczepaniak, D.; Osowiecka, K.; Sprawka, A.; Trąbska-Kluch, B.; Czeremszynska, B. Quality of life after whole brain radiotherapy compared with radiosurgery of the tumor bed: Results from a randomized trial. Clin. Transl. Oncol. 2018, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.D.; Braun, E.; Kryza-Lacombe, M.; Ho, K.-W.; Reiner, A.S.; Panageas, K.S.; Yahalom, J.; Sauter, C.S.; Abrey, L.E.; DeAngelis, L.M.; et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J. Neuro-Oncol. 2019, 144, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.D.; Shi, W.; Abrey, L.E.; DeAngelis, L.M.; Omuro, A.M.; Deutsch, M.B.; Thaler, H.T. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro-Oncology 2012, 14, 101–108. [Google Scholar] [CrossRef]
- Doolittle, N.D.; Korfel, A.; Lubow, M.A.; Schorb, E.; Schlegel, U.; Rogowski, S.; Fu, R.; Dósa, E.; Illerhaus, G.; Kraemer, D.F.; et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 2013, 81, 84–92. [Google Scholar] [CrossRef]
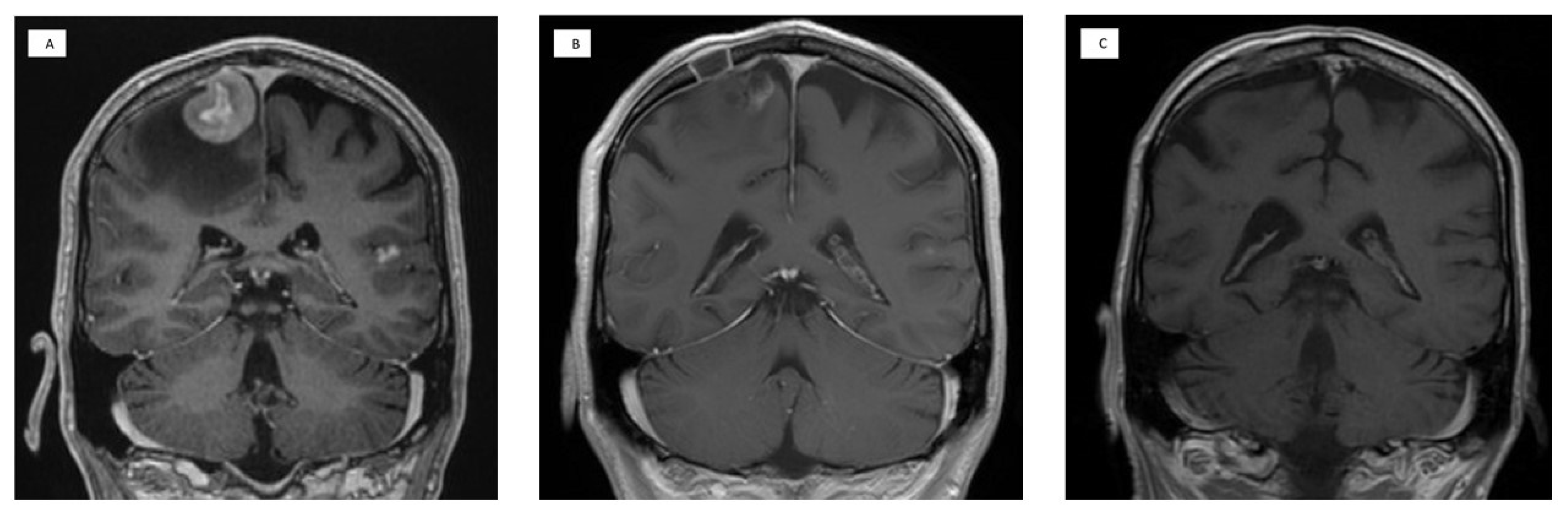
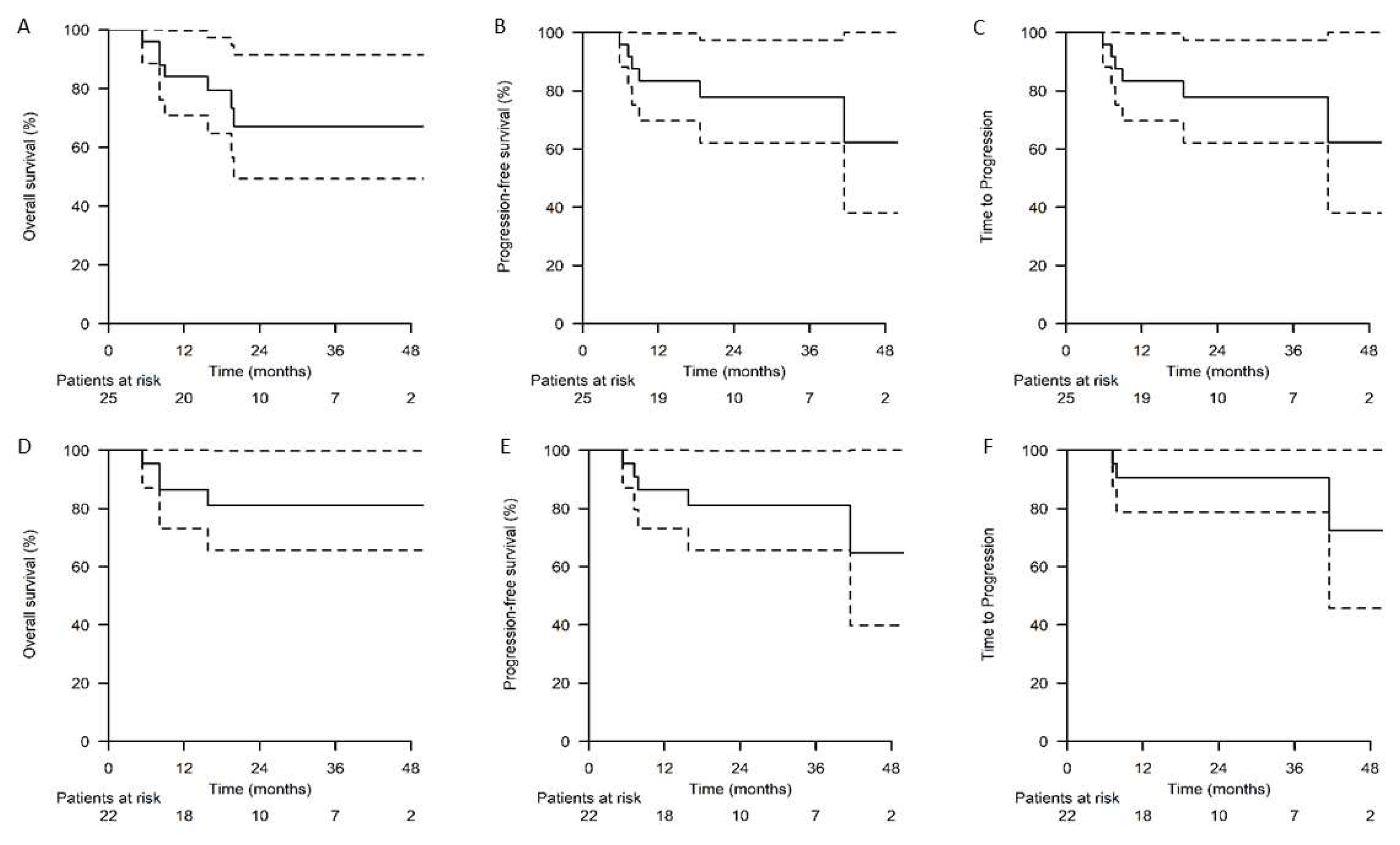
| Chemotherapy Agent | Dose | Route | Day of Cycle |
|---|---|---|---|
| Rituximab | 375 mg/m2 | intravenous | 0 |
| Methotrexate | 2500 mg/m2 | intra-arterial | 1–2 |
| Carboplatin | 200 mg/m2 | intra-arterial | 1–2 |
| Dexamethasone | 6 mg × 4–6 | peroral | 2–10 |
| Cytarabine | 40 mg | intrathecal | 14 |
| Cyclophosphamide | 330 mg/m2 | intravenous | 1–2 |
| Etoposide | 200 mg/m2 | intravenous | 1–2 |
| Sex | N (%) |
|---|---|
| Male | 12 (48) |
| Female | 13 (52) |
| Age at diagnosis | (mean + SD) |
| Years | 61.3 ± 10.9 |
| Performance status (WHO a) | N (%) |
| 0 | 4 (16) |
| 1 | 10 (40) |
| 2 | 5 (20) |
| 3 | 6 (24) |
| MSKCC b risk group | N (%) |
| 0 (age < 50 years) | 4 (16) |
| 1 (age ≥ 50 years and KPS c ≥ 70%) | 11 (44) |
| 2 (age ≥ 50 years and KPS c < 70%) | 10 (40) |
| Deep brain structure involvement | N (%) |
| Yes | 24 (96) |
| No | 1 (4) |
| Eye involvement | N (%) |
| No | 22 (88) |
| Yes | 3 (12) |
| Spinal involvement | N (%) |
| No | 23 (92) |
| Yes | 1 (4) |
| N/A | 1 (4) |
| Response | After MATRix Regimen | After BBBD Treatment | At Restaging |
|---|---|---|---|
| Complete response, N (%) | 0 (0) | 19 (76) | 22 (88) |
| Partial response, N (%) | 23 (92) | 5 (20) | 2 (8) |
| Stable disease, N (%) | 0 (0) | 0 (0) | 0 (0) |
| Progressive disease, N (%) | 1 (4) | 0 (0) | 1 (4) |
| Not evaluable, N (%) | 1 (4) | 1 (4) | 0 (0) |
| Hematological AE a | All Grades, N (%) | Grade III–IV, N (%) |
|---|---|---|
| Anemia | 25 (100) | 20 (80) |
| Neutropenia | 25 (100) | 25 (100) |
| WBC b decreased | 25 (100) | 23 (92) |
| Platelet level decreased | 25 (100) | 23 (92) |
| Infection | 21 (84) | 19 (76) |
| Red blood cell transfusion | 21 (84) | |
| Platelet transfusion | 17 (68) | |
| Neurological AE a | ||
| Tinnitus | 1 (4) | |
| Hearing loss | 0 (0) | |
| Retinopathy | 0 (0) | |
| CNS c ischemia (asymptomatic) | 5 (20) | |
| CNS c ischemia (symptomatic) | 0 (0) | |
| Other AE a | ||
| Mucositis | 16 (64) | 5 (20) |
| Nausea | 17 (68) | 3 (12) |
| Deep vein thrombosis | 2 (8) | |
| Pulmonary embolism | 4 (16) | |
| Osteoporotic fracture | 2 (8) | |
| High-dose treatment AE a | ||
| Infection | 25 (100) | 25 (100) |
| Mucositis | 23 (92) | 22 (88) |
| Red blood cell transfusion | 19 (76) | |
| Platelet transfusion | 25 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuitunen, H.K.; Rönkä, A.L.K.; Sonkajärvi, E.M.; Isokangas, J.-M.; Pyörälä, M.; Palosaari, K.A.A.; Jokimäki, A.S.; Partanen, A.E.; Littow, H.J.; Vakkala, M.A.; et al. Blood–Brain Barrier Disruption (BBBD)-Based Immunochemotherapy for Primary Central Nervous System Lymphoma (PCNSL), Early Results of a Phase II Study. Cancers 2023, 15, 1341. https://doi.org/10.3390/cancers15041341
Kuitunen HK, Rönkä ALK, Sonkajärvi EM, Isokangas J-M, Pyörälä M, Palosaari KAA, Jokimäki AS, Partanen AE, Littow HJ, Vakkala MA, et al. Blood–Brain Barrier Disruption (BBBD)-Based Immunochemotherapy for Primary Central Nervous System Lymphoma (PCNSL), Early Results of a Phase II Study. Cancers. 2023; 15(4):1341. https://doi.org/10.3390/cancers15041341
Chicago/Turabian StyleKuitunen, Hanne K., Aino L. K. Rönkä, Eila M. Sonkajärvi, Juha-Matti Isokangas, Marja Pyörälä, Kari A. A. Palosaari, Anna S. Jokimäki, Anu E. Partanen, Harri J. Littow, Merja A. Vakkala, and et al. 2023. "Blood–Brain Barrier Disruption (BBBD)-Based Immunochemotherapy for Primary Central Nervous System Lymphoma (PCNSL), Early Results of a Phase II Study" Cancers 15, no. 4: 1341. https://doi.org/10.3390/cancers15041341
APA StyleKuitunen, H. K., Rönkä, A. L. K., Sonkajärvi, E. M., Isokangas, J.-M., Pyörälä, M., Palosaari, K. A. A., Jokimäki, A. S., Partanen, A. E., Littow, H. J., Vakkala, M. A., Jantunen, E. J., Huttunen, M. E., Marin, K. J., Aromaa-Häyhä, A. M. K., Auvinen, P. K., Selander, T., Puhakka, I. K., & Kuittinen, O. M. (2023). Blood–Brain Barrier Disruption (BBBD)-Based Immunochemotherapy for Primary Central Nervous System Lymphoma (PCNSL), Early Results of a Phase II Study. Cancers, 15(4), 1341. https://doi.org/10.3390/cancers15041341





