Endometrial Cancer Arising in Adenomyosis (EC-AIA): A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Search Strategy
2.3. Search Selection
2.4. Risk of Bias Assessment
2.5. Data Extraction and Analysis
3. Results
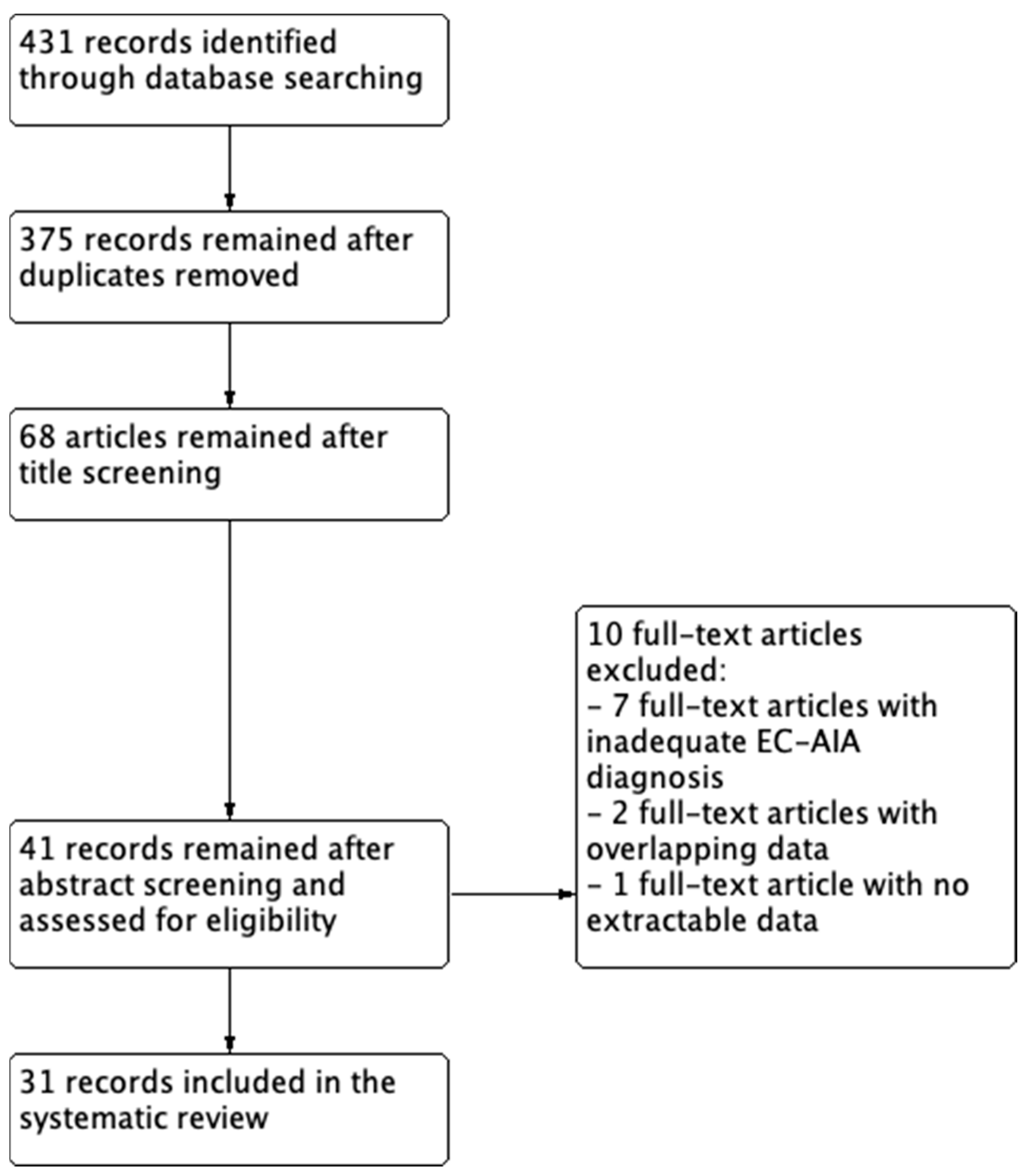
3.1. Study Selection
3.2. Characteristics of the Included Studies and Study Population
3.3. Assessment of Risk of Bias among Studies
4. Discussion
4.1. Main Findings and Interpretation
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Santoro, A.; Esposito, I.; Angelico, G.; Spadola, S.; Zannoni, G.F. Accuracy of One-Step Nucleic Acid Amplification in Detecting Lymph Node Metastases in Endometrial Cancer. Pathol. Oncol. Res. 2020, 26, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Troisi, J.; Boccia, D.; Travaglino, A.; Capuano, G.; Insabato, L.; Mollo, A.; Guida, M.; Zullo, F. Metabolomics in endometrial cancer diagnosis: A systematic review. Acta Obstet. Gynecol. Scand. 2020, 99, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Seracchioli, R.; Raimondo, D.; Maletta, M.; Travaglino, A.; Raimondo, I.; Giaquinto, I.; Orsini, B.; Insabato, L.; Pellicano, M.; et al. Prevalence of adenomyosis in endometrial cancer patients: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 303, 47–53. [Google Scholar] [CrossRef]
- Chao, X.; Wu, M.; Ma, S.; Tan, X.; Zhong, S.; Bi, Y.; Wu, H.; Lang, J.; Li, L. The clinicopathological characteristics and survival outcomes of endometrial carcinoma coexisting with or arising in adenomyosis: A pilot study. Sci. Rep. 2020, 10, 5984. [Google Scholar] [CrossRef]
- Colman, H.I.; Rosenthal, A.H. Carcinoma developing in areas of adenomyosis. Obstet. Gynecol. 1959, 14, 342–348. [Google Scholar]
- Matsuo, K.; Moeini, A.; Machida, H.; Scannell, C.A.; Bs, J.K.C.; Kakuda, M.; Adachi, S.; Garcia-Sayre, J.; Ueda, Y.; Roman, L.D. Tumor Characteristics and Survival Outcome of Endometrial Cancer Arising in Adenomyosis: An Exploratory Analysis. Ann. Surg. Oncol. 2015, 23, 959–967. [Google Scholar] [CrossRef]
- Machida, H.; Maeda, M.; Cahoon, S.S.; Scannell, C.A.; Garcia-Sayre, J.; Roman, L.D.; Matsuo, K. Endometrial cancer arising in adenomyosis versus endometrial cancer coexisting with adenomyosis: Are these two different entities? Arch. Gynecol. Obstet. 2017, 295, 1459–1468. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.; PRISMA-P Group. Preferred reporting items for systemic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Raimondo, D.; Neola, D.; Renzulli, F.; Santoro, A.; Insabato, L.; Casadio, P.; Zannoni, G.F.; Zullo, F.; et al. Prognostic value of myometrial invasion and TCGA groups of endometrial carcinoma. Gynecol. Oncol. 2021, 162, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M.; Suzuki, A.; Ozawa, M.; Fujita, K.; Sakakibara, A.; Kawamura, M.; Takahashi, S.; Fujii, H.; Hirano, T.; Okagaki, A.; et al. Adenocarcinomas arising from uterine adenomyosis: A Report of Four Cases. Int. J. Gynecol. Pathol. 2002, 21, 239–245. [Google Scholar] [CrossRef]
- Couto, D.; Mota, F.; Silva, T.; De Oliveira, C. Adenocarcinoma arising in adenomyosis: Report of an unusual case. Acta Obstet. Gynecol. Scand. 2004, 83, 406–408. [Google Scholar] [CrossRef]
- Takeuchi, K.; Yamanaka, Y.; Hamana, S.; Ohara, N.; Maruo, T. Invasive adenocarcinoma arising from uterine adenomyosis involving the rectosigmoid colon. Int. J. Gynecol. Cancer 2004, 14, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-I.; Chou, S.-Y.; Lin, S.-E.; Liang, S.-J.; Chiu, H.-C.; Hsu, C.-S. Very early stage adenocarcinoma arising from adenomyosis in the uterus. Taiwan. J. Obstet. Gynecol. 2006, 45, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Tateiwa, H.; Hamana, S.; Yoshida, S.; Kitazawa, S.; Maruo, T. Invasive adenocarcinoma arising from adenomyosis in a septate uterus. Acta Obstet. Gynecol. Scand. 2006, 85, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Izadi-Mood, N.; Samadi, N.; Sarmadi, S.; Eftekhar, Z. Papillary serous carcinoma arising from adenomyosis presenting as intramural leiomyoma. Arch. Iran. Med. 2007, 10, 258–260. [Google Scholar]
- Motohara, K.; Tashiro, H.; Ohtake, H.; Saito, F.; Ohba, T.; Katabuchi, H. Endometrioid adenocarcinoma arising in adenomyosis: Elucidation by periodic magnetic resonance imaging evaluations. Int. J. Clin. Oncol. 2008, 13, 266–270. [Google Scholar] [CrossRef]
- Puppa, G.; Shozu, M.; Perin, T.; Nomura, K.; Gloghini, A.; Campagnutta, E.; Canzonieri, V. Small primary adenocarcinoma in adenomyosis with nodal metastasis: A case report. BMC Cancer 2007, 7, 103. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamatani, S.; Suzuki, T.; Ikeda, K.; Kiyokawa, K.; Shiokawa, A.; Kushima, M.; Ota, H. Clear cell adenocarcinoma arising from a giant cystic adenomyosis: A case report with immunohistochemical analysis of laminin-5 gamma2 chain and p53 overexpression. Pathol.-Res. Pract. 2008, 204, 677–682. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Yasuda, M.; Kajiwara, H.; Nakamura, N.; Sato, S.; Nishijima, Y.; Mikami, M.; Osamura, R.Y. Clear cell adenocarcinoma arising from adenomyosis. Int. J. Gynecol. Pathol. 2009, 28, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Ansari, C.; Coakley, F.; Wang, Z.; Yeh, B.; Rabban, J.; Poder, L. Imaging of Mullerian adenosarcoma arising in adenomyosis. Clin. Radiol. 2009, 64, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Boes, A.S.; Tousseyn, T.; Vandenput, I.; Timmerman, D.; Vergote, I.; Moerman, P.; Amant, F. Pitfall in the diagnosis of endometrial cancer: Case report of an endometrioid adenocarcinoma arising from uterine adenomyosis. Eur. J. Gynaecol. Oncol. 2011, 32, 431–434. [Google Scholar] [PubMed]
- Heo, S.H.; Lee, K.-H.; Kim, J.W.; Jeong, Y.Y. Unusual manifestation of endometrioid adenocarcinoma arising from subserosal cystic adenomyosis of the uterus: Emphasis on MRI and positron emission tomography CT findings. Br. J. Radiol. 2011, 84, e212–e214. [Google Scholar] [CrossRef]
- Shin, Y.J.; Cho, S.-E.; Sung, C.O.; Choi, C.H.; Bae, D.-S. Clear cell adenocarcinoma arising from adenomyosis mimicking leiomyoma: A case report. Korean J. Obstet. Gynecol. 2011, 54, 561–565. [Google Scholar] [CrossRef]
- Elshafie, M.; Rahimi, S.; Ganesan, R.; Hirschowitz, L. Müllerian adenosarcoma arising in a subserosal adenomyoma. Int. J. Surg. Pathol. 2012, 21, 186–189. [Google Scholar] [CrossRef]
- Bae, H.S.; Kim, I.S.; Kang, J.S.; Song, J.Y. Endometrioid adenocarcinoma arising from adenomyosis after black cohosh with St John’s wort. J. Obstet. Gynaecol. 2014, 34, 213–214. [Google Scholar] [CrossRef]
- Kawamura, K.; Kaneki, E.; Ogawa, S.; Imamura, H.; Ohishi, Y.; Kobayashi, H.; Kato, K. Primary Uterine müllerian mucinous borderline tumor (mmbt) associated with adenomyosis. Int. J. Gynecol. Pathol. 2014, 33, 146–150. [Google Scholar] [CrossRef]
- Taga, S.; Sawada, M.; Nagai, A.; Yamamoto, D.; Hayase, R. A case of endometrioid adenocarcinoma arising from adenomyosis. Case Rep. Obstet. Gynecol. 2014, 2014, 569295. [Google Scholar] [CrossRef]
- Baba, A.; Yamazoe, S.; Dogru, M.; Ogawa, M.; Takamatsu, K.; Miyauchi, J. Clear cell adenocarcinoma arising from adenomyotic cyst: A case report and literature review. J. Obstet. Gynaecol. Res. 2015, 42, 217–223. [Google Scholar] [CrossRef]
- Mori, M.; Furusawa, A.; Kino, N.; Uno, M.; Ozaki, Y.; Yasugi, T. Rare case of endometrioid adenocarcinoma arising from cystic adenomyosis. J. Obstet. Gynaecol. Res. 2014, 41, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, K.; Hasegawa, K.; Kanamori, A.; Machida, H.; Kojima, M.; Fukasawa, I. Carcinosarcoma arising from uterine adenomyosis: A case report. J. Obstet. Gynaecol. Res. 2015, 42, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Chang, W.-H.; Liu, W.-M.; Wang, P.-H. Serous carcinoma arising from adenomyosis. Taiwan. J. Obstet. Gynecol. 2017, 56, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Park, J.Y. A Rare Case of Intramural Müllerian Adenosarcoma Arising from Adenomyosis of the Uterus. J. Pathol. Transl. Med. 2017, 51, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Kikuchi, A.; Sasagawa, M.; Honma, S. Two cases of endometrial cancer arising from adenomyosis during aromatase inhibitors therapy after mastectomy. J. Obstet. Gynaecol. 2017, 37, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Antovska, V.S.; Krstevska, I.; Trajanova, M.; Chelebieva, J.; Gosheva, I.; Zdravkovski, P.; Kostadinova-Kunovska, S.; Janevska, V.; Zdravkovski, P. Endometrioid Adenocarcinoma Arising in Adenomyoma in a Woman with a Genital Prolapse—Case Report. Open Access Maced. J. Med. Sci. 2018, 6, 1091–1094. [Google Scholar] [CrossRef]
- Bang, Y.J.; Lee, J.-M.; Pyeon, S.Y. Endometrial adenocarcinoma arising in adenomyosis: Case report and review of literature. Eur. J. Gynaecol. Oncol. 2020, 41, 858–862. [Google Scholar] [CrossRef]
- Izumi, Y.; Yamamoto, T.; Matsunaga, N.; Ota, T.; Owaki, Y.; Shinohara, K.; Tsuzuki, T.; Suzuki, K. Endometrial cancer arising from adenomyosis: Case report and literature review of MRI findings. Radiol. Case Rep. 2020, 15, 427–430. [Google Scholar] [CrossRef]
- Talia, K.L.F.; Naaman, Y.M.; McCluggage, W.G.F. Uterine Adenosarcoma Originating in Adenomyosis: Report of an Extremely Rare Phenomenon and Review of Published Literature. Int. J. Gynecol. Pathol. 2021, 40, 342–348. [Google Scholar] [CrossRef]
- Talwar, A.; Behera, P.; Ahuja, A.; Sarkar, B.; Phulware, R.H. Endometrial Serous Carcinoma Arising from Adenomyosis: A Clinico-Pathological Insight. J. Fam. Reprod. Health 2021, 15, 125–129. [Google Scholar] [CrossRef]
- Chikumi, J.; Oishi, T.; Nakaso, T.; Sawada, M.; Kudoh, A.; Komatsu, H.; Sato, S.; Taniguchi, F.; Harada, T. Endometrial Cancer Arising in Adenomyosis That Could Not Be Diagnosed by Endometrial Biopsy: A Case Report. Yonago Acta Med. 2022, 65, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Asami, Y.; Kobayashi-Kato, M.; Tanase, Y.; Uno, M.; Ishikawa, M.; Shiraishi, K.; Kato, T. Genetic features of endometrioid-type endometrial carcinoma arising in uterine adenomyosis. Virchows Arch. 2021, 481, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M.; Pluchino, N.; Petignat, P.; Bianchi, P.; Brosens, I.A.; Benagiano, G. Adenomyosis and Endometrial Cancer: Literature Review. Gynecol. Obstet. Investig. 2018, 83, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Casadio, P.; Raffone, A.; Maletta, M.; Travaglino, A.; Raimondo, D.; Raimondo, I.; Santoro, A.; Paradisi, R.; Zannoni, G.F.; Mollo, A.; et al. Clinical Characteristics of Patients with Endometrial Cancer and Adenomyosis. Cancers 2021, 13, 4918. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, D.; Raffone, A.; Travaglino, A.; Maletta, M.; Casadio, P.; Ambrosio, M.; Aru, A.C.; Santoro, A.; Zannoni, G.F.; Insabato, L.; et al. Impact of adenomyosis on the prognosis of patients with endometrial cancer. Int. J. Gynecol. Obstet. 2021, 157, 265–270. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Raimondo, D.; Maletta, M.; Salucci, P.; Santoro, A.; Zullo, F.; Zannoni, G.F.; Casadio, P.; Seracchioli, R.; et al. Histological Prognostic Factors of Endometrial Cancer in Patients with Adenomyosis: A Systematic Review and Meta-Analysis. Pathobiology 2022, 89, 127–134. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Mascolo, M.; Carotenuto, C.; Guida, M.; Mollo, A.; Insabato, L.; Zullo, F. Histopathological characterization of ProMisE molecular groups of endometrial cancer. Gynecol. Oncol. 2020, 157, 252–259. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Mascolo, M.; Guida, M.; Insabato, L.; Zannoni, G.F.; Zullo, F. Clear cell endometrial carcinoma and the TCGA classification. Histopathology 2019, 76, 336–338. [Google Scholar] [CrossRef]
- Koike, N.; Tsunemi, T.; Uekuri, C.; Akasaka, J.; Ito, F.; Shigemitsu, A.; Kobayashi, H. Pathogenesis and malignant transformation of adenomyosis (Review). Oncol. Rep. 2012, 29, 861–867. [Google Scholar] [CrossRef]


| Studies | Country | Study Design | Year |
|---|---|---|---|
| 2002 Koshiyama et al. | Japan | Case series | 1981–2001 |
| 2004 Couto et al. | Portugal | Case report | 2004 |
| 2004 Takeuchi et al. | Japan | Case report | 2004 |
| 2006 Hsu et al. | Taiwan | Case report | 2006 |
| 2006 Takeuchi et al. | Japan | Case report | 2006 |
| 2007 Izadi-Mood et al. | Iran | Case report | 2007 |
| 2007 Motohara et al. | Japan | Case report | 2007 |
| 2007 Puppa et al. | Italy | Case report | 2007 |
| 2008 Ohta et al. | Japan | Case report | 2008 |
| 2009 Hirabayashi et al. | Japan | Case report | 2009 |
| 2009 Jha et al. | United States of America | Case report | 2009 |
| 2011 Boes et al. | Belgium | Case report | 2011 |
| 2011 Heo et al. | Korea | Case report | 2011 |
| 2011 Shin et al. | Korea | Case report | 2011 |
| 2012 Elshafie et al. | United Kingdom | Case report | 2012 |
| 2014 Bae et al. | Korea | Case report | 2014 |
| 2014 Kawamura et al. | Japan | Case report | 2014 |
| 2014 Taga et al. | Japan | Case report | 2014 |
| 2015 Baba et al. | Japan | Case report | 2015 |
| 2015 Mori et al. | Japan | Case report | 2015 |
| 2016 Kiuchi et al. | Japan | Case report | 2016 |
| 2017 Chia-Hao Liu et al. | Taiwan | Case report | 2017 |
| 2017 Lee et al. | Korea | Case series | 2017 |
| 2017 Yanase et al. | Japan | Case series | 2013–2014 |
| 2018 Vesna et al. | Macedonia | Case report | 2018 |
| 2020 Bang et al. | Korea | Case report | 2020 |
| 2020 Izumi et al. | Japan | Case report | 2020 |
| 2020 Talia et al. | United Kingdom | Case report | 2020 |
| 2020 Talwar et al. | India | Case report | 2020 |
| 2022 Chikumi et al. | Japan | Case report | 2022 |
| 2022 Yoshida et al. | Japan | Case series | 2010–2020 |
| EC-AIA n (%) | |
|---|---|
| Number | 37 |
| Age, (years) mean ± SD | 57.9 ± 9.2 |
| Parity | |
| 0 | 6 (27.3) |
| ≥1 | 16 (72.7) |
| missing | 15 |
| Menopause | |
| yes | 23 (85.1) |
| no | 4 (14.8) |
| missing | 10 |
| Clinical manifestation | |
| Abnormal uterine bleeding | 15 (40.5) |
| Abdominal or pelvic pain | 10 (27) |
| No signs or symptoms | 8 (21.6) |
| Vaginal discharge | 1 (2.7) |
| Others | 3 (8.1) |
| Preoperative diagnosis | |
| Uterine sarcoma | 12 (32.4) |
| Atypical myoma | 6 (16.2) |
| Ovarian cancer | 6 (16.2) |
| Myoma | 4 (10.8) |
| Adenomyosis/adenomyoma | 4 (10.8) |
| None | 3 (8.1) |
| Endometrial cancer | 2 (5.4) |
| Surgical procedures | |
| Bilateral-salpingoophorectomy | 30 (81.0) |
| Pelvic lymphoadenectomy | 8 (21.6) |
| Pelvic and paraortic lymphoadenectomy | 5 (13.5) |
| Other additional surgical procedures | 9 (24.3) |
| EC-AIA (%) | |
|---|---|
| Number | 37 |
| FIGO stage | |
| IA | 12 (32.4) |
| IB | 10 (27) |
| IC | 3 (8.1) |
| II | 1 (2.7) |
| III | 5 (13.5) |
| IV | 6 (16.2) |
| Histotype | |
| Endometroid | 20 (57.1) |
| Clear cell | 5 (14.3) |
| Adenosarcoma | 4 (11.4) |
| Serous | 4 (11.4) |
| Carcinosarcoma | 1 (2.8) |
| Mullerian mucinous borderline tumor | 1 (2.8) |
| Missing | 2 |
| Grade | |
| Grade 1 | 10 (58.8) |
| Grade 2 | 3 (17.6) |
| Grade 3 | 4 (23.5) |
| Missing | 3 |
| Metastasis | |
| Pelvic and/or paraaortic nodal metastasis | 5 (13.6) |
| Other sites | 6 (16.2) |
| Immunohistochemical expression | |
| P53abn | 9 (45) |
| ER- PR- P53wt | 3 (15) |
| PR+ | 2 (10) |
| Others | 4 (2) |
| ER+ | 1 (5) |
| ER+ PR+ P53abn | 1 (5) |
| Missing | 17 |
| Adjuvant treatment | |
| Chemotherapy and/or radiotherapy | 21 (80.7) |
| Missing | 11 |
| Follow-up (months) mean ± SD | 15.4 (22) |
| Survival Outcomes | |
| Death | 1 (4.3) |
| Recurrence | 8 (34.8) |
| Missing | 14 |
| Studies | Histotype | Colman and Rosenthal Criteria * | Endometrium | Transition from Adenomyosis to Endometrial Cancer | Other Histological Features |
|---|---|---|---|---|---|
| 2002 Koshiyama et al. | Endometroid | b,c | Foci of adenocarcinoma | Yes | |
| 2004 Couto et al. | Endometroid | a,b,c | Atrophic | Yes | |
| 2004 Takeuchi et al. | Endometroid | a,b,c | Atrophic | No | |
| 2006 Hsu et al. | Endometroid | a,b,c | Atrophic | Yes | |
| 2006 Takeuchi et al. | Endometroid | a,b,c | Atrophic | Yes | Uterine septum |
| 2007 Izadi-Mood et al. | Serous | a,b,c | Atrophic | Not reported | |
| 2007 Motohara et al. | Endometroid | a,b,c | Atrophic | Yes | |
| 2007 Puppa et al. | Endometroid | a,b,c | Atrophic | Not reported | |
| 2008 Ohta et al. | Clear cell | a,b,c | Atrophic | Not reported | |
| 2009 Hirabayashi et al. | Clear cell | b,c | Foci of adenocarcinoma | Yes | |
| 2009 Jha et al. | Mullerian adenosarcoma | a,b,c | Atrophic | Not reported | |
| 2011 Boes et al. | Endometroid | a,b,c | Atrophic | Not reported | |
| 2011 Heo et al. | Endometroid | a,b,c | Atrophic | Yes | Arising from cystic adenomyosis |
| 2011 Shin et al. | Clear cell | b,c | Foci of adenocarcinoma | Yes | |
| 2012 Elshafie et al. | Mullerian adenosarcoma | a,b,c | Atrophic | Not reported | Arising in a subserosal adenomyoma |
| 2014 Bae et al. | Endometroid | b,c | Not reported | Not reported | |
| 2014 Kawamura et al. | Mullerian mucinous borderline tumor | a,b,c | Atrophic | Yes | |
| 2014 Taga et al. | Endometroid | a,b,c | Atrophic | Not reported | |
| 2015 Baba et al. | Clear cell | b,c | Foci of adenocarcinoma | Yes | Arising from cystic adenomyosis |
| 2015 Mori et al. | Endometroid | a,b,c | Atrophic | Not reported | Arising from cystic adenomyosis |
| 2016 Kiuchi et al. | Carcinosarcoma | a,b,c | Atrophic | Not reported | |
| 2017 Chia-Hao Liu et al. | Serous | a,b,c | Atrophic | Not reported | |
| 2017 Lee et al. | Mullerian adenosarcoma | a,b,c | Atrophic | Not reported | |
| 2017 Yanase et al. | Not reported | a,b,c | Atrophic | Yes | |
| 2018 Vesna et al. | Endometroid | b,c | Atypical Hyperplasia | Not reported | Uterine prolapse |
| 2020 Bang et al. | Endometroid | a,b,c | Atrophic | Not reported | Arising in an intramural adenomyoma |
| 2020 Izumi et al. | Endometroid | b,c | Not reported | Yes | |
| 2020 Talia et al. | Adenosarcoma | a,b,c | Atrophic | Yes | |
| 2020 Talwar et al. | Serous | a,b,c | Atrophic | Not reported | |
| 2022 Chikumi et al. | Endometroid | b,c | Foci of adenocarcinoma | Not reported | |
| 2022 Yoshida et al. | Endometroid | a,b,c | Atrophic | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffone, A.; Raimondo, D.; Maletta, M.; Travaglino, A.; Renzulli, F.; Neola, D.; De Laurentiis, U.; De Laurentiis, F.; Mabrouk, M.; Ianieri, M.M.; et al. Endometrial Cancer Arising in Adenomyosis (EC-AIA): A Systematic Review. Cancers 2023, 15, 1142. https://doi.org/10.3390/cancers15041142
Raffone A, Raimondo D, Maletta M, Travaglino A, Renzulli F, Neola D, De Laurentiis U, De Laurentiis F, Mabrouk M, Ianieri MM, et al. Endometrial Cancer Arising in Adenomyosis (EC-AIA): A Systematic Review. Cancers. 2023; 15(4):1142. https://doi.org/10.3390/cancers15041142
Chicago/Turabian StyleRaffone, Antonio, Diego Raimondo, Manuela Maletta, Antonio Travaglino, Federica Renzulli, Daniele Neola, Umberto De Laurentiis, Francesco De Laurentiis, Mohamed Mabrouk, Manuel Maria Ianieri, and et al. 2023. "Endometrial Cancer Arising in Adenomyosis (EC-AIA): A Systematic Review" Cancers 15, no. 4: 1142. https://doi.org/10.3390/cancers15041142
APA StyleRaffone, A., Raimondo, D., Maletta, M., Travaglino, A., Renzulli, F., Neola, D., De Laurentiis, U., De Laurentiis, F., Mabrouk, M., Ianieri, M. M., Seracchioli, R., Casadio, P., & Mollo, A. (2023). Endometrial Cancer Arising in Adenomyosis (EC-AIA): A Systematic Review. Cancers, 15(4), 1142. https://doi.org/10.3390/cancers15041142







