Evaluating the Risk of Inguinal Lymph Node Metastases before Surgery Using the Morphonode Predictive Model: A Prospective Diagnostic Study in Vulvar Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
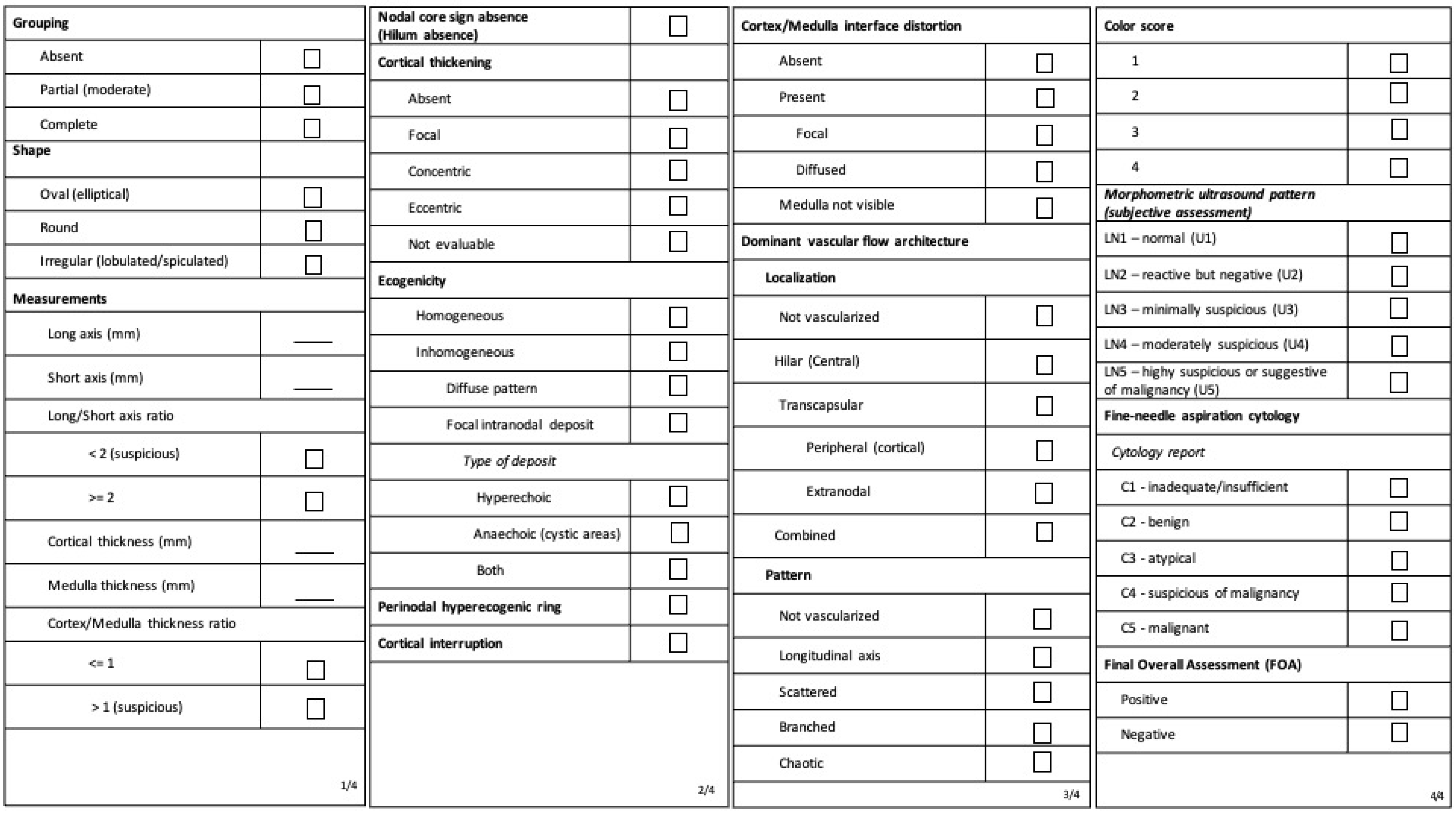
2.1. Ultrasound
2.2. Standard of Reference
2.3. Data Analysis and Sample Size
2.3.1. Sample Size
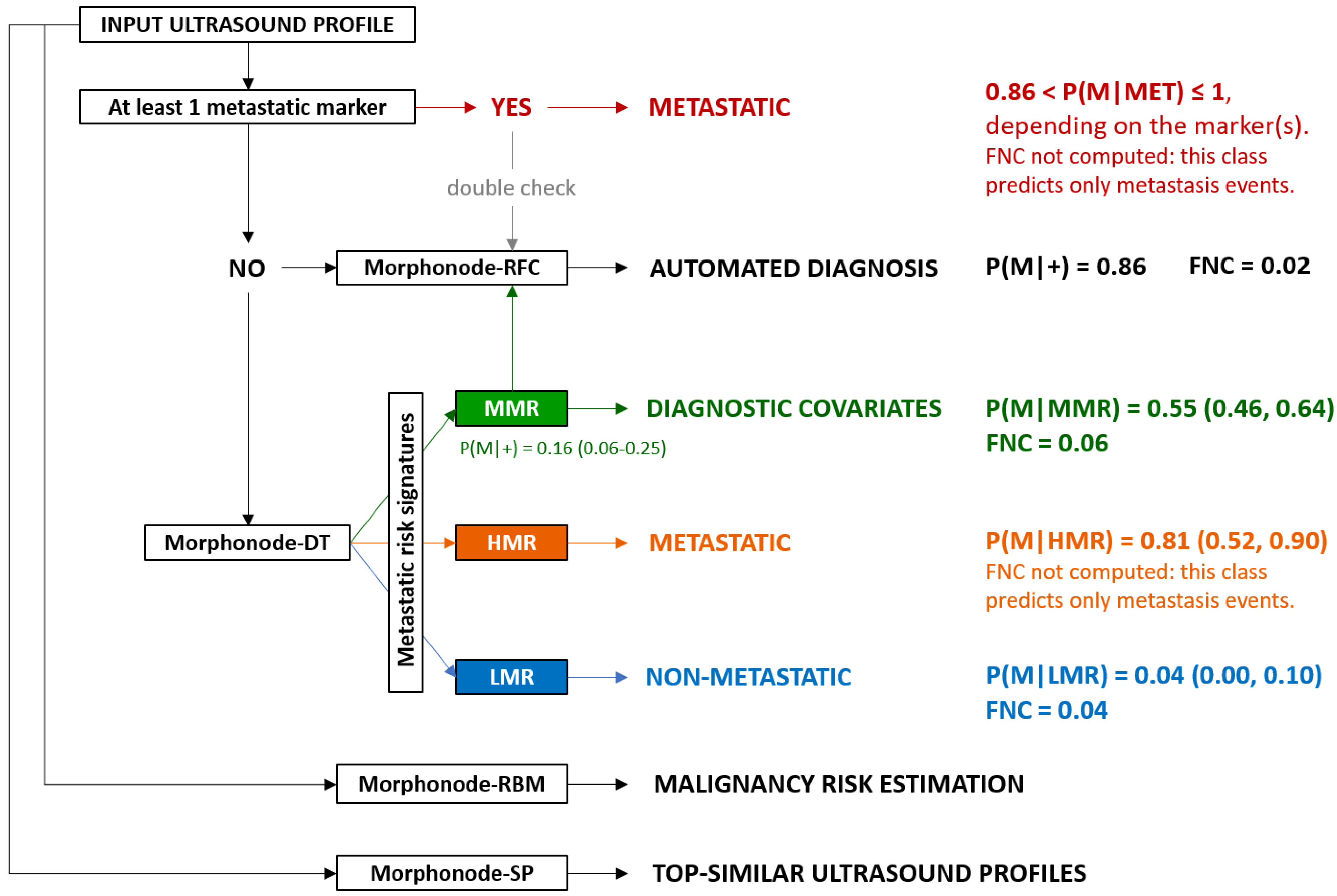
2.3.2. Malignancy Prediction through Random Forest Classifiers (RFCs): Morphonode–RFC
2.3.3. Malignancy Risk Evaluation: Morphonode–RBM
2.3.4. Decision Tree (DT) and Malignancy Signatures: Morphonode–DT
2.3.5. Prediction Error
2.3.6. Similarity Profiling: Morphonode–SP
2.3.7. Morphonode Predictive Model Implementation
3. Results
3.1. Study Population
3.2. Clinical, Surgical, Histopathologic and Ultrasound Features
3.3. Predictive Performances of Ultrasound Variables, Subjective Assessment and Morphonode–RFC
3.4. Malignancy Risk Thresholds and Morphonode–RBM Performances
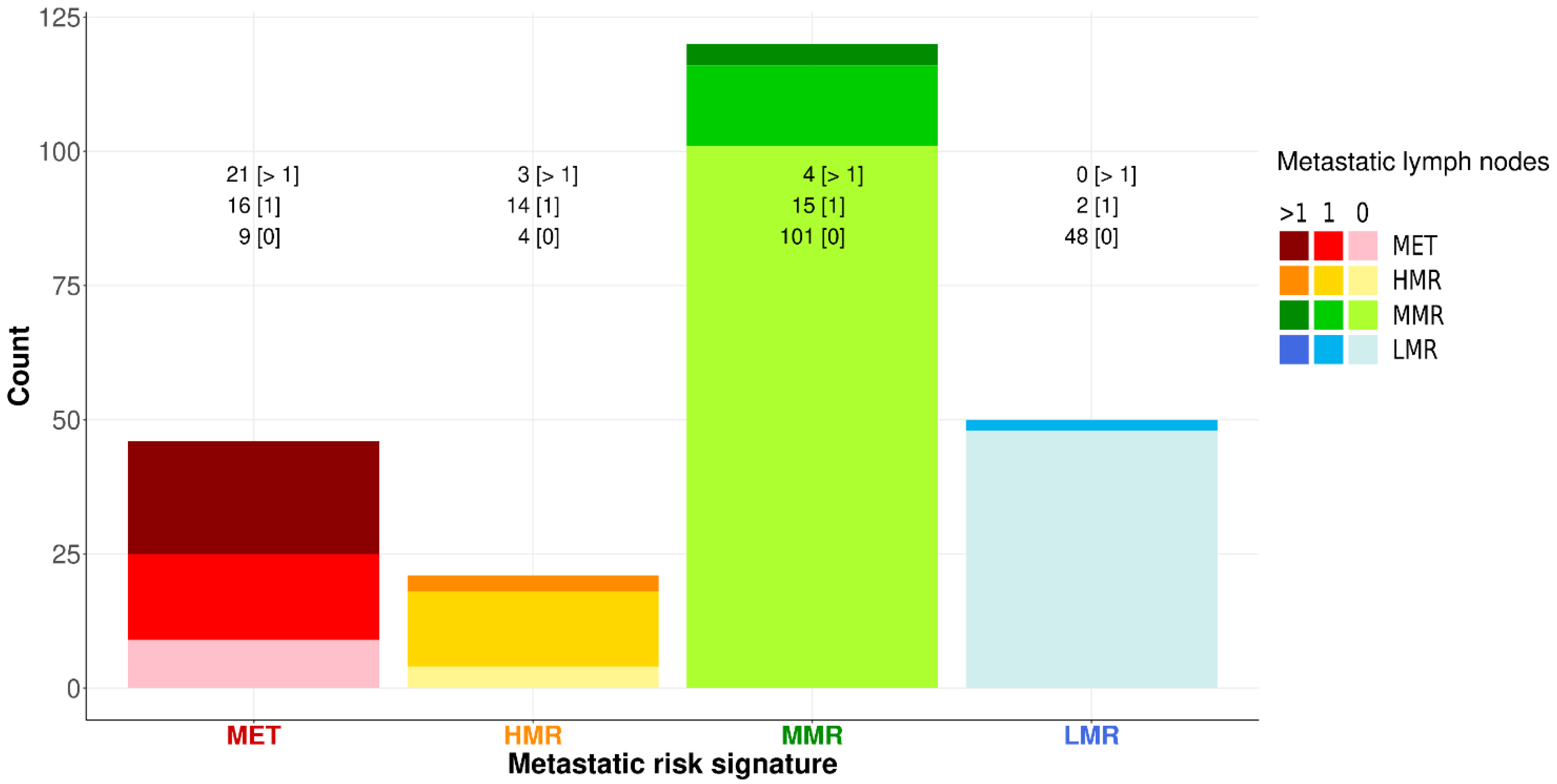
3.5. Morphonode–DT and Risk Signatures
3.6. Prediction Error
3.7. Similarity Search Module
- -
- Stratification into malignant or benign (Morphonode–RFC)
- -
- Point risk estimation (Morphonode–RBM)
- -
- Risk signature (Morphonode–DT)
- -
- Estimated prediction error of the combination of the three modules RFC, RMB and DT
- -
- Top five similar profiles (Morphonode–SP).
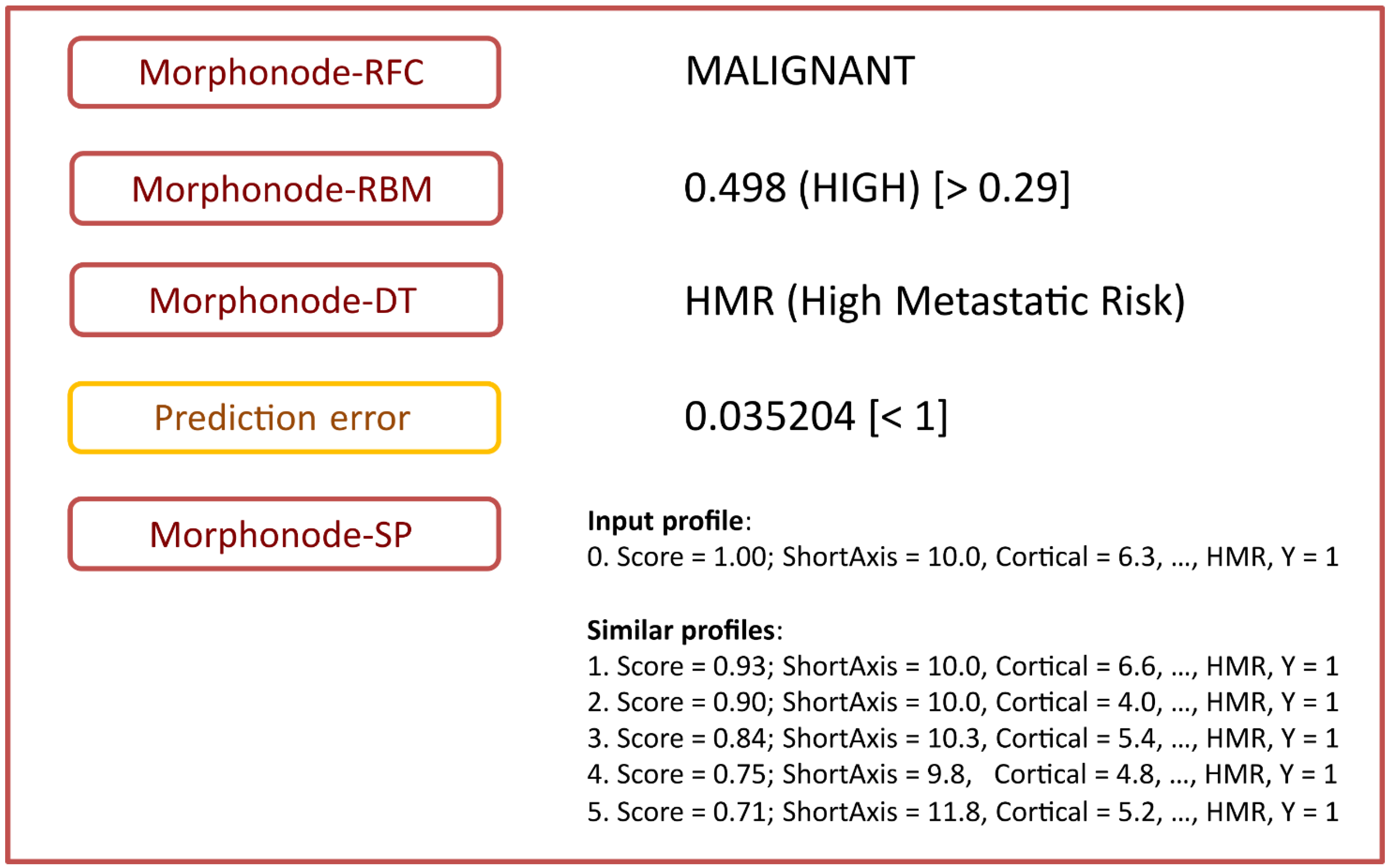
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Data Filtering and Imputation, Morphonode–RFC Ranking, Decision Tree Partition Criterion, Dichotomization
Appendix A.1. Data Filtering and Imputation
Appendix A.2. Morphonode–RFC Ranking
Appendix A.3. Decision Tree Partition Criterion
Appendix A.4. Dichotomization
References
- Land, R.; Herod, J.; Moskovic, E.; King, M.; Sohaib, S.A.; Trott, P.; Nasiri, N.; Shepherd, J.H.; Bridges, J.E.; Ind, T.E.; et al. Routine Computerized Tomography Scanning, Groin Ultrasound with or without Fine Needle Aspiration Cytology in the Surgical Management of Primary Squamous Cell Carcinoma of the Vulva. Int. J. Gynecol. Cancer 2006, 16, 312–317. [Google Scholar] [CrossRef]
- Kataoka, M.Y.; Sala, E.; Baldwin, P.; Reinhold, C.; Farhadi, A.; Hudolin, T.; Hricak, H. The accuracy of magnetic resonance imaging in staging of vulvar cancer: A retrospective multi-centre study. Gynecol. Oncol. 2010, 117, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Persiani, S.; Miccò, M.; Pignatelli, V.; Rodolfino, E.; Avesani, G.; Di Paola, V.; Panico, C.; Russo, L.; Fragomeni, S.M.; et al. MRI Staging in Locally Advanced Vulvar Cancer: From Anatomy to Clinico-Radiological Findings. A Multidisciplinary VulCan Team Point of View. J. Pers. Med. 2021, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Serrado, M.A.; Horta, M.; Cunha, T.M. State of the art in vulvar cancer imaging. Radiol. Bras. 2019, 52, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Bidzinski, M.; Brännström, M.; Landoni, F.; Mahner, S.; Mahantshetty, U.; Mirza, M.; Petersen, C.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer. Int. J. Gynecol. Cancer 2017, 27, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Collarino, A.; Garganese, G.; Valdés Olmos, R.A.; Stefanelli, A.; Perotti, G.; Mirk, P.; Fragomeni, S.M.; Ieria, F.P.; Scambia, G.; Giordano, A.; et al. Evaluation of Dual-Timepoint 18F-FDG PET/CT Imaging for Lymph Node Staging in Vulvar Cancer. J. Nucl. Med. 2017, 58, 1913–1918. [Google Scholar] [CrossRef]
- Rufini, V.; Garganese, G.; Ieria, F.P.; Pasciuto, T.; Fragomeni, S.M.; Gui, B.; Florit, A.; Inzani, F.; Zannoni, G.F.; Scambia, G.; et al. Diagnostic performance of preoperative [ 18 F]FDG-PET/CT for lymph node staging in vulvar cancer: A large single-centre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3303–3314. [Google Scholar] [CrossRef]
- Triumbari, E.K.A.; de Koster, E.J.; Rufini, V.; Fragomeni, S.M.; Garganese, G.; Collarino, A. 18F-FDG PET and 18F-FDG PET/CT in Vulvar Cancer: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2021, 46, 125–132. [Google Scholar] [CrossRef]
- Aide, N.; Markovina, S.; Ferrero, A. Is it time to include [18F]FDG-PET/CT in the diagnostic work-up for lymph node staging in cN0 vulvar cancer patients? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3043–3045. [Google Scholar] [CrossRef]
- Garganese, G.; Fragomeni, S.M.; Pasciuto, T.; Leombroni, M.; Moro, F.; Evangelista, M.T.; Bove, S.; Gentileschi, S.; Tagliaferri, L.; Paris, I.; et al. Ultrasound morphometric and cytologic preoperative assessment of inguinal lymph-node status in women with vulvar cancer: MorphoNode study. Ultrasound Obste. Gynecol. 2020, 55, 401–410. [Google Scholar] [CrossRef]
- Verri, D.; Moro, F.; Fragomeni, S.M.; Zaçe, D.; Bove, S.; Pozzati, F.; Gui, B.; Scambia, G.; Testa, A.C.; Garganese, G. The Role of Ultrasound in the Evaluation of Inguinal LymphNodes in Patients with Vulvar Cancer: A Systematic Reviewand Meta-Analysis. Cancers 2022, 14, 3082. [Google Scholar] [CrossRef]
- Fischerova, D.; Garganese, G.; Reina, H.; Fragomeni, S.M.; Cibula, D.; Nanka, O.; Rettenbacher, T.; Testa, A.C.; Epstein, E.; Guiggi, I.; et al. Terms, definitions and measurements to describe sonographic features of lymph nodes: Consensus opinion from the Vulvar International Tumor Analysis (VITA) group. Ultrasound Obste. Gynecol. 2021, 57, 861–879. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Vulvar Cancer Guidelines (Version 1.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 12 July 2021).
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaeco.l Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Vabalasid, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine learning algorithm validation, with a limited sample size. PLoS ONE 2019, 14, e0224365. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Pruim, R.J.; Kaplan, D.T.; Horton, N.J. The mosaic Package: Helping Students to “Think with Data” Using R. R J. 2017, 9, 77–102. [Google Scholar] [CrossRef]
- Therneau, T.; Atkinson, B. rpart: Recursive Partitioning and Regression Trees. R Package Version 4.1-15. 2019. Available online: https://CRAN.R-project.org/package=rpart (accessed on 23 November 2022).
- Moro, F.; Albanese, M.; Boldrini, L.; Chiappa, V.; Lenkowicz, J.; Bertolina, F.; Mascilini, F.; Moroni, R.; Gambacorta, M.A.; Raspagliesi, F.; et al. Developing and validating ultrasound-based radiomics models for predicting high-risk endometrial cancer. Ultrasound Obstet. Gynecol. 2021, 60, 256–268. [Google Scholar] [CrossRef]
- Christiansen, F.; Epstein, E.L.; Smedberg, E.; Åkerlund, M.; Smith, K.; Epstein, E. Ultrasound image analysis using deep neural networks for discriminating between benign and malignant ovarian tumors: Comparison with expert subjective assessment. Ultrasound Obstet. Gynecol. 2021, 57, 155–163. [Google Scholar] [CrossRef]
- Chiappa, V.; Interlenghi, M.; Salvatore, C.; Bertolina, F.; Bogani, G.; Ditto, A.; Martinelli, F.; Castiglioni, I.; Raspagliesi, F. Using rADioMIcs and machine learning with ultrasonography for the differential diagnosis of myometRiAL tumors (the ADMIRAL pilot study). Radiomics and differential diagnosis of myometrial tumors. Gynecol. Oncol. 2021, 161, 838–844. [Google Scholar] [CrossRef]
- Collarino, A.; Garganese, G.; Fragomeni, S.M.; Pereira Arias-Bouda, L.M.; Ieria, F.P.; Boellaard, R.; Rufini, V.; de Geus-Oei, L.F.; Scambia, G.; Valdés Olmos, R.A.; et al. Radiomics in vulvar cancer: First clinical experience using 18F-FDG PET/CT images. J. Nucl. Med. 2019, 60, 199–206. [Google Scholar] [CrossRef]
- de Gregorio, N.; Ebner, F.; Schwentner, L.; Friedl, T.W.; Deniz, M.; Látó, K.; Kreienberg, R.; Janni, W.; Varga, D. The role of preoperative ultrasound evaluation of inguinal lymph nodes in patients with vulvar malignancy. Gynecol. Oncol. 2013, 131, 113–117. [Google Scholar] [CrossRef]
- Pouwer, A.W.; Mus, R.; IntHout, J.; van der Zee, A.; Bulten, J.; Massuger, L.; de Hullu, J.A. The efficacy of ultrasound in the follow up after a negative sentinel lymph node in women with vulvar cancer: A prospective single-centre study. BJOG. 2018, 125, 1461–1468. [Google Scholar] [CrossRef]
- Zheng, X.; Yao, Z.; Huang, Y.; Yu, Y.; Wang, Y.; Liu, Y.; Mao, R.; Li, F.; Xiao, Y.; Wang, Y.; et al. Deep learning radiomics can predict axillary lymph node status in early-stage breast cancer. Nat. Commun. 2020, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, A.; Qu, E.; Sevrukov, A.; Liu, J.B.; Wang, S.; Lyshchik, A.; Yu, J.; Eisenbrey, J.R. Assessment of Axillary Lymph Nodes for Metastasis on Ultrasound Using Artificial Intelligence. Ultrason Imaging 2021, 43, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, Z.; Ouyang, J.; Tan, Y.; Chen, Y.; Gu, Y.; Mao, L.; Ren, W.; Wang, J.; Lin, L.; et al. Magnetic resonance imaging radiomics predicts preoperative axillary lymph node metastasis to support surgical decisions and is associated with tumor microenvironment in invasive breast cancer: A machine learning, multicenter study. EBioMedicine 2021, 69, 103460. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Cui, W.; Zheng, C.; Li, H.; Li, Y.; Lu, L.; Mao, J.; Zeng, W.; Yang, X.; et al. Preoperative prediction of axillary sentinel lymph node burden with multiparametric MRI-based radiomics nomogram in early-stage breast cancer. Eur. Radiol. 2021, 31, 5924–5939. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, E.J.; Kim, D.Y.; Jung, Y.J.; Heo, S.; Jang, Y.H.; An, S.H.; Lee, K. Application of deep learning to the diagnosis of cervical lymph node metastasis from thyroid cancer with CT: External validation and clinical utility for resident training. Eur Radiol. 2020, 30, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Song, B.I. A machine learning-based radiomics model for the prediction of axillary lymph-node metastasis in breast cancer. Breast Cancer 2021, 28, 664–671. [Google Scholar] [CrossRef]
- Garganese, G.; Tagliaferri, L.; Fragomeni, S.M.; Lancellotta, V.; Colloca, G.; Corrado, G.; Gentileschi, S.; Macchia, G.; Tamburrini, E.; Gambacorta, M.A.; et al. Personalizing vulvar cancer workflow in COVID-19 era: A proposal from Vul.Can MDT. J. Cancer Res. Clin. Oncol. 2020, 146, 2535–2545. [Google Scholar] [CrossRef]
- Lancellotta, V.; Macchia, G.; Garganese, G.; Fionda, B.; Fragomeni, S.M.; D’Aviero, A.; Casà, C.; Gui, B.; Gentileschi, S.; Corrado, G.; et al. The role of brachytherapy (interventional radiotherapy) for primary and/or recurrent vulvar cancer: A Gemelli Vul.Can multidisciplinary team systematic review. Clin. Transl. Oncol. 2021, 23, 1611–1619. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Lancellotta, V.; Casà, C.; Fragomeni, S.M.; Ferioli, M.; Gentileschi, S.; Caretto, A.A.; Corrado, G.; Gui, B.; Colloca, G.F.; et al. The Radiotherapy Role in the Multidisciplinary Management of Locally Advanced Vulvar Cancer: A Multidisciplinary VulCan Team Review. Cancers 2021, 13, 5747. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Lancellotta, V.; Casà, C.; Fragomeni, S.M.; Ferioli, M.; Gentileschi, S.; Caretto, A.A.; Corrado, G.; Gui, B.; Colloca, G.F.; et al. Clinical impact of SARS-CoV-2 infection among patients with vulvar cancer: The Gemelli Vul.Can multidisciplinary team. Int. J. Gynecol. Cancer 2022, 32, 127–132. [Google Scholar]
- Corrado, G.; Cutillo, G.; Fragomeni, S.M.; Bruno, V.; Tagliaferri, L.; Mancini, E.; Certelli, C.; Paris, I.; Vizza, E.; Scambia, G.; et al. Palliative electrochemotherapy in primary or recurrent vulvar cancer. Int. J. Gynecol. Cancer 2020, 30, 927–931. [Google Scholar] [CrossRef]
- Garganese, G.; Bove, S.; Zagaria, L.; Moro, F.; Fragomeni, S.M.; Ieria, F.P.; Gentileschi, S.; Romeo, P.; Di Giorgio, D.; Giordano, A.; et al. Fusion of ultrasound and 3D single-photon-emission computed tomography/computed tomography to identify sentinel lymph nodes in vulvar cancer: Feasibility study. Ultrasound Obstet. Gynecol. 2019, 54, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Gentileschi, S.; Servillo, M.; Garganese, G.; Fragomeni, S.M.; De Bonis, F.; Cina, A.; Scambia, G.; Salgarello, M. The lymphatic superficial circumflex iliac vessels deep branch perforator flap: A new preventive approach to lower limb lymphedema after groin dissection-preliminary evidence. Microsurgery 2017, 37, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Gentileschi, S.; Albanese, R.; Pino, V.; Stefanizzi, G.; Fragomeni, S.; Zagaria, L.; Ieria, F.P.; Salgarello, M.; Scambia, G.; Garganese, G. SPECT/CT and fusion ultrasound to target the efferent groin lymph node for lymphatic surgery. Microsurgery 2019, 39, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Garganese, G.; Collarino, A.; Fragomeni, S.M.; Rufini, V.; Perotti, G.; Gentileschi, S.; Evangelista, M.T.; Ieria, F.P.; Zagaria, L.; Bove, S.; et al. Groin sentinel node biopsy and18F-FDG PET/CT-supported preoperative lymph node assessment in cN0 patients with vulvar cancer currently unfit for minimally invasive inguinal surgery: The GroSNaPET study. Eur. J. Surg. Oncol. 2017, 43, 1776–1783. [Google Scholar] [CrossRef]
- Van der Zee, A.G.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; Van Dorst, E.B.; et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef]
- Te Grootenhuis, N.C.; van der Zee, A.G.; van Doorn, H.C.; van der Velden, J.; Vergote, I.; Zanagnolo, V.; Baldwin, P.J.; Gaarenstroom, K.N.; van Dorst, E.B.; Trum, J.W.; et al. Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol. Oncol. 2016, 140, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Pounds, R.; O’Neill, D.; Subba, K.; Garg, A.; Scerif, M.; Leong, E.; Nevin, J.; Kehoe, S.; Yap, J. The role of preoperative computerized tomography (CT) scan of the pelvis and groin in the management of clinically early staged vulva squamous cell carcinoma. Gynecol. Oncol. 2020, 157, 444–449. [Google Scholar] [CrossRef]
- Andersen, K.; Zobbe, V.; Thranov, I.R.; Pedersen, K.D. Relevance of computerized tomography in the preoperative evaluation of patients with vulvar cancer: A prospective study. Cancer Imaging 2015, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, A.; Bidzinski, M.; Rzepka, J.; Piatek, S. Sentinel lymph node in vulvar cancer. Chin. Clin. Oncol. 2021, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- ESGO Vulvar Cancer Guidelines Update-ESGO CONGRESS October 27–30 2022. 2022. Available online: https://guidelines.esgo.org/vulvar-cancer/guidelines/recommendations/ (accessed on 23 November 2022).




| Characteristic | All (n = 127) | N0—Negative Lymph Node at Histology (n = 71) | N1—Positive Lymph Node at Histology (n = 56) | Test [a] | Estimate [b] | CI 95% [c] | p-Value |
|---|---|---|---|---|---|---|---|
| Age (years): median (range) | 69 (32–95) | 71 (44–95) | 68 (32–89) | Wilcoxon | −2 | −6, 4 | 0.402 |
| Tumor site * | |||||||
| Anterior | 45/127 (35.4%) | 22 | 23 | proportions | 0.411, 0.310 | −0.083, 0.285 | 0.321 |
| Lateral | 61/127 (48%) | 34 | 27 | proportions | 0.482, 0.479 | −0.175, 0.182 | 0.999 |
| Posterior | 15/127 (11.8%) | 13 | 2 | proportions | 0.036, 0.183 | −0.266, −0.029 | 0.023 |
| Central | 5/127 (4%) | 2 | 3 | proportions | 0.054, 0.028 | −0.061, 0.112 | 0.786 |
| Absent | 1/127 (0.8%) | 0 | 1 | proportions | 0.018, 0.000 | −0.033, 0.069 | 0.905 |
| Focality | |||||||
| Unifocal | 106/127 (83.5%) | 63 | 43 | proportions | 0.768, 0.887 | −0.268, 0.029 | 0.119 |
| Multifocal | 21/127 (16.5%) | 8 | 13 | proportions | 0.232, 0.113 | −0.029, 0.268 | 0.119 |
| Type of vulvar surgery | |||||||
| Partial | 66/127 (51%) | 24 | 42 | proportions | 0.750, 0.338 | 0.238, 0.586 | <0.001 |
| Radical | 61/127 (49%) | 29 | 32 | proportions | 0.571, 0.408 | −0.026, 0.352 | 0.099 |
| Side of surgery | |||||||
| Monolateral | 16/127 (13%) | 12 | 4 | proportions | 0.071, 0.169 | −0.224, 0.029 | 0.169 |
| Bilateral | 111/127 (87%) | 59 | 52 | proportions | 0.929, 0.831 | −0.029, 0.224 | 0.169 |
| Histotype | |||||||
| Squamous | 110/127 (86.6%) | 63 | 46 | proportions | 0.821, 0.887 | −0.206, 0.074 | 0.423 |
| Paget | 9/127 (7%) | 5 | 4 | proportions | 0.071, 0.070 | −0.090, 0.092 | 0.999 |
| Melanoma | 5/127 (4%) | 2 | 3 | proportions | 0.054, 0.028 | −0.061, 0.112 | 0.786 |
| Basocellular | 1/127 (0.8%) | 0 | 1 | proportions | 0.018, 0.000 | −0.033, 0.069 | 0.905 |
| Adenocarcinoma | 1/127 (0.8%) | 1 | 0 | proportions | 0.000, 0.014 | −0.056, 0.027 | 0.999 |
| Sarcoma | 1/127 (0.8%) | 0 | 1 | proportions | 0.018, 0.000 | −0.033, 0.069 | 0.905 |
| Maximum tumor diameter (mm) | |||||||
| Median (range) | 30 (2–160) | 25.5 (0–100) | 41 (4–90) | Wilcoxon | 12 | 5, 20 | <0.001 |
| <20 mm | 28/122 (23%) | 22 | 6 | proportions | 0.107, 0.310 | −0.353, −0.052 | 0.012 |
| 20–40 mm | 52/122 (42.6%) | 31 | 21 | proportions | 0.375, 0.437 | −0.249, 0.126 | 0.604 |
| >40 mm | 42/122 (34.4%) | 15 | 27 | proportions | 0.482, 0.211 | 0.093, 0.449 | 0.002 |
| Grading (squamous histotype) | |||||||
| G1 | 20/110 | 14 | 6 | proportions | 0.107, 0.197 | −0.229, 0.049 | 0.255 |
| G2 | 63/110 | 34 | 29 | proportions | 0.518, 0.479 | −0.152, 0.230 | 0.797 |
| G3 | 19/110 | 7 | 12 | proportions | 0.214, 0.099 | −0.028, 0.260 | 0.118 |
| Depth of invasion ° (squamous histotype; mm) | |||||||
| Median (range) | 6 (0–19) | 5 (0–12) | 6 (0.9–19) | Wilcoxon | 2 | 0, 3 | 0.020 |
| <5 mm | 32 | 20 | 12 | proportions | 0.214, 0.282 | −0.233, 0.099 | 0.507 |
| >=5 mm | 54 | 23 | 31 | proportions | 0.554, 0.324 | 0.044, 0.415 | 0.016 |
| Lymphovascular invasion | |||||||
| Absent | 31/127 (24%) | 22 | 9 | proportions | 0.161, 0.310 | −0.309, 0.011 | 0.083 |
| Present | 96/127 (76%) | 49 | 47 | proportions | 0.839, 0.690 | −0.011, 0.309 | 0.083 |
| Stage | |||||||
| IB | 59 (46.5) | 58 | 1 | proportions | 0.018, 0.817 | −0.911, −0.687 | <0.001 |
| II | 4 (3.2) | 4 | 0 | proportions | 0.000, 0.056 | −0.126, 0.013 | 0.196 |
| III | 40 (31.5) | 0 | 40 | proportions | 0.714, 0.000 | 0.580, 0.849 | <0.001 |
| IV | 4 (3.2) | 0 | 4 | proportions | 0.071, 0.000 | −0.012, 0.155 | 0.076 |
| Relapse | 12 (9.4) | 5 | 7 | proportions | 0.125, 0.070 | −0.066, 0.176 | 0.460 |
| Post-RT/CT | 8 (6.3) | 4 | 4 | proportions | 0.071, 0.056 | −0.086, 0.116 | 0.999 |
| Variable | RFC Ranking | Discriminant | Metastatic Risk | Priority | Description |
|---|---|---|---|---|---|
| Short axis (mm) | * | +++ | ++ | Necessary | Excellent outcome predictor, good risk predictor |
| Cortical thickness (mm) | * | +++ | ++ | Necessary | Excellent outcome predictor, good risk predictor |
| Nodal core sign absence | +++ | + | * | Very high | Fair outcome predictor, excellent risk predictor |
| Perinodal hyperecogenic ring | ++ | + | * | Very high | Fair outcome predictor, excellent risk predictor |
| cortical interruption | ++ | − | * | Very high | Fair outcome predictor, excellent risk predictor |
| Echogenicity | +++ | ++ | +++ | High | Good outcome predictor, good risk predictor |
| Focal intranodal deposit | +++ | ++ | ++ | High | Good outcome predictor, good risk predictor |
| Vascular flow localization | +++ | + | − | High | Good outcome predictor |
| Cortical thickening | +++ | − | + | High | Good outcome predictor |
| Vascular flow architecture pattern | ++ | + | ++ | High | Fair outcome predictor, good risk predictor |
| Cortical–medullar interface distortion | ++ | ++ | ++ | High | Fair outcome predictor, good risk predictor |
| Shape | + | + | ++ | Low | Poorly informative |
| Grouping | + | − | + | Low | Poorly informative |
| Color score | + | + | − | Low | Poorly informative |
| Medulla (mm) | − | − | − | Unnecessary | Not informative |
| Long axis (mm) | − | − | − | Unnecessary | Not informative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragomeni, S.M.; Moro, F.; Palluzzi, F.; Mascilini, F.; Rufini, V.; Collarino, A.; Inzani, F.; Giacò, L.; Scambia, G.; Testa, A.C.; et al. Evaluating the Risk of Inguinal Lymph Node Metastases before Surgery Using the Morphonode Predictive Model: A Prospective Diagnostic Study in Vulvar Cancer Patients. Cancers 2023, 15, 1121. https://doi.org/10.3390/cancers15041121
Fragomeni SM, Moro F, Palluzzi F, Mascilini F, Rufini V, Collarino A, Inzani F, Giacò L, Scambia G, Testa AC, et al. Evaluating the Risk of Inguinal Lymph Node Metastases before Surgery Using the Morphonode Predictive Model: A Prospective Diagnostic Study in Vulvar Cancer Patients. Cancers. 2023; 15(4):1121. https://doi.org/10.3390/cancers15041121
Chicago/Turabian StyleFragomeni, Simona Maria, Francesca Moro, Fernando Palluzzi, Floriana Mascilini, Vittoria Rufini, Angela Collarino, Frediano Inzani, Luciano Giacò, Giovanni Scambia, Antonia Carla Testa, and et al. 2023. "Evaluating the Risk of Inguinal Lymph Node Metastases before Surgery Using the Morphonode Predictive Model: A Prospective Diagnostic Study in Vulvar Cancer Patients" Cancers 15, no. 4: 1121. https://doi.org/10.3390/cancers15041121
APA StyleFragomeni, S. M., Moro, F., Palluzzi, F., Mascilini, F., Rufini, V., Collarino, A., Inzani, F., Giacò, L., Scambia, G., Testa, A. C., & Garganese, G. (2023). Evaluating the Risk of Inguinal Lymph Node Metastases before Surgery Using the Morphonode Predictive Model: A Prospective Diagnostic Study in Vulvar Cancer Patients. Cancers, 15(4), 1121. https://doi.org/10.3390/cancers15041121







