How Biology Guides the Combination of Locoregional Interventional Therapies and Immunotherapy for Hepatocellular Carcinoma: Cytokines and Their Roles
Abstract
Simple Summary
Abstract
1. Introduction
2. Locoregional Interventional Therapies in Combination with ICI-Based Immunotherapy for HCC: Opportunities and Challenges
2.1. Ablative Therapies
2.2. Transarterial Therapies
3. Cytokines in the Combined Strategies of Locoregional Interventional Therapies and ICI-Based Immunotherapy: Role and Therapeutic Potentials
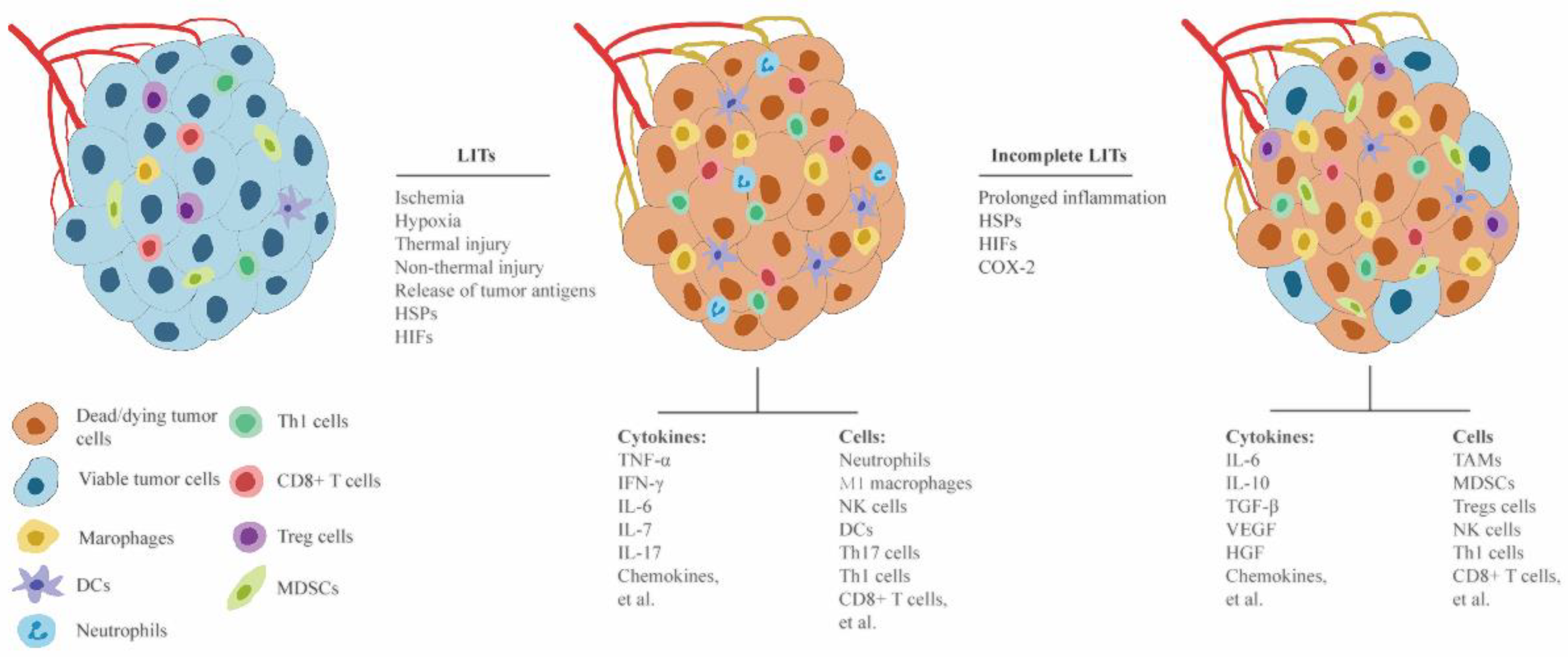
3.1. Different Roles of Complete and Incomplete LITs in Modulating Antitumor Immune Response and Tumor Progression
3.2. Roles of Cytokines in Modulating Local and Systemic Responses to LITs
3.2.1. Interferons (IFNs)
3.2.2. Interleukins (ILs)
3.2.3. Chemokines
3.2.4. TNF-α
3.2.5. TGF-β
3.2.6. VEGF
3.2.7. HGF
3.3. Specific Cytokines as Therapeutic Targets for Reducing ICI-Induced Immune-Related Adverse Event (irAEs)
4. Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BTLA | B- and T-lymphocyte attenuator |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL3 | C-C motif chemokine ligand 3 |
| CCL4 | C-C motif chemokine ligand 4 |
| CCL8 | C-C motif chemokine ligand 8 |
| c-MET | mesenchymal-epithelial transition factor |
| COX-2 | cyclooxygenase-2 |
| CTLA4 | cytotoxic T-lymphocyte antigen 4 |
| CXCL9 | C-X-C motif ligand 9 |
| CXCL14 | C-X-C motif ligand 14 |
| DCs | dendritic cells |
| EMT | epithelial-mesenchymal transition |
| EphA2 | ephrin type-A receptor 2 |
| HIFs | hypoxia-inducible factors |
| HIF-1α | hypoxia-inducible factor-1 alpha |
| HGF | hepatocyte growth factor |
| HSP-70 | heat shock protein-70 |
| HSPs | heat shock proteins |
| IFN-α | Interferon-alpha |
| IFN-β | Interferon-beta |
| IFN-γ | Interferon-gamma |
| IL-1 | interleukin-1 |
| IL-2 | interleukin-2 |
| IL-6 | interleukin-6 |
| IL-7 | interleukin-7 |
| IL-10 | interleukin-10 |
| IL-12 | interleukin-12 |
| IL-17 | interleukin-17 |
| LAG-3 | lymphocyte-activation gene 3 |
| MDSCs | myeloid-derived suppressor cells |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Kraehenbuehl, L.; Weng, C.H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.H.; Malagari, K.; Kulik, L.M. Role of locoregional therapies in the wake of systemic therapy. J. Hepatol. 2020, 72, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Brace, C.L.; Lee, F.T., Jr.; Goldberg, S.N. Principles of and advances in percutaneous ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef]
- Nault, J.C.; Sutter, O.; Nahon, P.; Ganne-Carrié, N.; Séror, O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J. Hepatol. 2018, 68, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Kim, J.K.; Kim, M.Y.; Rhim, H.; Han, J.K. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009, 49, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Ravetta, V.; Rosa, L.; Ghittoni, G.; Viera, F.T.; Garbagnati, F.; Silini, E.M.; Dionigi, P.; Calliada, F.; Quaretti, P.; et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: A long-term cohort study. Hepatology 2011, 53, 136–147. [Google Scholar] [CrossRef] [PubMed]
- N’Kontchou, G.; Mahamoudi, A.; Aout, M.; Ganne-Carrié, N.; Grando, V.; Coderc, E.; Vicaut, E.; Trinchet, J.C.; Sellier, N.; Beaugrand, M.; et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009, 50, 1475–1483. [Google Scholar] [CrossRef]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Yoon, J.H.; Kim, Y.J.; Han, J.K.; Choi, B.I. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014, 270, 900–909. [Google Scholar] [CrossRef]
- Lee, J.J.X.; Tai, D.W.; Choo, S.P. Locoregional therapy in hepatocellular carcinoma: When to start and when to stop and when to revisit. ESMO Open 2021, 6, 100129. [Google Scholar] [CrossRef]
- Liao, M.; Zhong, X.; Zhang, J.; Liu, Y.; Zhu, Z.; Wu, H.; Zeng, Y.; Huang, J. Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis: A prospective randomized trial. J. Surg. Oncol. 2017, 115, 971–979. [Google Scholar] [CrossRef]
- Weis, S.; Franke, A.; Mössner, J.; Jakobsen, J.C.; Schoppmeyer, K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst. Rev. 2013, 19, Cd003046. [Google Scholar] [CrossRef] [PubMed]
- Germani, G.; Pleguezuelo, M.; Gurusamy, K.; Meyer, T.; Isgrò, G.; Burroughs, A.K. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: A meta-analysis. J. Hepatol. 2010, 52, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; De Matthaeis, N.; Saviano, A.; De Sio, I.; Francica, G.; Brunello, F.; Cantamessa, A.; Giorgio, A.; Scognamiglio, U.; Fornari, F.; et al. Single hepatocellular carcinoma smaller than 2 cm: Are ethanol injection and radiofrequency ablation equally effective? Anticancer Res. 2015, 35, 325–332. [Google Scholar] [PubMed]
- Vietti Violi, N.; Duran, R.; Guiu, B.; Cercueil, J.P.; Aubé, C.; Digklia, A.; Pache, I.; Deltenre, P.; Knebel, J.F.; Denys, A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: A randomised controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 317–325. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.L.; Han, Z.Y.; Cheng, Z.G.; Liu, F.Y.; Zhai, H.Y.; Mu, M.J.; Liu, Y.M.; Liang, P. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: A phase III randomised controlled trial. Gut 2017, 66, 1172–1173. [Google Scholar] [CrossRef]
- Gupta, P.; Maralakunte, M.; Kumar, M.P.; Chandel, K.; Chaluvashetty, S.B.; Bhujade, H.; Kalra, N.; Sandhu, M.S. Overall survival and local recurrence following RFA, MWA, and cryoablation of very early and early HCC: A systematic review and Bayesian network meta-analysis. Eur. Radiol. 2021, 31, 5400–5408. [Google Scholar] [CrossRef]
- Erinjeri, J.P.; Clark, T.W. Cryoablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. 2010, 21, S187–S191. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [CrossRef]
- Wang, C.; Wang, H.; Yang, W.; Hu, K.; Xie, H.; Hu, K.Q.; Bai, W.; Dong, Z.; Lu, Y.; Zeng, Z.; et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015, 61, 1579–1590. [Google Scholar] [CrossRef]
- Xu, J.; Noda, C.; Erickson, A.; Mokkarala, M.; Charalel, R.; Ramaswamy, R.; Tao, Y.U.; Akinwande, O. Radiofrequency Ablation vs. Cryoablation for Localized Hepatocellular Carcinoma: A Propensity-matched Population Study. Anticancer Res. 2018, 38, 6381–6386. [Google Scholar] [CrossRef]
- Greten, T.F.; Mauda-Havakuk, M.; Heinrich, B.; Korangy, F.; Wood, B.J. Combined locoregional-immunotherapy for liver cancer. J. Hepatol. 2019, 70, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gu, L.; Lv, S.; Zhang, M.; Zhuang, L.; Zhang, Y.; Chen, P. Suppression of the transforming growth factor-β signaling pathway produces a synergistic effect of combination therapy with programmed death receptor 1 blockade and radiofrequency ablation against hepatic carcinoma in mice. Bioengineered 2022, 13, 9046–9058. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, H.; Li, Y.; Xiao, W.; Liu, Y.; Chen, R.; Zhu, Y.; Zheng, X.; Wu, C.; Chen, L. TIGIT Blockade Exerts Synergistic Effects on Microwave Ablation Against Cancer. Front Immunol. 2022, 13, 832230. [Google Scholar] [CrossRef]
- Shao, D.; Chen, Y.; Huang, H.; Liu, Y.; Chen, J.; Zhu, D.; Zheng, X.; Chen, L.; Jiang, J. LAG3 blockade coordinates with microwave ablation to promote CD8(+) T cell-mediated anti-tumor immunity. J. Transl. Med. 2022, 20, 433. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.; Wang, Y.; Liu, X.; Zhang, M.; Li, W. Antitumor Immunity Augmented by Combining Radiofrequency Ablation with Anti-CTLA-4 Therapy in a Subcutaneous Murine Hepatoma Model. J. Vasc. Interv. Radiol. 2020, 31, 1178–1186. [Google Scholar] [CrossRef]
- Lyu, N.; Kong, Y.; Li, X.; Mu, L.; Deng, H.; Chen, H.; He, M.; Lai, J.; Li, J.; Tang, H.; et al. Ablation Reboots the Response in Advanced Hepatocellular Carcinoma With Stable or Atypical Response During PD-1 Therapy: A Proof-of-Concept Study. Front Oncol. 2020, 10, 580241. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Xu, Y.; Xu, X.; Jiang, Q.; Ruan, J.; Wu, Y.; Zhou, Y.; Saw, P.E.; Luo, B. Targeting PI3Kγ/AKT Pathway Remodels LC3-Associated Phagocytosis Induced Immunosuppression After Radiofrequency Ablation. Adv. Sci. 2022, 9, e2102182. [Google Scholar] [CrossRef]
- Habib, A.; Desai, K.; Hickey, R.; Thornburg, B.; Lewandowski, R.; Salem, R. Transarterial approaches to primary and secondary hepatic malignancies. Nat. Rev. Clin. Oncol. 2015, 12, 481–489. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Geschwind, J.F.; Liapi, E.; Salem, R. Transcatheter intraarterial therapies: Rationale and overview. Radiology 2011, 259, 641–657. [Google Scholar] [CrossRef]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montañá, X.; Llovet, J.M.; et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef]
- Golfieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef]
- Lencioni, R.; de Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.F. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Salem, R. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma: One step forward? J. Clin. Oncol. 2013, 31, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, M.; Hiraoka, A.; Ochi, H.; Koizumi, Y.; Kisaka, Y.; Tokumoto, Y.; Abe, M.; Joko, K.; Michitaka, K.; Hiasa, Y. The efficacy of radiofrequency ablation combined with transcatheter hepatic chemoembolization for patients with BCLC stage B hepatocellular carcinoma: A multicenter retrospective study-propensity score matching. Hepatology 2016, 64, 685A. [Google Scholar]
- Lencioni, R.; Crocetti, L.; Petruzzi, P.; Vignali, C.; Bozzi, E.; Della Pina, C.; Bargellini, I.; Cioni, D.; Oliveri, F.; De Simone, P.; et al. Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: A pilot clinical study. J. Hepatol. 2008, 49, 217–222. [Google Scholar] [CrossRef]
- Peng, Z.W.; Zhang, Y.J.; Chen, M.S.; Xu, L.; Liang, H.H.; Lin, X.J.; Guo, R.P.; Zhang, Y.Q.; Lau, W.Y. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: A prospective randomized trial. J. Clin. Oncol. 2013, 31, 426–432. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chen, M.S.; Chen, Y.; Lau, W.Y.; Peng, Z. Long-term Outcomes of Transcatheter Arterial Chemoembolization Combined With Radiofrequency Ablation as an Initial Treatment for Early-Stage Hepatocellular Carcinoma. JAMA Netw. Open 2021, 4, e2126992. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Ren, M.; Lu, X.; Lu, G.; He, S. Efficacy and Safety of Radiofrequency Ablation Combined with Transcatheter Arterial Chemoembolization for Hepatocellular Carcinomas Compared with Radiofrequency Ablation Alone: A Time-to-Event Meta-Analysis. Korean J. Radiol. 2016, 17, 93–102. [Google Scholar] [CrossRef]
- Morimoto, M.; Numata, K.; Kondo, M.; Nozaki, A.; Moriya, S.; Takizawa, K.; Maeda, S.; Tanaka, K. Long-term outcome in patients with intermediate-sized hepatocellular carcinoma: A randomized controlled trial to determine the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Hepatology 2011, 54, 1366A–1367A. [Google Scholar] [CrossRef]
- Yin, X.; Tang, B.; Gan, Y.H.; Wang, Y.H.; Chen, Y.; Ge, N.L.; Chen, R.; Zhang, L.; Zhang, B.; Ren, Z. Randomized clinical trial of transcatheter arterial chemoembolization plus radiofrequency ablation versus transcatheter arterial chemoembolization for hepatocellular carcinoma with intermediate stage (BCLC stage B) hepatocellular carcinoma beyond Milan criteria. J. Clin. Oncol. 2019, 37, 4077. [Google Scholar] [CrossRef]
- Okamoto, T.; Endo, K.; Takikawa, Y. Efficacy of combination therapy with transcatheter arterial chemoembolization and radiofrequency ablation for intermediate-stage hepatocelluar carcinoma. J. Hepatol. 2019, 70, e615–e616. [Google Scholar] [CrossRef]
- English, K.; Brodin, N.P.; Shankar, V.; Zhu, S.; Ohri, N.; Golowa, Y.S.; Cynamon, J.; Bellemare, S.; Kaubisch, A.; Kinkhabwala, M.; et al. Association of Addition of Ablative Therapy Following Transarterial Chemoembolization With Survival Rates in Patients With Hepatocellular Carcinoma. JAMA Netw. Open 2020, 3, e2023942. [Google Scholar] [CrossRef]
- Shi, F.; Wu, M.; Lian, S.S.; Mo, Z.Q.; Gou, Q.; Xu, R.D.; Li, H.L.; Huang, Z.M.; Wu, P.H.; Chen, X.M. Radiofrequency Ablation Following Downstaging of Hepatocellular Carcinoma by Using Transarterial Chemoembolization: Long-term Outcomes. Radiology 2019, 293, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef]
- Kudo, M.; Han, G.; Finn, R.S.; Poon, R.T.; Blanc, J.F.; Yan, L.; Yang, J.; Lu, L.; Tak, W.Y.; Yu, X.; et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology 2014, 60, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Kasugai, H.; Shioyama, Y.; Tanaka, K.; Kudo, M.; Saisho, H.; Osaki, Y.; Sata, M.; Fujiyama, S.; Kumada, T.; et al. Transarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: A randomized phase III trial. J. Hepatol. 2009, 51, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Imanaka, K.; Chida, N.; Nakachi, K.; Tak, W.Y.; Takayama, T.; Yoon, J.H.; Hori, T.; Kumada, H.; Hayashi, N.; et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2117–2127. [Google Scholar] [CrossRef]
- Yu, S.C.; Hui, J.W.; Hui, E.P.; Chan, S.L.; Lee, K.F.; Mo, F.; Wong, J.; Ma, B.; Lai, P.; Mok, T.; et al. Unresectable hepatocellular carcinoma: Randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology 2014, 270, 607–620. [Google Scholar] [CrossRef]
- Ikeda, M.; Kudo, M.; Aikata, H.; Nagamatsu, H.; Ishii, H.; Yokosuka, O.; Torimura, T.; Morimoto, M.; Ikeda, K.; Kumada, H.; et al. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: A phase III randomized trial. J. Gastroenterol. 2018, 53, 281–290. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Shi, F.; Mai, Q.; Wang, L.; Wang, F.; Zhuang, W.; Chen, X.; Chen, H.; Xu, B.; et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: A retrospective study. J. Cancer Res. Clin. Oncol. 2022, 148, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Fang, S.; Wu, F.; Chen, W.; Chen, M.; Weng, Q.; Wu, X.; Song, J.; Zhao, Z.; Ji, J. Efficacy and Safety of TACE Combined With Sorafenib Plus Immune Checkpoint Inhibitors for the Treatment of Intermediate and Advanced TACE-Refractory Hepatocellular Carcinoma: A Retrospective Study. Front Mol. Biosci. 2020, 7, 609322. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, B.; Kim, E.; D’Alessio, A.; Cedillo, M.; Sinha, I.; Debnath, N.; Kudo, M.; Nishida, N.; Saeed, A.; Hildebrand, H.; et al. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: Evaluation of safety and efficacy in a retrospective, propensity score-matched study. J. Immunother. Cancer 2022, 10, 4205. [Google Scholar] [CrossRef]
- Jin, Z.; Zhong, B.; Chen, J.; Zhu, H.; Teng, G. Transarterial chemoembolization plus Camrelizumab and Apatinib for hepatocellular carcinoma: A multicenter, retrospective, cohort study. CardioVascular Interv. Radiol. 2022, 45, S8. [Google Scholar] [CrossRef]
- Shi, L.; Wang, J.; Ding, N.; Zhang, Y.; Zhu, Y.; Dong, S.; Wang, X.; Peng, C.; Zhou, C.; Zhou, L.; et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 2019, 10, 5421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bei, J.; Liu, M.; Huang, J.; Xie, L.; Huang, W.; Cai, M.; Guo, Y.; Lin, L.; Zhu, K. Sublethal heat stress-induced O-GlcNAcylation coordinates the Warburg effect to promote hepatocellular carcinoma recurrence and metastasis after thermal ablation. Cancer Lett. 2021, 518, 23–34. [Google Scholar] [CrossRef]
- Wu, H.; Li, S.S.; Zhou, M.; Jiang, A.N.; He, Y.; Wang, S.; Yang, W.; Liu, H. Palliative Radiofrequency Ablation Accelerates the Residual Tumor Progression Through Increasing Tumor-Infiltrating MDSCs and Reducing T-Cell-Mediated Anti-Tumor Immune Responses in Animal Model. Front Oncol. 2020, 10, 1308. [Google Scholar] [CrossRef]
- Zeng, X.; Liao, G.; Li, S.; Liu, H.; Zhao, X.; Li, S.; Lei, K.; Zhu, S.; Chen, Z.; Zhao, Y.; et al. Eliminating METTL1-mediated accumulation of PMN-MDSCs prevents hepatocellular carcinoma recurrence after radiofrequency ablation. Hepatology 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Moussa, M.; Mwin, D.; Liao, H.; Atac, M.F.; Markezana, A.; Galun, E.; Goldberg, S.N.; Ahmed, M. Myofibroblasts: A key promoter of tumorigenesis following radiofrequency tumor ablation. PLoS ONE 2022, 17, e0266522. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kwon, J.H.; Moon, Y.H.; Kim, Y.B.; Yu, Y.S.; Lee, N.; Choi, K.Y.; Kim, Y.S.; Park, Y.K.; Kim, B.W.; et al. Influence of preoperative transcatheter arterial chemoembolization on gene expression in the HIF-1α pathway in patients with hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 1507–1515. [Google Scholar] [CrossRef]
- Tong, Y.; Yang, H.; Xu, X.; Ruan, J.; Liang, M.; Wu, J.; Luo, B. Effect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasion. Cancer Sci. 2017, 108, 753–762. [Google Scholar] [CrossRef]
- Liang, B.; Zheng, C.S.; Feng, G.S.; Wu, H.P.; Wang, Y.; Zhao, H.; Qian, J.; Liang, H.M. Correlation of hypoxia-inducible factor 1alpha with angiogenesis in liver tumors after transcatheter arterial embolization in an animal model. Cardiovasc. Interv. Radiol. 2010, 33, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Kong, J.; Pan, B.; Ke, S.; Dong, S.; Li, X.; Zhou, A.; Zheng, L.; Sun, W.B. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS ONE 2012, 7, e37266. [Google Scholar] [CrossRef]
- Wu, L.; Fu, Z.; Zhou, S.; Gong, J.; Liu, C.A.; Qiao, Z.; Li, S. HIF-1α and HIF-2α: Siblings in promoting angiogenesis of residual hepatocellular carcinoma after high-intensity focused ultrasound ablation. PLoS ONE 2014, 9, e88913. [Google Scholar] [CrossRef] [PubMed]
- Albakova, Z.; Mangasarova, Y. The HSP Immune Network in Cancer. Front Immunol. 2021, 12, 796493. [Google Scholar] [CrossRef]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Waldmann, T.A. Cytokines in Cancer Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028472. [Google Scholar] [CrossRef]
- Song, X.; Li, N.; Liu, Y.; Wang, Z.; Wang, T.; Tan, S.; Li, C.; Qiu, C.; Gao, L.; Asano, K.; et al. CD169-positive macrophages enhance abscopal effect of radiofrequency ablation therapy in liver cancer. Transl. Oncol. 2022, 15, 101306. [Google Scholar] [CrossRef]
- Cortes, A.; Chang, S.; Polak, U.; Sheth, R.; Hicks, M.; Avritscher, R. Increased CD4+Th17 cells after transarterial hepatic artery embolization in an orthotopic rat model of hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2018, 29, S189. [Google Scholar] [CrossRef]
- Bulvik, B.E.; Rozenblum, N.; Gourevich, S.; Ahmed, M.; Andriyanov, A.V.; Galun, E.; Goldberg, S.N. Irreversible Electroporation versus Radiofrequency Ablation: A Comparison of Local and Systemic Effects in a Small-Animal Model. Radiology 2016, 280, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, B.; Liang, P.; Yu, X.; Su, L.; Yu, D.; Ji, X.; Yu, G. Significance of changes in local immunity in patients with hepatocellular carcinoma after percutaneous microwave coagulation therapy. Chin. Med. J. 2002, 115, 1367–1371. [Google Scholar] [PubMed]
- Dong, B.W.; Zhang, J.; Liang, P.; Yu, X.L.; Su, L.; Yu, D.J.; Ji, X.L.; Yu, G. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int. J. Hyperth. 2003, 19, 119–133. [Google Scholar] [CrossRef]
- Erös de Bethlenfalva-Hora, C.; Mertens, J.C.; Piguet, A.C.; Kettenbach, J.; Schmitt, J.; Terracciano, L.; Weimann, R.; Dufour, J.F.; Geier, A. Radiofrequency ablation suppresses distant tumour growth in a novel rat model of multifocal hepatocellular carcinoma. Clin. Sci. 2014, 126, 243–252. [Google Scholar] [CrossRef]
- Xia, J.Z.; Xie, F.L.; Ran, L.F.; Xie, X.P.; Fan, Y.M.; Wu, F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med. Biol. 2012, 38, 1363–1371. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, L.; Yuan, J.; Babikr, F.; Freywald, A.; Chibbar, R.; Moser, M.; Zhang, W.; Zhang, B.; Fu, Z.; et al. TLR9 agonist enhances radiofrequency ablation-induced CTL responses, leading to the potent inhibition of primary tumor growth and lung metastasis. Cell Mol. Immunol. 2019, 16, 820–832. [Google Scholar] [CrossRef]
- Tischfield, D.J.; Gurevich, A.; Johnson, O.; Gatmaytan, I.; Nadolski, G.J.; Soulen, M.C.; Kaplan, D.E.; Furth, E.; Hunt, S.J.; Gade, T.P.F. Transarterial Embolization Modulates the Immune Response within Target and Nontarget Hepatocellular Carcinomas in a Rat Model. Radiology 2022, 303, 215–225. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Qin, J.; Huang, J.; Wang, F.; Xu, G.P.; Lv, Y.T.; Zhang, J.B.; Shen, L.M. Zoledronic acid inhibits infiltration of tumor-associated macrophages and angiogenesis following transcatheter arterial chemoembolization in rat hepatocellular carcinoma models. Oncol. Lett. 2017, 14, 4078–4084. [Google Scholar] [CrossRef]
- Virmani, S.; Rhee, T.K.; Ryu, R.K.; Sato, K.T.; Lewandowski, R.J.; Mulcahy, M.F.; Kulik, L.M.; Szolc-Kowalska, B.; Woloschak, G.E.; Yang, G.Y.; et al. Comparison of hypoxia-inducible factor-1alpha expression before and after transcatheter arterial embolization in rabbit VX2 liver tumors. J. Vasc. Interv. Radiol. 2008, 19, 1483–1489. [Google Scholar] [CrossRef]
- Yamada, S.; Utsunomiya, T.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Kanamoto, M.; Iwahashi, S.; Saito, Y.; Takasu, C.; et al. Expressions of hypoxia-inducible factor-1 and epithelial cell adhesion molecule are linked with aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation therapy. Ann. Surg. Oncol. 2014, 21 (Suppl. S3), S436–S442. [Google Scholar] [CrossRef]
- Wan, J.; Wu, W.; Huang, Y.; Ge, W.; Liu, S. Incomplete radiofrequency ablation accelerates proliferation and angiogenesis of residual lung carcinomas via HSP70/HIF-1α. Oncol. Rep. 2016, 36, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, K.; Myllymäki, L.; Jansner, K.; Bruun, A.; Stenram, U.; Tranberg, K.G. Heat shock protein 70 (HSP70) after laser thermotherapy of an adenocarcinoma transplanted into rat liver. Anticancer Res. 2003, 23, 3703–3712. [Google Scholar] [PubMed]
- Velez, E.; Goldberg, S.N.; Kumar, G.; Wang, Y.; Gourevitch, S.; Sosna, J.; Moon, T.; Brace, C.L.; Ahmed, M. Hepatic Thermal Ablation: Effect of Device and Heating Parameters on Local Tissue Reactions and Distant Tumor Growth. Radiology 2016, 281, 782–792. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef]
- Gao, H.J.; Zhang, Y.J.; Liang, H.H.; Li, P.; Peng, Z.W.; Pang, X.H.; Chen, M.S. Radiofrequency ablation does not induce the significant increase of CD4(+) CD25(+) Foxp3(+) regulatory T cells compared with surgical resection in Hepal-6 tumor model. Arch. Immunol. Ther. Exp. 2013, 61, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Mazmishvili, K.; Jayant, K.; Janikashvili, N.; Kikodze, N.; Mizandari, M.; Pantsulaia, I.; Paksashvili, N.; Sodergren, M.H.; Reccia, I.; Pai, M.; et al. Study to evaluate the immunomodulatory effects of radiofrequency ablation compared to surgical resection for liver cancer. J. Cancer 2018, 9, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Goldberg, S.N.; Wang, Y.; Velez, E.; Gourevitch, S.; Galun, E.; Ahmed, M. Hepatic radiofrequency ablation: Markedly reduced systemic effects by modulating periablational inflammation via cyclooxygenase-2 inhibition. Eur. Radiol. 2017, 27, 1238–1247. [Google Scholar] [CrossRef]
- Song, S.; He, X.; Zeng, Z.; Zhang, H.; Yao, Q.; Yang, F.; Zheng, C.; Guo, X. Blocking transforming growth factor-beta reduces the migration and invasion of the residual tumour after TAE. Am. J. Transl. Res. 2019, 11, 2155–2167. [Google Scholar]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Gani, R.A.; Lesmana, L.A.; Kresno, S.B.; Pandelaki, J.; Suwarto, S. The Association between Peripheral Th17, Th1, IL-17, and IFN-γ Levels and TACE Response in Patients with Unresectable Hepatocellular Carcinoma with or without Cirrhosis. Acta. Med. Indones. 2020, 52, 326–333. [Google Scholar] [PubMed]
- Jekarl, D.W.; Lee, S.; Kwon, J.H.; Nam, S.W.; Kim, M.; Kim, Y.; Jang, J.W. Complex interaction networks of cytokines after transarterial chemotherapy in patients with hepatocellular carcinoma. PLoS ONE 2019, 14, e0224318. [Google Scholar] [CrossRef]
- Mo, Z.; Lu, H.; Mo, S.; Fu, X.; Chang, S.; Yue, J. Ultrasound-guided radiofrequency ablation enhances natural killer-mediated antitumor immunity against liver cancer. Oncol. Lett. 2018, 15, 7014–7020. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, W.; Xue, M.; Zhong, B.; Zhang, S.; Wang, Y.; Yao, W.; Zhao, Y.; Li, J. Postintervention Interleukin-6 (IL-6) Level, Rather than the Pretreatment or Dynamic Changes of IL-6, as an Early Practical Marker of Tumor Response in Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. Oncologist 2019, 24, e1489–e1495. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kawata, S.; Nagase, T.; Maeda, Y.; Yamasaki, E.; Kiso, S.; Ishiguro, H.; Matsuzawa, Y. Interleukin-6 in transcatheter arterial embolization for patients with hepatocellular carcinoma. Effects of serine protease inhibitor. Cancer 1994, 73, 53–57. [Google Scholar] [CrossRef]
- Gai, X.; Zhou, P.; Xu, M.; Liu, Z.; Zheng, X.; Liu, Q. Hyperactivation of IL-6/STAT3 pathway leaded to the poor prognosis of post-TACE HCCs by HIF-1α/SNAI1 axis-induced epithelial to mesenchymal transition. J. Cancer 2020, 11, 570–582. [Google Scholar] [CrossRef]
- Markezana, A.; Goldberg, S.N.; Kumar, G.; Zorde-Khvalevsky, E.; Gourevtich, S.; Rozenblum, N.; Galun, E.; Ahmed, M. Incomplete thermal ablation of tumors promotes increased tumorigenesis. Int. J. Hyperth. 2021, 38, 263–272. [Google Scholar] [CrossRef]
- Ke, S.; Ding, X.M.; Kong, J.; Gao, J.; Wang, S.H.; Cheng, Y.; Sun, W.B. Low temperature of radiofrequency ablation at the target sites can facilitate rapid progression of residual hepatic VX2 carcinoma. J. Transl. Med. 2010, 8, 73. [Google Scholar] [CrossRef]
- Kumar, G.; Goldberg, S.N.; Gourevitch, S.; Levchenko, T.; Torchilin, V.; Galun, E.; Ahmed, M. Targeting STAT3 to Suppress Systemic Pro-Oncogenic Effects from Hepatic Radiofrequency Ablation. Radiology 2018, 286, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, X.; Ding, J.; Duan, B.; Lu, S. Inflammation and cancer: Inhibiting the progression of residual hepatic VX2 carcinoma by anti-inflammatory drug after incomplete radiofrequency ablation. Int. J. Clin. Exp. Pathol. 2015, 8, 13945–13956. [Google Scholar] [PubMed]
- Wang, H.; Zhang, G.; Fan, W.; Wu, Y.; Zhang, J.; Xue, M.; Zhao, Y.; Yao, W.; Li, J. Clinical Significance of Peripheral Blood Lymphocyte Subtypes and Cytokines in Patients with Hepatocellular Carcinoma Treated with TACE. Cancer Manag. Res. 2022, 14, 451–464. [Google Scholar] [CrossRef]
- Avritscher, R.; Jo, N.; Polak, U.; Cortes, A.C.; Nishiofuku, H.; Odisio, B.C.; Takaki, H.; Tam, A.L.; Melancon, M.P.; Yevich, S.; et al. Hepatic Arterial Bland Embolization Increases Th17 Cell Infiltration in a Syngeneic Rat Model of Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2020, 43, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Nakamoto, Y.; Baba, T.; Nakagawa, H.; Mizukoshi, E.; Naito, M.; Mukaida, N.; Kaneko, S. Antitumor effect after radiofrequency ablation of murine hepatoma is augmented by an active variant of CC Chemokine ligand 3/macrophage inflammatory protein-1alpha. Cancer Res. 2010, 70, 6556–6565. [Google Scholar] [CrossRef]
- Schaller, T.H.; Batich, K.A.; Hotchkiss, K.; Cui, X.; Sanchez-Perez, L.; Sampson, J.H. Abstract A80: The effects of CCL3 on dendritic cell migration and immune cell activation. Cancer Immunol. Res. 2020, 8, A80. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, C.; Liang, B.; Zhao, H.; Qian, J.; Liang, H.; Feng, G. Hepatocellular necrosis, apoptosis, and proliferation after transcatheter arterial embolization or chemoembolization in a standardized rabbit model. J. Vasc. Interv. Radiol. 2011, 22, 1606–1612. [Google Scholar] [CrossRef]
- Ali, M.Y.; Grimm, C.F.; Ritter, M.; Mohr, L.; Allgaier, H.P.; Weth, R.; Bocher, W.O.; Endrulat, K.; Blum, H.E.; Geissler, M. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J. Hepatol. 2005, 43, 817–822. [Google Scholar] [CrossRef]
- Li, K.; Niu, Y.; Yuan, Y.; Qiu, J.; Shi, Y.; Zhong, C.; Qiu, Z.; Li, K.; Lin, Z.; Huang, Z.; et al. Insufficient ablation induces E3-ligase Nedd4 to promote hepatocellular carcinoma progression by tuning TGF-β signaling. Oncogene 2022, 41, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, H.; Jia, G.; Li, Y.; Liu, X.; Ren, W. Insufficient radiofrequency ablation promotes human hepatoma SMMC7721 cell proliferation by stimulating vascular endothelial growth factor overexpression. Oncol. Lett. 2015, 9, 1893–1896. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, J.; Gu, H. Amarogentin Inhibits Liver Cancer Cell Angiogenesis after Insufficient Radiofrequency Ablation via Affecting Stemness and the p53-Dependent VEGFA/Dll4/Notch1 Pathway. Biomed. Res. Int. 2020, 2020, 5391058. [Google Scholar] [CrossRef]
- Li, H.; Zhao, B.; Liu, Y.; Deng, W.; Zhang, Y. Angiogenesis in residual cancer and roles of HIF-1α, VEGF, and MMP-9 in the development of residual cancer after radiofrequency ablation and surgical resection in rabbits with liver cancer. Folia Morphol. 2020, 79, 71–78. [Google Scholar] [CrossRef]
- Park, J.H.; Ryu, S.H.; Kim, J.A.; Kim, Y.M.; Lee, J.H.; Kim, Y.S.; Moon, J.S. Association between serial changes of serum vascular endothelial growth factor/insulin-like growth factor-2 levels and the short period recurrence of hepatocellular carcinoma after transcatheter arterial chemoembolization; A prospective study. Hepatology 2009, 50, 1146A. [Google Scholar] [CrossRef]
- Xiao, E.H.; Guo, D.; Bian, D.J. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J. Gastroenterol. 2009, 15, 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.F.; Yi, J.L.; Li, X.R.; Deng, W.; Yang, Z.F.; Tian, G. Angiogenesis in rabbit hepatic tumor after transcatheter arterial embolization. World J. Gastroenterol. 2004, 10, 1885–1889. [Google Scholar] [CrossRef]
- Sun, X.D.; Du, Y.Q.; Chen, W.J. Effects of endostar on VEGF expression after TACE in hepatocellular carcinoma. J. Pract. Oncol. 2013, 28, 41–44. [Google Scholar]
- Li, W.; Kong, S.; Su, J.; Huang, J.; Xue, H. Efficacy of transcatheter arterial chemoembolization combined with sorafenib in inhibiting tumor angiogenesis in a rabbit VX2 liver cancer model. J. Interv. Med. 2020, 3, 27–33. [Google Scholar] [CrossRef]
- Shi, Y.L.; Xu, T.; Li, L.P.; Chen, X.P. Over-expression of VEGF and MMP-9 in residual tumor cells of hepatocellular carcinoma after embolization with lipidol. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Chen, S.; Wei, G.; Li, Y.; Liao, J.; Jin, H.; Zou, Y.; Huang, M.; Peng, Z.; Guo, Y.; et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019, 460, 29–40. [Google Scholar] [CrossRef]
- Guo, J.H.; Zhu, X.; Li, X.T.; Yang, R.J. Impact of serum vascular endothelial growth factor on prognosis in patients with unresectable hepatocellular carcinoma after transarterial chemoembolization. Chin. J. Cancer Res. 2012, 24, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiang, H.; Jiang, X.; Tan, H.; Meng, Q.; Sun, B.; Xu, R.; Krissansen, G.W. Antisense hypoxia-inducible factor-1alpha augments transcatheter arterial embolization in the treatment of hepatocellular carcinomas in rats. Hum. Gene Ther. 2009, 20, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lai, L.; Liu, S.; Zhou, C.; Wu, C.; Huang, M.; Lin, Q. Targeting HIF-1α and VEGF by lentivirus-mediated RNA interference reduces liver tumor cells migration and invasion under hypoxic conditions. Neoplasma 2016, 63, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Markezana, A.; Ahmed, M.; Kumar, G.; Zorde-Khvalevsky, E.; Rozenblum, N.; Galun, E.; Goldberg, S.N. Moderate hyperthermic heating encountered during thermal ablation increases tumor cell activity. Int. J. Hyperth. 2020, 37, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Muktiali, M.; Ren, B.; Hu, Y.; Li, D.; Li, Z.; Li, D.; Xie, Y.; Tao, M.; et al. Effect of microwave ablation treatment of hepatic malignancies on serum cytokine levels. BMC Cancer 2020, 20, 812. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, X.; Yu, Z. The effect of high intensity focused ultrasound on the treatment of liver cancer and patients’ immunity. Cancer Biomark. 2019, 24, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hou, X.; Cai, H.; Zhuang, X. Effects of microwave ablation on T-cell subsets and cytokines of patients with hepatocellular carcinoma. Minim Invasive Ther. Allied Technol. 2017, 26, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Kim, M.J.; Jang, J.W.; Oh, B.S.; Kwon, J.H.; Chung, K.W.; Jung, H.S.; Jekarl, D.W.; Lee, S. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine 2013, 64, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ronald, J.; Nixon, A.B.; Marin, D.; Gupta, R.T.; Janas, G.; Chen, W.; Suhocki, P.V.; Pabon-Ramos, W.; Sopko, D.R.; Starr, M.D.; et al. Pilot Evaluation of Angiogenesis Signaling Factor Response after Transcatheter Arterial Embolization for Hepatocellular Carcinoma. Radiology 2017, 285, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.H.; Li, H.; Han, X.W.; Ren, J.Z.; Li, F.Y.; Ju, S.G.; Chen, P.F.; Kuang, D.L. Upregulation of IL-6 is involved in moderate hyperthermia induced proliferation and invasion of hepatocellular carcinoma cells. Eur. J. Pharm. 2018, 833, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Kong, L.; Kong, J.; Ke, S.; Gao, J.; Ding, X.; Zheng, L.; Sun, H.; Sun, W. After insufficient radiofrequency ablation, tumor-associated endothelial cells exhibit enhanced angiogenesis and promote invasiveness of residual hepatocellular carcinoma. J. Transl. Med. 2012, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, B.; Wang, Y.; Wang, W.; Chang, H.; Li, D.; Li, Y.; Song, Z. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition mediated by interleukin-6/signal transducer and activator of transcription 3/Snail pathway in the H22 cells. J. Cancer Res. Ther. 2020, 16, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Ypsilantis, P.; Lambropoulou, M.; Anagnostopoulos, C.; Tsigalou, C.; Vasiliadis, C.; Kortsaris, A.; Papadopoulos, N.; Simopoulos, C. Pringle maneuver exacerbates systemic inflammatory response and multiple-organ injury induced by extended liver radiofrequency ablation. Hum. Exp. Toxicol. 2011, 30, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, G.G.; Lai, P.B.S. Targeting hepatocyte growth factor/c-mesenchymal-epithelial transition factor axis in hepatocellular carcinoma: Rationale and therapeutic strategies. Med. Res. Rev. 2021, 41, 507–524. [Google Scholar] [CrossRef]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef]
- Chen, X.J.; Deng, Y.R.; Wang, Z.C.; Wei, W.F.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Liang, L.J.; Zhong, M.; Liang, L.; et al. Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 2019, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, T.H.; Loosen, S.H.; Schulze-Hagen, M.; Gorgulho, J.; Kandler, J.; Joerdens, M.; Demir, M.; Mohr, R.; Bruners, P.; Kuhl, C.; et al. Macrophage migration inhibitory factor predicts an unfavorable outcome after transarterial chemoembolization for hepatic malignancies. Clin. Transl. Sci. 2021, 14, 1853–1863. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Gu, Y.; Srimathveeravalli, G.; Cai, L.; Ueshima, E.; Maybody, M.; Yarmohammadi, H.; Zhu, Y.S.; Durack, J.C.; Solomon, S.B.; Coleman, J.A.; et al. Pirfenidone inhibits cryoablation induced local macrophage infiltration along with its associated TGFb1 expression and serum cytokine level in a mouse model. Cryobiology 2018, 82, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Yan, Z.; Wu, Y.; Chen, Y.; Qu, P.; Xu, X.; Yuan, P.; Huang, X.; Xing, J.; Zhang, H.; et al. Transarterial chemoembolization aggravated peritumoral fibrosis via hypoxia-inducible factor-1α dependent pathway in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2015, 30, 925–932. [Google Scholar] [CrossRef]
- Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.S.; Ye, M.L.; Shen, F.; Liu, W.; Hu, H.S.; Li, S.W.; Wu, H.W.; Chen, Q.H.; Zhou, W.B. Overexpression and correlation of HIF-2α, VEGFA and EphA2 in residual hepatocellular carcinoma following high-intensity focused ultrasound treatment: Implications for tumor recurrence and progression. Exp. Ther. Med. 2017, 13, 3529–3534. [Google Scholar] [CrossRef]
- Li, Z.; Xue, T.Q.; Chen, X.Y. Predictive values of serum VEGF and CRP levels combined with contrast enhanced MRI in hepatocellular carcinoma patients after TACE. Am. J. Cancer Res. 2016, 6, 2375–2385. [Google Scholar]
- Xu, M.; Xie, X.H.; Xie, X.Y.; Xu, Z.F.; Liu, G.J.; Zheng, Y.L.; Huang, G.L.; Wang, W.; Zheng, S.G.; Lü, M.D. Sorafenib suppresses the rapid progress of hepatocellular carcinoma after insufficient radiofrequency ablation therapy: An experiment in vivo. Acta Radiol. 2013, 54, 199–204. [Google Scholar] [CrossRef]
- Sergio, A.; Cristofori, C.; Cardin, R.; Pivetta, G.; Ragazzi, R.; Baldan, A.; Girardi, L.; Cillo, U.; Burra, P.; Giacomin, A.; et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am. J. Gastroenterol. 2008, 103, 914–921. [Google Scholar] [CrossRef]
- Wu, T.; Yao, Y.; Sun, R.; Wang, H.; Yin, X.; Zhang, J.; Zhou, Q.; Huangfu, C. Arterial Infusion of Rapamycin in the Treatment of Rabbit Hepatocellular Carcinoma to Improve the Effect of TACE. Open Life Sci. 2018, 13, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Rhee, T.K.; Young, J.Y.; Larson, A.C.; Haines, G.K., 3rd; Sato, K.T.; Salem, R.; Mulcahy, M.F.; Kulik, L.M.; Paunesku, T.; Woloschak, G.E.; et al. Effect of transcatheter arterial embolization on levels of hypoxia-inducible factor-1alpha in rabbit VX2 liver tumors. J. Vasc. Interv. Radiol. 2007, 18, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Ammendola, M.; Marech, I.; Laterza, A.; Abbate, I.; Oakley, C.; Vacca, A.; Sacco, R.; Gadaleta, C.D. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J. Gastroenterol. 2015, 21, 6018–6025. [Google Scholar] [CrossRef]
- Blumenschein, G.R., Jr.; Mills, G.B.; Gonzalez-Angulo, A.M. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J. Clin. Oncol. 2012, 30, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, L.; Dansi, P.; Matteucci, E.; Desiderio, M.A. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis 2001, 22, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Valpione, S.; Pasquali, S.; Campana, L.G.; Piccin, L.; Mocellin, S.; Pigozzo, J.; Chiarion-Sileni, V. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J. Transl. Med. 2018, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Bentebibel, S.E.; Daher, M.; Haymaker, C.; Wani, K.; Saberian, C.; Ogata, D.; et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022, 40, 509–523. [Google Scholar] [CrossRef]
- Moi, L.; Bouchaab, H.; Mederos, N.; Nguyen-Ngoc, T.; Perreau, M.; Fenwick, C.; Vaucher, J.; Sempoux, C.; Peters, S.; Obeid, M. Personalized Cytokine-Directed Therapy With Tocilizumab for Refractory Immune Checkpoint Inhibitor-Related Cholangiohepatitis. J. Thorac. Oncol. 2021, 16, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Stroud, C.R.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pract. 2019, 25, 551–557. [Google Scholar] [CrossRef]
- Holmstroem, R.B.; Nielsen, O.H.; Jacobsen, S.; Riis, L.B.; Theile, S.; Bjerrum, J.T.; Vilmann, P.; Johansen, J.S.; Boisen, M.K.; Eefsen, R.H.L.; et al. COLAR: Open-label clinical study of IL-6 blockade with tocilizumab for the treatment of immune checkpoint inhibitor-induced colitis and arthritis. J. Immunother. Cancer 2022, 10, e005111. [Google Scholar] [CrossRef]
- Horvat, T.Z.; Adel, N.G.; Dang, T.O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef]
- Monsour, E.P.; Pothen, J.; Balaraman, R. A Novel Approach to the Treatment of Pembrolizumab-induced Psoriasis Exacerbation: A Case Report. Cureus 2019, 11, e5824. [Google Scholar] [CrossRef] [PubMed]
| Sponsor | Acronym | Intervention | Population | Sample Size | Primary Endpoints | Expected End | Trial Registration ID |
|---|---|---|---|---|---|---|---|
| Merck Sharp & Dohme LLC (Rahway, NJ, USA) | LEAP-012 | TACE plus lenvatinib plus pembrolizumab versus TACE plus placebo | Intermediate stage HCC | 450 | OS and PFS | 31 December 2029 | NCT04246177 |
| Hoffmann-La Roche (Basel, Switzerland) | - | Atezolizumab plus bevacizumab plus TACE versus TACE | Intermediate stage HCC | 342 | PFS and OS | 28 February 2029 | NCT04712643 |
| AstraZeneca (Cambridge, UK) | EMERALD-1 | Durvalumab plus bevacizumab plus TACE versus Durvalumab plus TACE versus TACE plus placebo | Locoregional HCC | 724 | PFS | 19 August 2024 | NCT03778957 |
| AstraZeneca | EMERALD-3 | Tremelimumab plus durvalumab plus lenvatinib plus TACE versus tremelimumab plus durvalumab plus TACE versus TACE | Locoregional HCC | 525 | PFS | 29 January 2027 | NCT05301842 |
| Bristol-Myers Squibb (New York, NY, USA) | CheckMate 74W | Nivolumab plus ipilimumab plus TACE versus nivolumab plus TACE versus TACE | Intermediate HCC | 26 | TTTP and OS | 29 January 2024 | NCT04340193 |
| The Clatterbridge Cancer Centre NHS Foundation Trust (Birkenhead, UK) | TACE-3 | Nivolumab plus TACE/TAE versus TACE/TAE | Intermediate stage HCC | 522 | OS and TTTP | June 2026 | NCT04268888 |
| Jiangsu HengRui Medicine Co., Ltd. (Lianyungang, China) | - | Camrelizumab plus Apatinib plus versus TACE | Incurable HCC | 360 | PFS | 30 July 2026 | NCT05320692 |
| Zhongda Hospital | - | Penpulimab plus anlotinib plus TACE versus penpulimab plus anlotinib | Advanced stage HCC | 109 | PFS | 31 March 2024 | NCT05344924 |
| AstraZeneca | EMERALD-2 | Curative therapy (resection of ablation) plus durvalumab plus bevacizumab versus Curative therapy (resection of ablation) plus durvalumab versus Curative therapy (resection of ablation) plus placebo | Early/intermediate stage HCC | 908 | RFS | 31 May 2024 | NCT03847428 |
| Merck Sharp & Dohme LLC | KEYNOTE-937 | Curative therapy (resection of ablation) plus Pembrolizumab versus Curative therapy (resection of ablation) plus placebo | Early/intermediate stage HCC | 950 | RFS | 31 August 2029 | NCT03867084 |
| Bristol-Myers Squibb | CheckMate 9DX | Curative therapy (resection of ablation) plus nivolumab versus Curative therapy (resection of ablation) plus placebo | Early/intermediate stage HCC | 545 | RFS | 16 December 2025 | NCT03383458 |
| Hoffmann-La Roche | IMbrave050 | Curative therapy (resection of ablation) plus atezolizumab plus bevacizumab versus none | Early/intermediate /advanced stage HCC | 668 | RFS | 16 July 2027 | NCT04102098 |
| Cytokine | Procedure | Location | Pathological Actions | Ref. |
|---|---|---|---|---|
| IFN-γ | TACE, ablative therapies | Intratumor, peripheral blood | Cytotoxic T cells activation and function; Macrophage activation; Upregulation of PD-L1 expression | [42,91,97,117] |
| IL-6 | TAE; ablative therapies | Intratumor; peripheral blood | Prolonged inflammation, tumor cell undergoing EMT; tumor cell proliferation, invasion, and migration; angiogenesis | [80,105,118,119,120,121,122,123,124] |
| IL-7 | Ablative therapies | Intratumor | T cell infiltration and activation | [91] |
| IL-10 | Ablative therapies | Intratumor; peripheral blood | Intratumor; peripheral blood | [125] |
| IL-17 | TAE | Intratumor | Inflammation | [126] |
| CCL2 | Ablative therapies | Intratumor | Monocyte and TAM infiltration; prolonged inflammation | [78] |
| CCL8 | Ablative therapies | Intratumor | TAM infiltration | [91] |
| CXCL14 | Ablative therapies | Intratumor | Intratumor | |
| CCL3 | Ablative therapies | Intratumor | CD4+ T cell and CD8+ T cell infiltration | [91,127,128] |
| CCL4 | Ablative therapies | Intratumor | CD4+ T cell and CD8+ T cell infiltration | [91,127] |
| TNF-α | Ablative therapies | Intratumor; peripheral blood | Inflammation; CCL2 induction | [97,117,124,129,130] |
| TGF-β | TAE; ablative therapies | Intratumor | MSDCs infiltration; inhibition of CD8+T cell infiltration; tumor cell undergoing EMT; tumor cells survival, proliferation, invasion, and migration | [43,80,81,84,111,131] |
| VEGF | TACE; ablative therapies | Intratumor; peripheral blood | Angiogenesis; tumor cell stemness | [85,86,87,101,105,121,123,132,133,134,135,136,137,138,139,140,141,142,143,144] |
| HGF | Ablative therapies | Intratumor | Tumor cell proliferation, invasion, and migration | [82,105,121,122,123,145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Zeng, C.H.; An, C.; Liu, Y.; Shin, J.H.; Li, X. How Biology Guides the Combination of Locoregional Interventional Therapies and Immunotherapy for Hepatocellular Carcinoma: Cytokines and Their Roles. Cancers 2023, 15, 1324. https://doi.org/10.3390/cancers15041324
Fu Y, Zeng CH, An C, Liu Y, Shin JH, Li X. How Biology Guides the Combination of Locoregional Interventional Therapies and Immunotherapy for Hepatocellular Carcinoma: Cytokines and Their Roles. Cancers. 2023; 15(4):1324. https://doi.org/10.3390/cancers15041324
Chicago/Turabian StyleFu, Yan, Chu Hui Zeng, Chao An, Yue Liu, Ji Hoon Shin, and Xiao Li. 2023. "How Biology Guides the Combination of Locoregional Interventional Therapies and Immunotherapy for Hepatocellular Carcinoma: Cytokines and Their Roles" Cancers 15, no. 4: 1324. https://doi.org/10.3390/cancers15041324
APA StyleFu, Y., Zeng, C. H., An, C., Liu, Y., Shin, J. H., & Li, X. (2023). How Biology Guides the Combination of Locoregional Interventional Therapies and Immunotherapy for Hepatocellular Carcinoma: Cytokines and Their Roles. Cancers, 15(4), 1324. https://doi.org/10.3390/cancers15041324






