A Comprehensive View of the Cancer-Immunity Cycle (CIC) in HPV-Mediated Cervical Cancer and Prospects for Emerging Therapeutic Opportunities
Abstract
Simple Summary
Abstract
1. Introduction
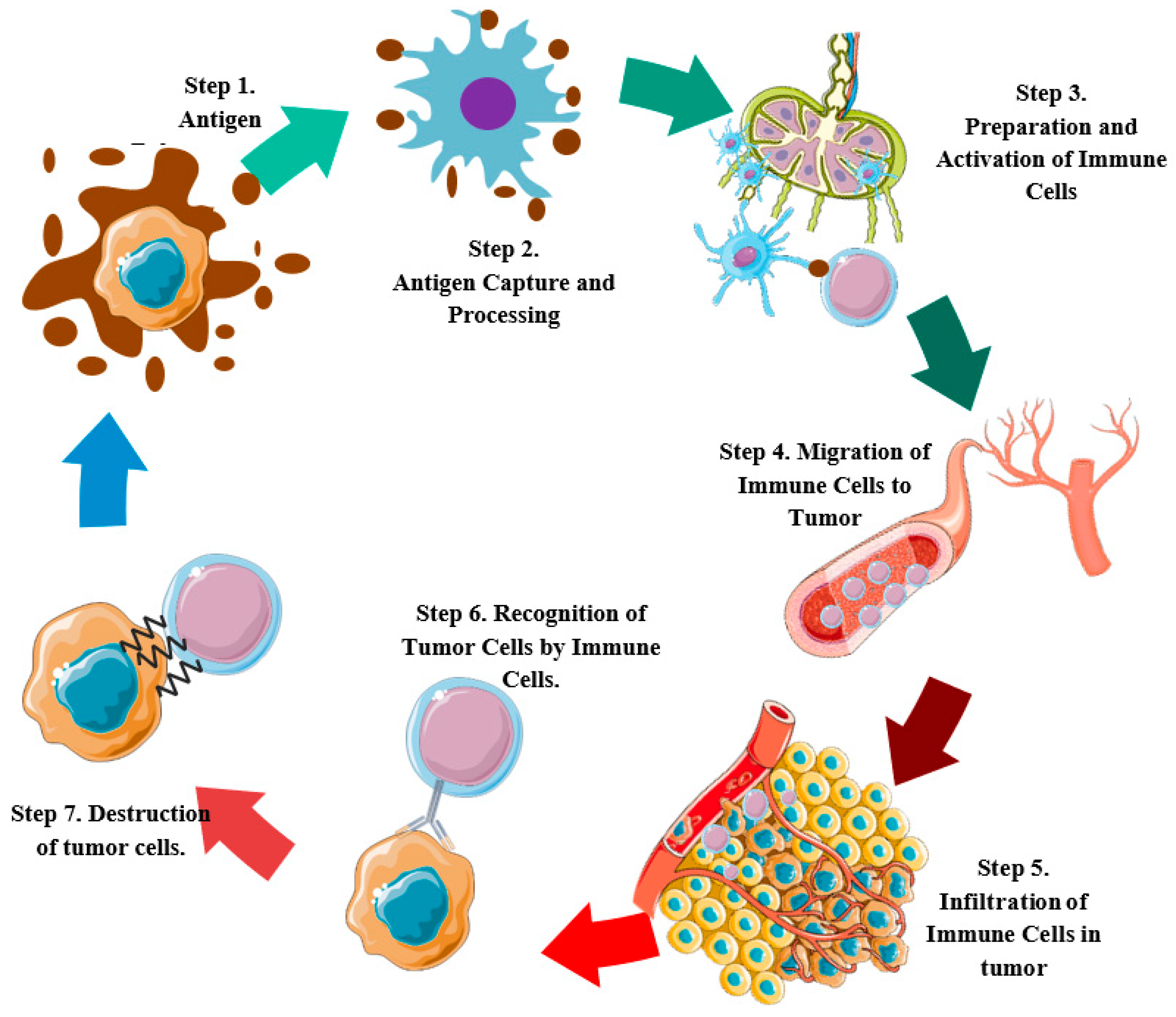
2. Molecular Events in the Cancer-Immunity Cycle and Development of Cervical Tumors
2.1. Cell Death and Antigen Release (Step 1)
2.2. Capture and Antigen Processing (Step 2)
2.3. Priming and Activation of Immune Cells (Step 3)
2.4. Migration of Immune Cells to the Tumor (Step 4)
2.5. Infiltration of Immune Cells into the Tumor (Step 5)
2.6. Recognition of Tumor Cells by Immune Cells (Step 6)
2.7. Destruction of Tumor Cells (Step 7)
3. CIC-Targeted Immunotherapies in Cervical Cancer
3.1. DNA Vaccines
3.2. DCs Based Vaccines
3.3. T-Cell-Based Vaccines
3.4. Non-Coding RNA-Based Therapies
3.5. CRISPR/Cas9 Gene Editing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155 (Suppl. S1), 28–44. [Google Scholar] [CrossRef]
- Wiemann, B.; Starnes, C.O. Coley’s toxins, tumor necrosis factor and cancer research: A historical perspective. Pharmacol. Ther. 1994, 64, 529–564. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Guo, Z.S. The 2018 Nobel Prize in medicine goes to cancer immunotherapy. BMC Cancer 2018, 18, 1086. [Google Scholar] [CrossRef]
- Cancer Immunotherapy Pioneers Win Medicine Nobel. Available online: https://www.science.org/content/article/cancer-immunotherapy-pioneers-win-medicine-nobel (accessed on 16 December 2022).
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef] [PubMed]
- Maskey, N.; Maskey, N.; Thapa, N.; Thapa, N.; Maharjan, M.; Maharjan, M.; Shrestha, G.; Shrestha, G.; Maharjan, N.; Maharjan, N.; et al. Infiltrating CD4 and CD8 lymphocytes in HPV infected uterine cervical milieu. Cancer Manag. Res. 2019, 11, 7647–7655. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Contribution of regulatory T cells to cancer: A review. J. Cell. Physiol. 2018, 234, 7983–7993. [Google Scholar] [CrossRef]
- Moynihan, K.D.; Irvine, D.J. Roles for Innate Immunity in Combination Immunotherapies. Cancer Res. 2017, 77, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature 2019, 574, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kametani, Y.; Miyamoto, A.; Seki, T.; Ito, R.; Habu, S.; Tokuda, Y. The significance of humanized mouse models for the evaluation of the humoral immune response against cancer vaccines. Pers. Med. Univ. 2018, 7, 13–18. [Google Scholar] [CrossRef]
- Varn, F.S.; Mullins, D.W.; Arias-Pulido, H.; Fiering, S.; Cheng, C. Adaptive immunity programmes in breast cancer. Immunology 2016, 150, 25–34. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G.; Dougan, S.K. Cancer Immunotherapy: Beyond Checkpoint Blockade. Annu. Rev. Cancer Biol. 2019, 3, 55–75. [Google Scholar] [CrossRef]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774. [Google Scholar] [CrossRef]
- Horton, B.L.; Fessenden, T.B.; Spranger, S. Tissue Site and the Cancer Immunity Cycle. Trends Cancer 2019, 5, 593–603. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, C.; Liu, J.; Li, G. Resistance Mechanism of PD-1/PD-L1 Blockade in the Cancer-Immunity Cycle. OncoTargets Ther. 2020, 13, 83–94. [Google Scholar] [CrossRef]
- Zhu, Y.; An, X.; Zhang, X.; Qiao, Y.; Zheng, T.; Li, X. STING: A master regulator in the cancer-immunity cycle. Mol. Cancer 2019, 18, 152. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Wang, X.; Zhang, C.; Jiang, X. Natural killer cell awakening: Unleash cancer-immunity cycle against glioblastoma. Cell Death Dis. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- de Freitas, A.C.; Coimbra, E.C.; Leitão, M.D.C.G. Molecular targets of HPV oncoproteins: Potential biomarkers for cervical carcinogenesis. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2014, 1845, 91–103. [Google Scholar] [CrossRef]
- Smola, S. Immunopathogenesis of HPV-Associated Cancers and Prospects for Immunotherapy. Viruses 2017, 9, 254. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Terrassoux, L.; Claux, H.; Bacari, S.; Meignan, S.; Furlan, A. A Bloody Conspiracy—Blood Vessels and Immune Cells in the Tumor Microenvironment. Cancers 2022, 14, 4581. [Google Scholar] [CrossRef] [PubMed]
- Bayik, D.; Lathia, J.D. Cancer stem cell–immune cell crosstalk in tumour progression. Nat. Rev. Cancer 2021, 21, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Rs, J. The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions—Evidence for Estrogen as an Immunomodulator. Front. Cell. Infect. Microbiol. 2021, 11, 649815. [Google Scholar]
- Gorvel, L.; Olive, D. Tumor associated macrophage in HPV+ tumors: Between immunosuppression and inflammation. Semin. Immunol. 2023, 65, 101671. [Google Scholar] [CrossRef] [PubMed]
- Nahand, J.S.; Moghoofei, M.; Salmaninejad, A.; Bahmanpour, Z.; Karimzadeh, M.; Nasiri, M.; Mirzaei, H.R.; Pourhanifeh, M.H.; Bokharaei-Salim, F.; Mirzaei, H.; et al. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: A review. Int. J. Cancer 2019, 146, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, M.L.M.; Carmo, T.I.T.D.; Zanini, D.; Cardoso, A.M. Inflammatory profile in cervical cancer: Influence of purinergic signaling and possible therapeutic targets. Inflamm. Res. 2022, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, F.; Teles, A.M.; Nascimento, M.D.D.S.B.; DOS Santos, A.P.A.; Lopes, F.F.; Paiva, A.; Brito, H.O.; GIL DA Costa, R. Human Papillomavirus Modulates Matrix Metalloproteinases During Carcinogenesis: Clinical Significance and Role of Viral Oncoproteins. In Vivo 2022, 36, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-J.; Sun, S.-G.; Tang, X.-Y.; Lin, Y.-Y.; Hua, K.-Q. Extracellular vesicular Wnt7b mediates HPV E6-induced cervical cancer angiogenesis by activating the β-catenin signaling pathway. J. Exp. Clin. Cancer Res. 2020, 39, 260. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Yadav, J.; Thakur, K.; Aggarwal, N.; Tripathi, T.; Chhokar, A.; Singh, T.; Jadli, M.; Bharti, A.C. Exosomes from cervical cancer cells facilitate pro-angiogenic endothelial reconditioning through transfer of Hedgehog–GLI signaling components. Cancer Cell Int. 2021, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Kuzet, S.-E.; Gaggioli, C. Fibroblast activation in cancer: When seed fertilizes soil. Cell Tissue Res. 2016, 365, 607–619. [Google Scholar] [CrossRef]
- Hao, N.-B.; Lü, M.-H.; Fan, Y.-H.; Cao, Y.-L.; Zhang, Z.-R.; Yang, S.-M. Macrophages in Tumor Microenvironments and the Progression of Tumors. J. Immunol. Res. 2012, 2012, e948098. [Google Scholar] [CrossRef]
- Xu, L.; Cai, H.; Zhu, N.; Zheng, B. Interleukin-22 derived from cervical cancer-associated fibroblasts accelerates senescence of normal fibroblasts and promotes expression of tumorigenesis-related factors in HeLa cells. Eur. J. Gynaecol. Oncol. 2020, 41, 192–199. [Google Scholar] [CrossRef]
- Chen, X.-J.; Han, L.-F.; Wu, X.-G.; Wei, W.-F.; Wu, L.-F.; Yi, H.-Y.; Yan, R.-M.; Bai, X.-Y.; Zhong, M.; Yu, Y.-H.; et al. Clinical Significance of CD163+ and CD68+ Tumor-associated Macrophages in High-risk HPV-related Cervical Cancer. J. Cancer 2017, 8, 3868–3875. [Google Scholar] [CrossRef]
- Lai, Y.; Wahyuningtyas, R.; Aui, S.; Chang, K. AutocrineVEGFsignalling on M2 macrophages regulatesPD-L1 expression for immunomodulation of T cells. J. Cell. Mol. Med. 2018, 23, 1257–1267. [Google Scholar] [CrossRef]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef]
- Walch-Rückheim, B.; Mavrova, R.; Henning, M.; Vicinus, B.; Kim, Y.-J.; Bohle, R.M.; Juhasz-Böss, I.; Solomayer, E.-F.; Smola, S. Stromal Fibroblasts Induce CCL20 through IL6/C/EBPβ to Support the Recruitment of Th17 Cells during Cervical Cancer Progression. Cancer Res. 2015, 75, 5248–5259. [Google Scholar] [CrossRef]
- Murata, T.; Mizushima, H.; Chinen, I.; Moribe, H.; Yagi, S.; Hoffman, R.M.; Kimura, T.; Yoshino, K.; Ueda, Y.; Enomoto, T.; et al. HB-EGF and PDGF Mediate Reciprocal Interactions of Carcinoma Cells with Cancer-Associated Fibroblasts to Support Progression of Uterine Cervical Cancers. Cancer Res. 2011, 71, 6633–6642. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.-Y.; Yang, J.-T.; Huang, T.-H.; Liu, H.-W. Crosstalk with Cancer-Associated Fibroblasts Increases the Growth and Radiation Survival of Cervical Cancer Cells. Radiat. Res. 2014, 181, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Fullár, A.; Dudás, J.; Oláh, L.; Hollósi, P.; Papp, Z.; Sobel, G.; Karászi, K.; Paku, S.; Baghy, K.; Kovalszky, I. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiology 2020, 88, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liao, G.; Li, G. Regulatory effects of COL1A1 on apoptosis induced by radiation in cervical cancer cells. Cancer Cell Int. 2017, 17, 73. [Google Scholar] [CrossRef]
- Huang, X.; Shi, H.; Shi, X.; Jiang, X. LncRNA FBXL19-AS1 promotes proliferation and metastasis of cervical cancer through upregulating COL1A1 as a sponge of miR-193a-5p. J. Biol. Res. 2021, 28, 20. [Google Scholar] [CrossRef]
- Zheng, J.; Dai, X.; Chen, H.; Fang, C.; Chen, J.; Sun, L. Down-regulation of LHPP in cervical cancer influences cell proliferation, metastasis and apoptosis by modulating AKT. Biochem. Biophys. Res. Commun. 2018, 503, 1108–1114. [Google Scholar] [CrossRef]
- Jiang, F.; Bian, G.; Li, J. ASPP2 promotes cell apoptosis in cervical cancer through inhibiting autophagy. Exp. Ther. Med. 2022, 24, 726. [Google Scholar] [CrossRef]
- Zhou, Y.; Shu, C.; Huang, Y. Fibronectin promotes cervical cancer tumorigenesis through activating FAK signaling pathway. J. Cell. Biochem. 2019, 120, 10988–10997. [Google Scholar] [CrossRef]
- Yang, Y.; Han, A.; Wang, X.; Yin, X.; Cui, M.; Lin, Z. Lipid metabolism regulator human hydroxysteroid dehydrogenase-like 2 (HSDL2) modulates cervical cancer cell proliferation and metastasis. J. Cell. Mol. Med. 2021, 25, 4846–4859. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Liu, M.; Ning, Q.; Xiang, Z.; Zheng, X.; Tang, S.; Mo, Z. MiR-26a-5p regulates proliferation, apoptosis, migration and invasion via inhibiting hydroxysteroid dehydrogenase like-2 in cervical cancer cell. BMC Cancer 2022, 22, 876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, T.; Wang, Y.; Yu, J.; Liu, Y.; Lin, Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol. Ther. 2015, 17, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Lin, Z. MiR-21 is involved in cervical squamous cell tumorigenesis and regulates CCL20. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zhao, H. Analysis of diagnostic and prognostic value of lncRNA MEG3 in cervical cancer. Oncol. Lett. 2020, 20, 183. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, W.; Chen, L.; Liu, L. MicroRNA-433 Inhibits Cell Growth and Induces Apoptosis in Human Cervical Cancer through PI3K/AKT Signaling by Targeting FAK. Available online: https://www.spandidos-publications.com/10.3892/or.2018.6718 (accessed on 16 December 2022).
- Zhang, J.; Gao, Y. CCAT-1 promotes proliferation and inhibits apoptosis of cervical cancer cells via the Wnt signaling pathway. Oncotarget 2017, 8, 68059–68070. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, I.; Thavathiru, E.; Ramalingam, S.; Natarajan, G.; Mills, W.K.; Benbrook, D.M.; Zuna, R.; Lightfoot, S.; Reis, A.; Anant, S.; et al. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene 2011, 31, 2725–2737. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, J.; Liu, L. The long noncoding RNA PCGEM1 promotes cell proliferation, migration and invasion via targeting the miR-182/FBXW11 axis in cervical cancer. Cancer Cell Int. 2019, 19, 304. [Google Scholar] [CrossRef]
- Jones, D.L.; Alani, R.M.; Münger, K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997, 11, 2101–2111. [Google Scholar] [CrossRef]
- Cabeça, T.K.; Abreu, A.D.M.; Andrette, R.; Lino, V.D.S.; Morale, M.G.; Aguayo, F.; Termini, L.; Villa, L.L.; Lepique, A.P.; Boccardo, E. HPV-Mediated Resistance to TNF and TRAIL Is Characterized by Global Alterations in Apoptosis Regulatory Factors, Dysregulation of Death Receptors, and Induction of ROS/RNS. Int. J. Mol. Sci. 2019, 20, 198. [Google Scholar] [CrossRef]
- Gómez-Gómez, Y.; Organista-Nava, J.; Ocadiz-Delgado, R.; García-Villa, E.; Leyva-Vazquez, M.A.; Illades-Aguiar, B.; Lambert, P.F.; García-Carrancá, A.; Gariglio, P. The expression of miR-21 and miR-143 is deregulated by the HPV16 E7 oncoprotein and 17β-estradiol. Int. J. Oncol. 2016, 49, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, S.; Zhao, Z.; Mao, X.; Huang, J.; Wu, Z.; Zheng, L.; Wang, Q. MicroRNA-27b up-regulated by human papillomavirus 16 E7 promotes proliferation and suppresses apoptosis by targeting polo-like kinase2 in cervical cancer. Oncotarget 2016, 7, 19666–19679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Fan, L.-P. Long non-coding RNA CRNDE enhances cervical cancer progression by suppressing PUMA expression. Biomed. Pharmacother. 2019, 117, 108726. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Munger, K. The Role of Long Noncoding RNAs in Human Papillomavirus-associated Pathogenesis. Pathogens 2020, 9, 289. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Bai, L.; Li, W.; Zheng, W.; Xu, D.; Chen, N.; Cui, J. Promising targets based on pattern recognition receptors for cancer immunotherapy. Pharmacol. Res. 2020, 159, 105017. [Google Scholar] [CrossRef]
- Douzandeh-Mobarrez, B.; Kariminik, A.; Arababadi, M.K.; Kheirkhah, B. TLR9 in the Human Papilloma Virus Infections: Friend or Foe? Viral Immunol. 2022, 35, 457–464. [Google Scholar] [CrossRef]
- Manjgaladze, K.; Tevdorashvili, G.; Muzashvili, T.; Gachechiladze, M.; Burkadze, G. TLR9 expression, angerhans cell density and lymphocytic infiltration in progressing cervical intraepithelial neoplasia. Georgian Med. News 2019, 296, 126–130. [Google Scholar]
- Cannella, F.; Pierangeli, A.; Scagnolari, C.; Cacciotti, G.; Tranquilli, G.; Stentella, P.; Recine, N.; Antonelli, G. TLR9 is expressed in human papillomavirus-positive cervical cells and is overexpressed in persistent infections. Immunobiology 2015, 220, 363–368. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Liu, L.; Chen, Z.; Qiang, F.; Kan, Y.; Shen, Y.; Wu, J.; Shen, H.; Hu, Z. A Genetic Variant in the Promoter Region of Toll-Like Receptor 9 and Cervical Cancer Susceptibility. DNA Cell Biol. 2012, 31, 766–771. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Xu, D.; Li, X. The Relationship of the TLR9 and TLR2 Genetic Polymorphisms with Cervical Cancer Risk: A Meta-Analysis of Case-Control Studies. Pathol. Oncol. Res. 2018, 26, 307–315. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, C.A.; Rosa, B.; Jackson, R.; Niccoli, S.; Escott, N.G.; Zehbe, I. Toll-Like Receptor Transcriptome in the HPV-Positive Cervical Cancer Microenvironment. J. Immunol. Res. 2011, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, G.; Wang, X.; Huang, Y.; Ding, J.; Huang, J.; Hong, L. TLR4 May Accelerate Hypoxia Reaction to Promote the Occurrence and Progress of Cervical Lesions by Infected Pathogenic Microorganisms Other than HPV. J. Cancer Ther. 2013, 04, 549–553. [Google Scholar] [CrossRef]
- Iuliano, M.; Mangino, G.; Chiantore, M.V.; Fiorucci, G.; Accardi, R.; Tommasino, M.; Romeo, G. The Role of Microenvironment in Tumorigenesis: Focus on Dendritic Cells in Human Papillomavirus E6, E7-transformed keratinocytes. Cancer Cell Microenviron. 2015, 2, e874. [Google Scholar] [CrossRef]
- Dai, W.; Gui, L.; Du, H.; Li, S.; Wu, R. The association of cervicovaginal Langerhans cells with clearance of human papillomavirus. Front. Immunol. 2022, 13, 918190. [Google Scholar] [CrossRef]
- Leong, C.-M.; Doorbar, J.; Nindl, I.; Yoon, H.-S.; Hibma, M.H. Deregulation of E-cadherin by human papillomavirus is not confined to high-risk, cancer-causing types. Br. J. Dermatol. 2010, 163, 1253–1263. [Google Scholar] [CrossRef]
- Guess, J.C.; McCance, D.J. Decreased Migration of Langerhans Precursor-like Cells in Response to Human Keratinocytes Expressing Human Papillomavirus Type 16 E6/E7 Is Related to Reduced Macrophage Inflammatory Protein-3alpha Production. J. Virol. 2005, 79, 14852–14862. [Google Scholar] [CrossRef]
- Jiang, B.; Xue, M. Correlation of E6 and E7 levels in high-risk HPV16 type cervical lesions with CCL20 and Langerhans cells. Genet. Mol. Res. 2015, 14, 10473–10481. [Google Scholar] [CrossRef]
- Fernandes, A.T.G.; Carvalho, M.O.O.; Avvad-Portari, E.; Rocha, N.P.; Russomano, F.; Roma, E.H.; Bonecini-Almeida, M.D.G. A prognostic value of CD45RA+, CD45RO+, CCL20+ and CCR6+ expressing cells as ‘immunoscore’ to predict cervical cancer induced by HPV. Sci. Rep. 2021, 11, 8782. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-kappa B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; McCance, D.J. Down Regulation of the Interleukin-8 Promoter by Human Papillomavirus Type 16 E6 and E7 through Effects on CREB Binding Protein/p300 and P/CAF. J. Virol. 2002, 76, 8710–8721. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Tummers, B.; Meyers, C.; Biryukov, J.L.; Alam, S.; Backendorf, C.; Jha, V.; Offringa, R.; van Ommen, G.-J.B.; Melief, C.J.M.; et al. Human Papillomavirus (HPV) Upregulates the Cellular Deubiquitinase UCHL1 to Suppress the Keratinocyte’s Innate Immune Response. PLoS Pathog. 2013, 9, e1003384. [Google Scholar] [CrossRef] [PubMed]
- Vandermark, E.R.; Deluca, K.A.; Gardner, C.R.; Marker, D.F.; Schreiner, C.N.; Strickland, D.A.; Wilton, K.M.; Mondal, S.; Woodworth, C.D. Human papillomavirus type 16 E6 and E 7 proteins alter NF-kB in cultured cervical epithelial cells and inhibition of NF-kB promotes cell growth and immortalization. Virology 2012, 425, 53–60. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, Z.J.; Jolly, C.; Androphy, E.J.; Mercer, A.; Matthews, C.M.; Hibma, M.H. Transcriptional Repression of E-Cadherin by Human Papillomavirus Type 16 E6. PLoS ONE 2012, 7, e48954. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Diener, N.; Zahner, S.P.; Tripp, C.; Backer, R.A.; Karram, K.; Jiang, A.; Mellman, I.; Stoitzner, P.; Clausen, B.E. E-Cadherin is Dispensable to Maintain Langerhans Cells in the Epidermis. J. Investig. Dermatol. 2019, 140, 132–142.e3. [Google Scholar] [CrossRef] [PubMed]
- Leo, P.J.; Madeleine, M.M.; Wang, S.; Schwartz, S.M.; Newell, F.; Pettersson-Kymmer, U.; Hemminki, K.; Hallmans, G.; Tiews, S.; Steinberg, W.; et al. Correction: Defining the genetic susceptibility to cervical neoplasia—A genome-wide association study. PLoS Genet. 2018, 14, e1007257. [Google Scholar] [CrossRef]
- Campo, M.; Graham, S.; Cortese, M.; Ashrafi, G.; Araibi, E.; Dornan, E.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 2010, 407, 137–142. [Google Scholar] [CrossRef]
- Li, C.; Dai, S.; Yan, Z.; Zhang, X.; Liu, S.; Wang, X.; Wang, J.; Shi, L.; Yao, Y. Genetic polymorphisms of proteasome subunit genes of the MHC-I antigen-presenting system are associated with cervical cancer in a Chinese Han population. Hum. Immunol. 2020, 81, 445–451. [Google Scholar] [CrossRef]
- de Moura, E.L.; dos Santos, I.F.; de Freitas, P.P.; da Silva, D.M.; dos Santos, A.C.M.; Neto, A.B.L.; e Silva, A.C.P.; Barbosa, N.R.; Nascimento, C.A.; Balliano, T.L.; et al. Polymorphisms in Toll-like receptors genes changes the host’s immune response and is associated with cervical cancer. Immunobiology 2022, 227, 152187. [Google Scholar] [CrossRef]
- Parsaeian, S.F.; Asadian, F.; Karimi-Zarchi, M.; Setayesh, S.; Javaheri, A.; Tabatabaie, R.S.; Dastgheib, S.A.; Golestanpour, H.; Neamatzadeh, H. A Meta-Analysis for Association of XRCC3 rs861539, MTHFR rs1801133, IL-6 rs1800795, IL-12B rs3212227, TNF-α rs1800629, and TLR9 rs352140 Polymorphisms with Susceptibility to Cervical Carcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 3419–3431. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, W.; Ou, M.; Guo, C.; Ye, X.; Liu, Y.; Wang, M.; Zhang, B.; Zhang, N.; Huang, S.; et al. Interaction between susceptibility loci in cGAS-STING pathway, MHC gene and HPV infection on the risk of cervical precancerous lesions in Chinese population. Oncotarget 2016, 7, 84228–84238. [Google Scholar] [CrossRef] [PubMed]
- Das, A.P.; Saini, S.; Agarwal, S.M. A comprehensive meta-analysis of non-coding polymorphisms associated with precancerous lesions and cervical cancer. Genomics 2022, 114, 110323. [Google Scholar] [CrossRef] [PubMed]
- Pahne-Zeppenfeld, J.; Schröer, N.; Walch-Rückheim, B.; Oldak, M.; Gorter, A.; Hegde, S.; Smola, S. Cervical Cancer Cell-Derived Interleukin-6 Impairs CCR7-Dependent Migration of MMP-9-Expressing Dendritic Cells. Available online: https://pubmed.ncbi.nlm.nih.gov/24136650/?dopt=Abstract (accessed on 3 June 2020).
- Song, Z.; Lin, Y.; Ye, X.; Feng, C.; Lu, Y.; Yang, G.; Dong, C. Expression of IL-1α and IL-6 is Associated with Progression and Prognosis of Human Cervical Cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 4475–4481. [Google Scholar] [CrossRef] [PubMed]
- Artaza-Irigaray, C.; Molina-Pineda, A.; Aguilar-Lemarroy, A.; Ortiz-Lazareno, P.; Limón-Toledo, L.P.; Pereira-Suárez, A.L.; Rojo-Contreras, W.; Jave-Suárez, L.F. E6/E7 and E6* From HPV16 and HPV18 Upregulate IL-6 Expression Independently of p53 in Keratinocytes. Front. Immunol. 2019, 10, 1676. [Google Scholar] [CrossRef]
- Mueller, S.N.; Germain, R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 2009, 9, 618–629. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Woodham, A.W.; Skeate, J.G.; Rijkee, L.K.; Taylor, J.R.; Brand, H.E.; Muderspach, L.I.; Roman, L.D.; Yessaian, A.A.; Pham, H.Q.; et al. Langerhans cells from women with cervical precancerous lesions become functionally responsive against human papillomavirus after activation with stabilized Poly-I:C. Clin. Immunol. 2015, 161, 197–208. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.; Liu, X.; Wen, H.; Li, W.; Yao, X. Langerhans cells mediate the skin-induced tolerance to ovalbumin via Langerin in a murine model. Allergy 2019, 74, 1738–1747. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Gu, Y.; Liu, B.-G.; Tang, H.; Hua, Y.; Wang, J. Oncogenic role of Tc17 cells in cervical cancer development. World J. Clin. Cases 2020, 8, 11–19. [Google Scholar] [CrossRef]
- Tan, J.; Yang, L.; Zhao, H.; Ai, Y.; Ren, L.; Zhang, F.; Dong, W.; Shi, R.; Sun, D.; Feng, Y. The role of NFATc1/c-myc/PKM2/IL-10 axis in activating cervical cancer tumor-associated M2 macrophage polarization to promote cervical cancer progression. Exp. Cell Res. 2022, 413, 113052. [Google Scholar] [CrossRef]
- Chattopadhyay, G.; Shevach, E.M. Antigen-Specific Induced T Regulatory Cells Impair Dendritic Cell Function via an IL-10/MARCH1–Dependent Mechanism. J. Immunol. 2013, 191, 5875–5884. [Google Scholar] [CrossRef]
- Paiva, I.; Gil Da Costa, R.M.; Ribeiro, J.; Sousa, H.; Bastos, M.; Rocha, A.F.C.; Oliveira, P.A.; Medeiros, R. A Role for MicroRNA-155 Expression in Microenvironment Associated to HPV-Induced Carcinogenesis in K14-HPV16 Transgenic Mice. PLoS ONE 2015, 10, e0116868. [Google Scholar] [CrossRef]
- Wang, F.; Shan, S.; Huo, Y.; Xie, Z.; Fang, Y.; Qi, Z.; Chen, F.; Li, Y.; Sun, B. MiR-155-5p inhibits PDK1 and promotes autophagy via the mTOR pathway in cervical cancer. Int. J. Biochem. Cell Biol. 2018, 99, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Miao, Y.; Romoff, N.; Frazer, I.H. Epithelium Expressing the E7 Oncoprotein of HPV16 Attracts Immune-Modulatory Dendritic Cells to the Skin and Suppresses Their Antigen-Processing Capacity. PLoS ONE 2016, 11, e0152886. [Google Scholar] [CrossRef] [PubMed]
- Bashaw, A.A.; Teoh, S.M.; Tuong, Z.K.; Leggatt, G.R.; Frazer, I.H.; Chandra, J. HPV16 E7-Driven Epithelial Hyperplasia Promotes Impaired Antigen Presentation and Regulatory T-Cell Development. J. Investig. Dermatol. 2019, 139, 2467–2476.e3. [Google Scholar] [CrossRef] [PubMed]
- Jemon, K.; Leong, C.-M.; Ly, K.; Young, S.L.; McLellan, A.D.; Hibma, M.H. Suppression of the CD8 T cell response by human papillomavirus type 16 E7 occurs in Langerhans cell-depleted mice. Sci. Rep. 2016, 6, 34789. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, C.; Luci, C.; Rol, N.; Rousseau, D.; Kissenpfennig, A.; Malissen, B.; Czerkinsky, C.; Anjuère, F. Langerhans Cells Prime IL-17–Producing T Cells and Dampen Genital Cytotoxic Responses following Mucosal Immunization. J. Immunol. 2010, 184, 4842–4851. [Google Scholar] [CrossRef]
- Rotman, J.; Heeren, A.M.; Gassama, A.A.; Lougheed, S.M.; Pocorni, N.; Stam, A.G.; Bleeker, M.C.; Zijlmans, H.J.; Mom, C.H.; Kenter, G.G.; et al. Adenocarcinoma of the Uterine Cervix Shows Impaired Recruitment of cDC1 and CD8+ T Cells and Elevated β-Catenin Activation Compared with Squamous Cell Carcinoma. Clin. Cancer Res. 2020, 26, 3791–3802. [Google Scholar] [CrossRef]
- Cicchini, L.; Westrich, J.A.; Xu, T.; Vermeer, D.W.; Berger, J.N.; Clambey, E.T.; Lee, D.; Song, J.I.; Lambert, P.F.; Greer, R.O.; et al. Suppression of Antitumor Immune Responses by Human Papillomavirus through Epigenetic Downregulation of CXCL14. MBio 2016, 7, e00270-16. [Google Scholar] [CrossRef]
- Westrich, J.A.; Vermeer, D.W.; Colbert, P.L.; Spanos, W.C.; Pyeon, D. The multifarious roles of the chemokine CXCL14 in cancer progression and immune responses. Mol. Carcinog. 2020, 59, 794–806. [Google Scholar] [CrossRef]
- Brewitz, A.; Eickhoff, S.; Dähling, S.; Quast, T.; Bedoui, S.; Kroczek, R.A.; Kurts, C.; Garbi, N.; Barchet, W.; Iannacone, M.; et al. CD8+ T Cells Orchestrate pDC-XCR1+ Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity 2017, 46, 205–219. [Google Scholar] [CrossRef]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments in Melanoma. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- Zuk, J.; O’Sullivan, C.; Stefanutti, E.; Kovalenko, M.; Greenstein, A.; Barry-Hamilton, V.; Mikaelian, I.; Degenhardt, J.; Yue, P.; Smith, V.; et al. MMP-9 Inhibition Promotes Anti-Tumor Immunity through Disruption of Biochemical and Physical Barriers to T-Cell Trafficking to Tumors. PLoS ONE 2018, 13, e0207255. [Google Scholar] [CrossRef]
- Hou, F.; Li, Z.; Ma, D.; Zhang, W.; Zhang, Y.; Zhang, T.; Kong, B.; Cui, B. Distribution of Th17 cells and Foxp3-expressing T cells in tumor-infiltrating lymphocytes in patients with uterine cervical cancer. Clin. Chim. Acta 2012, 413, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Kol, A.; Lubbers, J.M.; Terwindt, A.L.; Workel, H.H.; Plat, A.; Wisman, G.B.A.; Bart, J.; Nijman, H.W.; De Bruyn, M. Combined STING levels and CD103+ T cell infiltration have significant prognostic implications for patients with cervical cancer. Oncoimmunology 2021, 10, 1936391. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Weeks, A.; Yuzhalin, A.E. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers 2020, 12, 3331. [Google Scholar] [CrossRef]
- Kurnia, I.; Rauf, S.; Hatta, M.; Arifuddin, S.; Hidayat, Y.M.; Natzir, R.; Kaelan, C.; Bukhari, A.; Pelupessy, N.U.; Patelonggi, I.J. Molecular Patho-mechanisms of cervical cancer (MMP1). Ann. Med. Surg. 2022, 77, 103415. [Google Scholar] [CrossRef]
- Yu, J.; Xie, Q.; Zhou, H.; Peng, Y.; Lu, H.; Yao, T.; Lin, Z. Survivin, MMP-2, and MMP-9 Expression in Different Types of Cervical Lesions and Correlation Analysis. Int. J. Clin. Exp. Pathol. 2016, 7, 5445–5451. [Google Scholar]
- Lubowicka, E.; Gacuta, E.; Zajkowska, M.; Głażewska, E.K.; Przylipiak, A.; Chrostek, L.; Zbucka-Krętowska, M.; Ławicki, S. The plasma levels and diagnostic utility of matrix metalloproteinase-9 and CA 125 in cervical cancer patients. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2017, 43, 10–14. [Google Scholar]
- Guo, H.; Dai, Y.; Wang, A.; Wang, C.; Sun, L.; Wang, Z. Association between expression of MMP-7 and MMP-9 and pelvic lymph node and para-aortic lymph node metastasis in early cervical cancer. J. Obstet. Gynaecol. Res. 2018, 44, 1274–1283. [Google Scholar] [CrossRef]
- Li, X.; Ning, Y.; Liu, D.; Yan, A.; Wang, Z.; Wang, S.; Miao, M.; Zhu, H.; Jia, Y. Metabolic mechanism of phenyllactic acid naturally occurring in Chinese pickles. Food Chem. 2015, 186, 265–270. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sui, L.; Wang, J.; Li, F. Phenyllactic acid promotes cell migration and invasion in cervical cancer via IKK/NF-κB-mediated MMP-9 activation. Cancer Cell Int. 2019, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, T.; Wu, J.; Wang, Y.; Hong, Y.; Zhou, H. Transferrin receptor-involved HIF-1 signaling pathway in cervical cancer. Cancer Gene Ther. 2019, 26, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Uchino, J.; Chihara, Y.; Tamiya, N.; Kaneko, Y.; Yamada, T.; Takayama, K. Tumor Neovascularization and Developments in Therapeutics. Cancers 2019, 11, 316. [Google Scholar] [CrossRef]
- Stone, S.C.; Rossetti, R.A.M.; Lima, A.M.; Lepique, A.P. HPV associated tumor cells control tumor microenvironment and leukocytosis in experimental models. Immunity Inflamm. Dis. 2014, 2, 63–75. [Google Scholar] [CrossRef]
- Piersma, S.J. Immunosuppressive Tumor Microenvironment in Cervical Cancer Patients. Cancer Microenviron. 2011, 4, 361–375. [Google Scholar] [CrossRef]
- Dibbern, M.E.; Bullock, T.N.; Jenkins, T.M.; Duska, L.R.; Stoler, M.H.; Mills, A.M. Loss of MHC Class I Expression in HPV-associated Cervical and Vulvar Neoplasia. Am. J. Surg. Pathol. 2020, 44, 1184–1191. [Google Scholar] [CrossRef]
- Cicchini, L.; Blumhagen, R.Z.; Westrich, J.A.; Myers, M.E.; Warren, C.J.; Siska, C.; Raben, D.; Kechris, K.J.; Pyeon, D. High-Risk Human Papillomavirus E7 Alters Host DNA Methylome and Represses HLA-E Expression in Human Keratinocytes. Sci. Rep. 2017, 7, 3633. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Huang, Y.; Dong, X.; Xiang, Z.; Zou, J.; Wu, L.; Lu, W. circEYA1 Functions as a Sponge of miR-582-3p to Suppress Cervical Adenocarcinoma Tumorigenesis via Upregulating CXCL14. Mol. Ther.-Nucleic Acids 2020, 22, 1176–1190. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Wang, Y.; Wu, X. The Role of Galectins in Cervical Cancer Biology and Progression. Available online: https://www.hindawi.com/journals/bmri/2018/2175927/ (accessed on 15 December 2020).
- Tao, J.; Dai, J.; Hou, S. Association between B7-H1 and cervical cancer: B7-H1 impairs the immune response in human cervical cancer cells. Exp. Ther. Med. 2017, 14, 4125–4133. [Google Scholar] [CrossRef]
- Meng, Y.; Liang, H.; Hu, J.; Liu, S.; Hao, X.; Wong, M.S.K.; Li, X.; Hu, L. PD-L1 Expression Correlates with Tumor Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy In Cervical Cancer. J. Cancer 2018, 9, 2938–2945. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yan, L.; Liu, N.; Xu, M.; Cai, H. IFI16 promotes cervical cancer progression by upregulating PD-L1 in immunomicroenvironment through STING-TBK1-NF-kB pathway. Biomed. Pharmacother. 2020, 123, 109790. [Google Scholar] [CrossRef]
- Chen, Z.; Pang, N.; Du, R.; Zhu, Y.; Fan, L.; Cai, D.; Ding, Y.; Ding, J. Elevated Expression of Programmed Death-1 and Programmed Death Ligand-1 Negatively Regulates Immune Response against Cervical Cancer Cells. Mediat. Inflamm. 2016, 2016, e6891482. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, J.; Tian, H.; Du, W.; Zhao, L.; Feng, J.; Yuan, D.; Li, Z. Increased expression of PD-L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol. Med. Rep. 2017, 15, 1063–1070. [Google Scholar] [CrossRef]
- Sunthamala, N.; Thierry, F.; Teissier, S.; Pientong, C.; Kongyingyoes, B.; Tangsiriwatthana, T.; Sangkomkamhang, U.; Ekalaksananan, T. E2 Proteins of High Risk Human Papillomaviruses Down-Modulate STING and IFN-κ Transcription in Keratinocytes. PLoS ONE 2014, 9, e91473. [Google Scholar] [CrossRef]
- Luo, X.; Donnelly, C.; Gong, W.; Heath, B.R.; Hao, Y.; Donnelly, L.A.; Moghbeli, T.; Tan, Y.S.; Lin, X.; Bellile, E.; et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Investig. 2020, 130, 1635–1652. [Google Scholar] [CrossRef]
- Cigno, I.L.; Calati, F.; Borgogna, C.; Zevini, A.; Albertini, S.; Martuscelli, L.; De Andrea, M.; Hiscott, J.; Landolfo, S.; Gariglio, M. Human Papillomavirus E7 Oncoprotein Subverts Host Innate Immunity via SUV39H1-Mediated Epigenetic Silencing of Immune Sensor Genes. J. Virol. 2020, 94, e01812-19. [Google Scholar] [CrossRef]
- Peng, J.; Qi, S.; Wang, P.; Li, W.; Song, L.; Liu, C.; Li, F. Meta-analysis of downregulated E-cadherin as a poor prognostic biomarker for cervical cancer. Future Oncol. 2016, 12, 715–726. [Google Scholar] [CrossRef]
- Zacapala-Gómez, A.E.; Navarro-Tito, N.; Alarcón-Romero, L.D.C.; Ortuño-Pineda, C.; Illades-Aguiar, B.; Castañeda-Saucedo, E.; Ortiz-Ortiz, J.; Garibay-Cerdenares, O.L.; Jiménez-López, M.A.; Mendoza-Catalán, M.A. Ezrin and E-cadherin expression profile in cervical cytology: A prognostic marker for tumor progression in cervical cancer. BMC Cancer 2018, 18, 349. [Google Scholar] [CrossRef] [PubMed]
- Cepek, K.L.; Shaw, S.K.; Parker, C.M.; Russell, G.J.; Morrow, J.S.; Rimm, D.L.; Brenner, M.B. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 1994, 372, 190–193. [Google Scholar] [CrossRef] [PubMed]
- French, J.J.; Cresswell, J.; Wong, W.K.; Seymour, K.; Charnley, R.M.; Kirby, J.A. T cell adhesion and cytolysis of pancreatic cancer cells: A role for E-cadherin in immunotherapy? Br. J. Cancer 2002, 87, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’H, A.; Jalil, A.; Vergnon, I.; Chansac, B.L.M.; Lazar, V.; Bismuth, G.; Chouaib, S.; Mami-Chouaib, F. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 2007, 204, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’H, A.; Jalil, A.; Franciszkiewicz, K.; Validire, P.; Vergnon, I.; Mami-Chouaib, F. Minimal Engagement of CD103 on Cytotoxic T Lymphocytes with an E-Cadherin-Fc Molecule Triggers Lytic Granule Polarization via a Phospholipase Cγ–Dependent Pathway. Cancer Res. 2011, 71, 328–338. [Google Scholar] [CrossRef]
- Mami-Chouaib, F.; Blanc, C.; Corgnac, S.; Hans, S.; Malenica, I.; Granier, C.; Tihy, I.; Tartour, E. Resident memory T cells, critical components in tumor immunology. J. Immunother. Cancer 2018, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Ferns, D.M.; Kema, I.P.; Buist, M.R.; Nijman, H.W.; Kenter, G.G.; Jordanova, E.S. Indoleamine-2,3-dioxygenase (IDO) metabolic activity is detrimental for cervical cancer patient survival. Oncoimmunology 2015, 4, e981457. [Google Scholar] [CrossRef] [PubMed]
- Hascitha, J.; Priya, R.; Jayavelu, S.; Dhandapani, H.; Selvaluxmy, G.; Singh, S.S.; Rajkumar, T. Analysis of Kynurenine/Tryptophan ratio and expression of IDO1 and 2 mRNA in tumour tissue of cervical cancer patients. Clin. Biochem. 2016, 49, 919–924. [Google Scholar] [CrossRef]
- Sato, N.; Saga, Y.; Mizukami, H.; Wang, D.; Takahashi, S.; Nonaka, H.; Fujiwara, H.; Takei, Y.; Machida, S.; Takikawa, O.; et al. Downregulation of indoleamine-2,3-dioxygenase in cervical cancer cells suppresses tumor growth by promoting natural killer cell accumulation. Oncol. Rep. 2012, 28, 1574–1578. [Google Scholar] [CrossRef]
- Mittal, D.; Kassianos, A.J.; Tran, L.S.; Bergot, A.-S.; Gosmann, C.; Hofmann, J.; Blumenthal, A.; Leggatt, G.R.; Frazer, I.H. Indoleamine 2,3-Dioxygenase Activity Contributes to Local Immune Suppression in the Skin Expressing Human Papillomavirus Oncoprotein E7. J. Investig. Dermatol. 2013, 133, 2686–2694. [Google Scholar] [CrossRef]
- Rowhani-Rahbar, A.; Hawes, S.E.; Sow, P.S.; Toure, P.; Feng, Q.; Dem, A.; Dembele, B.; Critchlow, C.W.; N’Doye, I.; Kiviat, N.B. The Impact of HIV Status and Type on the Clearance of Human Papillomavirus Infection among Senegalese Women. J. Infect. Dis. 2007, 196, 887–894. [Google Scholar] [CrossRef]
- Chikandiwa, A.; Pisa, P.T.; Muller, E.E.; Tamalet, C.; Mayaud, P.; Chersich, M.F.; Delany-Moretlwe, S. Incidence, Persistence, Clearance, and Correlates of Genital Human Papillomavirus Infection and Anogenital Warts in a Cohort of Men Living with Human Immunodeficiency Virus in South Africa. Sex. Transm. Dis. 2019, 46, 347–353. [Google Scholar] [CrossRef]
- Garcia-Chagollan, M.; Jave-Suarez, L.F.; Haramati, J.; Bueno-Topete, M.R.; Aguilar-Lemarroy, A.; Estrada-Chávez, C.; Bastidas-Ramirez, B.E.; Pereira-Suarez, A.L.; Del Toro-Arreola, S. An approach to the immunophenotypic features of circulating CD4+NKG2D+ T cells in invasive cervical carcinoma. J. Biomed. Sci. 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Asadzadeh, Z.; Mohammadi, H.; Safarzadeh, E.; Hemmatzadeh, M.; Mahdian-Shakib, A.; Jadidi-Niaragh, F.; Azizi, G.; Baradaran, B. The paradox of Th17 cell functions in tumor immunity. Cell. Immunol. 2017, 322, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, D.; Zhang, Y.; Tian, Y.; Wang, X.; Qiao, Y.; Cui, B. The imbalance of Th17/Treg in patients with uterine cervical cancer. Clin. Chim. Acta 2011, 412, 894–900. [Google Scholar] [CrossRef]
- Punt, S.; Van Vliet, M.E.; Spaans, V.M.; De Kroon, C.D.; Fleuren, G.J.; Gorter, A.; Jordanova, E.S. FoxP3(+) and IL-17(+) cells are correlated with improved prognosis in cervical adenocarcinoma. Cancer Immunol. Immunother. 2015, 64, 745–753. [Google Scholar] [CrossRef]
- Chauhan, S.R.; Singhal, P.G.; Sharma, U.; Bandil, K.; Chakraborty, K.; Bharadwaj, M. Th9 cytokines curb cervical cancer progression and immune evasion. Hum. Immunol. 2019, 80, 1020–1025. [Google Scholar] [CrossRef]
- Miao, B.-P.; Zhang, R.-S.; Sun, H.-J.; Yu, Y.-P.; Chen, T.; Li, L.-J.; Liu, J.-Q.; Liu, J.; Yu, H.-Q.; Zhang, M.; et al. Inhibition of squamous cancer growth in a mouse model by Staphylococcal enterotoxin B-triggered Th9 cell expansion. Cell. Mol. Immunol. 2015, 14, 371–379. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, S.; Son, M.-J.; Kim, G.; Singh, P.; Kim, H.N.; Choi, H.-G.; Yoo, H.J.; Ko, Y.B.; Lee, B.S.; et al. Dual oxidase 1 and NADPH oxidase 2 exert favorable effects in cervical cancer patients by activating immune response. BMC Cancer 2019, 19, 1078. [Google Scholar] [CrossRef]
- Hussein, W.M.; Liu, T.-Y.; Maruthayanar, P.; Mukaida, S.; Moyle, P.M.; Wells, J.W.; Toth, I.; Skwarczynski, M. Double conjugation strategy to incorporate lipid adjuvants into multiantigenic vaccines. Chem. Sci. 2016, 7, 2308–2321. [Google Scholar] [CrossRef]
- Hussein, W.M.; Mukaida, S.; Liu, T.-Y.; Toth, I. Fluorinated Lipids Conjugated to Peptide Antigens do not Induce Immune Responses Against Cervical Cancer. Vaccin. Res.-Open J. 2017, 2, 7–12. [Google Scholar] [CrossRef]
- Ajorloo, M.; Alamdary, A.; Soleimanjahi, H.; El Boulani, A.; Khanizadeh, S.; Nikoo, H.R. Immunization of mice by the co-administration of codon-optimized HPV16 E7 and lL12 genes against HPV16-associated cervical cancer. Microb. Pathog. 2019, 132, 20–25. [Google Scholar] [CrossRef]
- Tahamtan, A.; Barati, M.; Tabarraei, A.; Mohebbi, S.R.; Shirian, S.; Gorji, A.; Ghaemi, A. Antitumor Immunity Induced by Genetic Immunization with Chitosan Nanoparticle Formulated Adjuvanted for HPV-16 E7 DNA Vaccine. Iran. J. Immunol. 2018, 15, 269–280. [Google Scholar] [CrossRef]
- Cheng, M.; Farmer, E.; Huang, C.; Lin, J.; Hung, C.-F.; Wu, T.-C. Therapeutic DNA Vaccines for Human Papillomavirus and Associated Diseases. Hum. Gene Ther. 2018, 29, 971–996. [Google Scholar] [CrossRef]
- Hasan, Y.; Furtado, L.; Tergas, A.; Lee, N.; Brooks, R.; McCall, A.; Golden, D.; Jolly, S.; Fleming, G.; Morrow, M.; et al. A Phase 1 Trial Assessing the Safety and Tolerability of a Therapeutic DNA Vaccination Against HPV16 and HPV18 E6/E7 Oncogenes After Chemoradiation for Cervical Cancer. Int. J. Radiat. Oncol. 2020, 107, 487–498. [Google Scholar] [CrossRef]
- Cui, Z.; Huang, L. Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: Therapeutic effect against cervical cancer. Cancer Immunol. Immunother. 2005, 54, 1180–1190. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, S.; Qiu, J.; Miao, J.; Yang, B.; Jeang, J.; Hung, C.-F.; Wu, T.-C. Intravaginal HPV DNA vaccination with electroporation induces local CD8+ T-cell immune responses and antitumor effects against cervicovaginal tumors. Gene Ther. 2015, 22, 528–535. [Google Scholar] [CrossRef]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 921–932. [Google Scholar] [CrossRef]
- Karimi, H.; Soleimanjahi, H.; Abdoli, A.; Banijamali, R.S. Combination therapy using human papillomavirus L1/E6/E7 genes and archaeosome: A nanovaccine confer immuneadjuvanting effects to fight cervical cancer. Sci. Rep. 2020, 10, 5787. [Google Scholar] [CrossRef]
- Toth, I.; Khongkow, M.; Liu, T.-Y.; Bartlett, S.; Hussein, W.M.; Nevagi, R.; Jia, Z.; Monteiro, M.J.; Wells, J.; Ruktanonchai, U.R.; et al. Liposomal formulation of polyacrylate-peptide conjugate as a new vaccine candidate against cervical cancer. Precis. Nanomed. 2018, 1, 183–193. [Google Scholar] [CrossRef]
- Medina-Alarcón, K.P.; Voltan, A.R.; Fonseca-Santos, B.; Moro, I.J.; Souza, F.D.O.; Chorilli, M.; Soares, C.P.; dos Santos, A.G.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Highlights in nanocarriers for the treatment against cervical cancer. Mater. Sci. Eng. C 2017, 80, 748–759. [Google Scholar] [CrossRef]
- Cordeiro, M.N.; Lima, R.D.C.P.D.; Paolini, F.; Melo, A.R.D.S.; Campos, A.P.F.; Venuti, A.; De Freitas, A.C. Current research into novel therapeutic vaccines against cervical cancer. Expert Rev. Anticancer Ther. 2018, 18, 365–376. [Google Scholar] [CrossRef]
- Liu, W.J.; Gao, F.; Zhao, K.N.; Zhao, W.; Fernando, G.J.; Thomas, R.; Frazer, I.H. Codon Modified Human Papillomavirus Type 16 E7 DNA Vaccine Enhances Cytotoxic T-Lymphocyte Induction and Anti-tumour Activity. Virology 2002, 301, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Ge, J.; Lu, F.; Ren, S.; Zhao, Z.; Pu, X.; Chen, X.; Sun, J.; Gu, Y. A novel therapeutic vaccine composed of a rearranged human papillomavirus type 16 E6/E7 fusion protein and Fms-like tyrosine kinase-3 ligand induces CD8+ T cell responses and antitumor effect. Vaccine 2017, 35, 6459–6467. [Google Scholar] [CrossRef] [PubMed]
- van de Wall, M.N. Design and Delivery Strategies of Alphavirus Replicon-Based Cervical Cancer Vaccines. Ph.D. Thesis, Rijksuniversiteit Groningen, Groningen, The Netherlands, 2018. [Google Scholar]
- Xiao, M.; Feng, Y.; Cao, G.; Liu, C.; Zhang, Z. A novel MtHSP70-FPR1 fusion protein enhances cytotoxic T lymphocyte responses to cervical cancer cells by activating human monocyte-derived dendritic cells via the p38 MAPK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Degen, W.G.J.; Schijns, V.E.J.C. Chapter 4ȁ4Host-Derived Cytokines and Chemokines as Vaccine Adjuvants. In Immunopotentiators in Modern Vaccines, 2nd ed.; Schijns, V.E.J.C., O’Hagan, D.T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 65–84. ISBN 978-0-12-804019-5. [Google Scholar]
- Sun, Y.; Peng, S.; Yang, A.; Farmer, E.; Wu, T.-C.; Hung, C.-F. Coinjection of IL2 DNA enhances E7-specific antitumor immunity elicited by intravaginal therapeutic HPV DNA vaccination with electroporation. Gene Ther. 2017, 24, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Pourhossein, B.; Ghaemi, A.; Fazeli, M.; Azadmanesh, K.; Mahmoodi, M.; Mirshafiey, A.; Shahmahmoodi, S. Evaluation of therapeutic potency of human papillomavirus-16 E7 DNA vaccine alone and with interleukin-18 as a genetic adjuvant. Sci. Med. 2018, 28, 30555. [Google Scholar] [CrossRef]
- Yin, W.; Duluc, D.; Joo, H.; Oh, S. Dendritic Cell Targeting Vaccine for HPV-Associated Cancer. Cancer Cell Microenviron. 2017, 3, e14823. [Google Scholar] [CrossRef]
- Böttcher, J.P.; e Sousa, C.R. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Chen, S.; Lv, M.; Fang, S.; Ye, W.; Gao, Y.; Xu, Y. Poly(I:C) enhanced anti-cervical cancer immunities induced by dendritic cells-derived exosomes. Int. J. Biol. Macromol. 2018, 113, 1182–1187. [Google Scholar] [CrossRef]
- Cao, G.; Cui, R.; Liu, C.; Zhang, G.; Zhang, Z. MTBHsp70-exFPR1-pulsed Dendritic Cells Enhance the Immune Response against Cervical Cancer. J. Cancer 2019, 10, 6364–6373. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Woodham, A.W.; Naylor, P.H.; Egan, J.E.; Berinstein, N.L.; Kast, W.M. Immunostimulatory Activity of the Cytokine-Based Biologic, IRX-2, on Human Papillomavirus-Exposed Langerhans Cells. J. Interferon Cytokine Res. 2016, 36, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Dammeijer, F.; Aerts, J.G.J.V.; Vroman, H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front. Immunol. 2018, 9, 2804. [Google Scholar] [CrossRef]
- van Poelgeest, M.I.E.; Visconti, V.V.; Aghai, Z.; van Ham, V.J.; Heusinkveld, M.; Zandvliet, M.L.; Valentijn, A.R.P.M.; Goedemans, R.; van der Minne, C.E.; Verdegaal, E.M.E.; et al. Potential use of lymph node-derived HPV-specific T cells for adoptive cell therapy of cervical cancer. Cancer Immunol. Immunother. 2016, 65, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete Regression of Metastatic Cervical Cancer After Treatment with Human Papillomavirus–Targeted Tumor-Infiltrating T Cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.; Klebanoff, C.A.; Kammula, U.; Sherry, R.M.; et al. Treatment of metastatic human papillomavirus-associated epithelial cancers with adoptive transfer of tumor-infiltrating T cells. J. Clin. Oncol. 2018, 36, 3004. [Google Scholar] [CrossRef]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-infiltrating Lymphocyte Therapy for Human Papillomavirus–associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef]
- Dor1an, S.L.; Stevanović, S.; Adhikary, S.; Gartner, J.J.; Jia, L.; Kwong, M.L.M.; Faquin, W.C.; Hewitt, S.M.; Sherry, R.M.; Yang, J.C.; et al. T-Cell Receptor Gene Therapy for Human Papillomavirus–Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. J. Clin. Oncol. 2019, 37, 2759–2768. [Google Scholar] [CrossRef]
- Helman, S.R.; Stevanovic, S.; Campbell, T.E.; Kwong, M.L.M.; Doran, S.L.; Faquin, W.C.; Hinrichs, C.S. Human Papillomavirus T-Cell Cross-reactivity in Cervical Cancer: Implications for Immunotherapy Clinical Trial Design. JAMA Netw. Open 2018, 1, e180706. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Shaath, H.; Elango, R.; Alajez, N.M. Noncoding RNAs as potential mediators of resistance to cancer immunotherapy. Semin. Cancer Biol. 2019, 65, 65–79. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yu, J.; Chen, L.; Tao, T.; Yi, S.; Hanley, S.J.B.; Yue, J.; Watari, H.; Sakuragi, N. Control of PD-L1 expression by miR-140/142/340/383 and oncogenic activation of the OCT4–miR-18a pathway in cervical cancer. Oncogene 2018, 37, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Saglam, O.; Conejo-Garcia, J. PD-1/PD-L1 immune checkpoint inhibitors in advanced cervical cancer. Integr. Cancer Sci. Ther. 2018, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; Le Tourneau, C. Pembrolizumab in cervical cancer: Latest evidence and clinical usefulness. Ther. Adv. Med. Oncol. 2017, 9, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-E.; Tang, W.-F.; Xu, Y.; Wan, F.-R.; Chen, A.-H. Ultrasound-Mediated Co-Delivery of miR-34a and sPD-1 Complexed with Microbubbles for Synergistic Cancer Therapy. Cancer Manag. Res. 2020, 12, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Liu, L.; Zhang, H.; Xiao, J.; Hann, S.S. Regulations of miR-183-5p and Snail-Mediated Shikonin-Reduced Epithelial-Mesenchymal Transition in Cervical Cancer Cells. Drug Des. Dev. Ther. 2020, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, J.; Zhu, X.; Yuan, H. MiR-126 reverses drug resistance to TRAIL through inhibiting the expression of c-FLIP in cervical cancer. Gene 2017, 627, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, J.; Wang, X.; Liu, Y.; Wang, C.; Fan, R. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol. Ther. 2018, 19, 391–399. [Google Scholar] [CrossRef]
- Inturi, R.; Jemth, P. CRISPR/Cas9-based inactivation of human papillomavirus oncogenes E6 or E7 induces senescence in cervical cancer cells. Virology 2021, 562, 92–102. [Google Scholar] [CrossRef]
- Yoshiba, T.; Saga, Y.; Urabe, M.; Uchibor, R.; Matsubara, S.; Fujiwara, H.; Mizukami, H. CRISPR/Cas9-mediated cervical cancer treatment targeting human papillomavirus E6. Oncol. Lett. 2018, 17, 2197–2206. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Wang, T.; He, D.; Tian, R.; Cui, Z.; Tian, X.; Gao, Q.; Ma, X.; Yang, J.; et al. In vitro and in vivo growth inhibition of human cervical cancer cells via human papillomavirus E6/E7 mRNAs’ cleavage by CRISPR/Cas13a system. Antivir. Res. 2020, 178, 104794. [Google Scholar] [CrossRef]
- Shaikh, M.H.; Bortnik, V.; McMillan, N.A.; Idris, A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb. Pathog. 2019, 132, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Bortnik, V.; Wu, M.; Julcher, B.; Salinas, A.; Nikolic, I.; Simpson, K.J.; McMillan, N.A.; Idris, A. Loss of HPV type 16 E7 restores cGAS-STING responses in human papilloma virus-positive oropharyngeal squamous cell carcinomas cells. J. Microbiol. Immunol. Infect. 2020, 54, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Lu, J.; Liu, Y.-H.; Chen, W.; Li, X. Synergistic antitumor effect on cervical cancer by rational combination of PD1 blockade and CRISPR-Cas9-mediated HPV knockout. Cancer Gene Ther. 2019, 27, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Qiang, R.; Lu, J.; Tuo, X.; Yang, X.; Li, X. CRISPR/Cas9-HPV-liposome enhances antitumor immunity and treatment of HPV infection-associated cervical cancer. J. Med. Virol. 2022, 95, e28144. [Google Scholar] [CrossRef] [PubMed]
- Ehrke-Schulz, E.; Heinemann, S.; Schulte, L.; Schiwon, M.; Ehrhardt, A. Adenoviral Vectors Armed with PAPILLOMAVIRUs Oncogene Specific CRISPR/Cas9 Kill Human-Papillomavirus-Induced Cervical Cancer Cells. Cancers 2020, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Miho, E.; Yermanos, A.; Weber, C.R.; Berger, C.T.; Reddy, S.T.; Greiff, V. Computational Strategies for Dissecting the High-Dimensional Complexity of Adaptive Immune Repertoires. Front. Immunol. 2018, 9, 224. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, C.; Rieth, J.M.; Wangmo, D.; Subramanian, S. Novel Methods to Overcome Acquired Resistance to Immunotherapy. In Current Applications for Overcoming Resistance to Targeted Therapies; Szewczuk, M.R., Qorri, B., Sambi, M., Eds.; Resistance to Targeted Anti-Cancer Therapeutics; Springer International Publishing: Cham, Switzerland, 2019; Volume 20, pp. 97–129. ISBN 978-3-030-21476-0. [Google Scholar]
| Steps of CIC | Upregulated Genes | Downregulated Genes | Alterations | References |
|---|---|---|---|---|
| Cell death and antigen release | COL1A1 FN1 HSDL2 miR-21-5p CCAT-1 | LHPP ASPP2 LncMEG3 MiR-433 | Decreased apoptosis. | [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] |
| Capture and antigen processing | TLR3 TLR4 | TLR2 CD11B CD207 CCL2 CCL20 CXCL14 E-cadherin | Decreased number of APCs. Decreased differentiation and maturation of APCs. | [68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] |
| Priming and activation of immune cells | Il-6. Il-10. | CCR7 | Increased priming of T-cells Foxp3+. Decreased migration of APCs to lymph nodes. Decreased priming of Th1 and CD8+ cells. | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110] |
| Migration of immune cells to tumors | IL-6 | CXCL14 CXCL9 CXCL10 CXCL11 CCL4 β-Catenin | The migration of TH17 pro-tumorigenic cells CD4/IL17/CCR6+. | [41,101,111,112,113,114,115] |
| Infiltration of immune cells in the tumor | IL-6 IL-10 TGF-β Fibronectin 1 MMP9 HIF-1α | STING | Matrix remodeling. Denser matrix. Abnormal neovascularization. Presence of Th17 and Foxp3+ cells. Reduced CD103+T-cells | [51,101,115,116,117,118,119,120,121,122,123,124,125,126,127,128] |
| Recognition of tumor cells by immune cells | March1 Ubiquitin Ligase 3 IL-10 Galectin 3 | HLA-A HLA-B HLA-C HLA-E HLA-G CXCL14 CircEYA1 | Decreased recognition of tumor cells. | [103,106,112,129,130,131,132,133] |
| Destruction of tumor cells | ICOSLG CD276 VTCN2 PD-L1 TGF-β IL-10 TIM-3 LAG-3 IDO1 Galectin 1 IFI16 NLRX1 | IFN-γ E-cadherin | Circulating CD4+ NKG2D+ T-cells with CD28+ decreases. Presence of Tregs cells. Decrease of Th1 cells. CD8+ ‘exhausted’ T-cells. | [20,110,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, J.P.; Carvalho, B.M.; Coimbra, E.C. A Comprehensive View of the Cancer-Immunity Cycle (CIC) in HPV-Mediated Cervical Cancer and Prospects for Emerging Therapeutic Opportunities. Cancers 2023, 15, 1333. https://doi.org/10.3390/cancers15041333
Avila JP, Carvalho BM, Coimbra EC. A Comprehensive View of the Cancer-Immunity Cycle (CIC) in HPV-Mediated Cervical Cancer and Prospects for Emerging Therapeutic Opportunities. Cancers. 2023; 15(4):1333. https://doi.org/10.3390/cancers15041333
Chicago/Turabian StyleAvila, Jonathan Peña, Bruno Melo Carvalho, and Eliane Campos Coimbra. 2023. "A Comprehensive View of the Cancer-Immunity Cycle (CIC) in HPV-Mediated Cervical Cancer and Prospects for Emerging Therapeutic Opportunities" Cancers 15, no. 4: 1333. https://doi.org/10.3390/cancers15041333
APA StyleAvila, J. P., Carvalho, B. M., & Coimbra, E. C. (2023). A Comprehensive View of the Cancer-Immunity Cycle (CIC) in HPV-Mediated Cervical Cancer and Prospects for Emerging Therapeutic Opportunities. Cancers, 15(4), 1333. https://doi.org/10.3390/cancers15041333






