Imaging and Histopathological Analysis of Microvascular Angiogenesis in Photodynamic Therapy for Oral Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Photosensitizers
2.2. Laser Delivery Systems
2.3. In Vivo Chicken Chorioallantoic Membranes (CAM) Model
2.4. In Vivo Stimulated Malignant Oral Lesions in Animal Model
3. Results
3.1. In Vivo Chicken Chorioallantoic Membrane (CAM) Model
3.2. In Vivo Stimulated Malignant Oral Lesions in Animal Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spikes, J.D. The historical development of ideas on applications of photosensitized reactions in the health sciences. In Primary Photo-Processes in Biology and Medicine; Benasson, R.V., Jori, G., Land, E.J., Truscott, T.G., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 209–227. [Google Scholar]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy: A new concept in medical treatment. Braz. J. Med. Biol. Res. 2000, 33, 869–880. [Google Scholar] [CrossRef]
- Chang, C.J.; Sun, C.H.; Liaw, L.H.L.; Nelson, J.S.; Berns, M.W. In vitro and in vivo photosensitizing capabilities of 5-ALA compared to Photofrin® in vascular endothelial cells. Lasers Surg. Med. 1999, 24, 178–186. [Google Scholar]
- Knighton, D.; Ausprunk, D.; Tapper, D.; Folkman, J. Avascular and vascular phases of tumor growth in the chick embryo. Br. J. Cancer 1977, 35, 347–356. [Google Scholar] [CrossRef]
- Debefve, E.; Pegaz, B.; van den Bergh, H.; Wagnières, G.; Lange, N.; Ballini, J.P. Video monitoring of neovessel occlusion induced by photodynamic therapy with verteporfin (Visudyne®), in the CAM model. Angiogenesis 2008, 11, 235–243. [Google Scholar] [CrossRef]
- Buzzá, H.H.; Zangirolami, A.C.; Davis, A.; Gómez-García, P.A.; Kurachi, C. Fluorescence analysis of a tumor model in the chorioallantoic membrane used for the evaluation of different photosensitizers for photodynamic therapy. Photodiagn. Photodyn. Ther. 2017, 19, 78–83. [Google Scholar] [CrossRef]
- Arthuzo, G.; Bagnato, V.S.; Buzzá, H.H. Study of destruction effect of blood vessels after photodynamic therapy in a model of chorioallantoic membrane. In Proceedings of the 17th International Photodynamic Association World Congress, Cambridge, MA, USA, 28 June–4 July 2019; SPIE: Bellingham, WA, USA, 2019; Volume 11070, pp. 388–395. [Google Scholar]
- Kimel, S.; Svaasand, L.O.; Hammer-Wilson, M.; Gottfried, V.; Cheng, S.; Svaasand, E.; Berns, M.W. Demonstration of synergistic effects of hyperthermia and photodynamic therapy using the chick chorioallantoic membrane model. Lasers Surg. Med. 1992, 12, 432–440. [Google Scholar] [CrossRef]
- Yang, O.; Cuccia, D.; Choi, B. Real-time blood flow visualization using the graphics processing unit. J. Biomed. Opt. 2011, 16, 016009. [Google Scholar] [CrossRef]
- Lin, M.S.; Hwang, P.S.; Chang, C.J. Topical application of Photofrin® for oral neoplasms. Opt. Quantum Electron. 2005, 37, 1353–1365. [Google Scholar]
- Nelson, J.S.; Liaw, L.H.; Orenstein, A.; Robert, W.G.; Berns, M.W. Mechanism of tumor destruction following photodynamic therapy with hematoporphyrin derivative, chlorine and phthalocyanine. J. Natl. Cancer Inst. 1988, 80, 1599–1605. [Google Scholar]
- Chang, C.J.; Yang, J.Y.; Weng, C.J.; Wei, F.C. Pilot in vitro toxicity study of 5-ALA and Photofrin® in microvascular endothelial cells cultures. J. Clin. Laser Med. Surg. 1997, 15, 83–87. [Google Scholar]
- Chang, C.J.; Ma, S.F.; Wei, F.C. Photosensitizing capabilities of Photofrin® in vascular endothelial cells. Formosa J. Surg. 2001, 34, 287–293. [Google Scholar]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976, 36, 2326–2369. [Google Scholar]
- Berns, M.W.; Coffey, J.; Wile, A.G. Laser photoradiation therapy of cancer: Possible role of hyperthermia. Lasers Surg. Med. 1984, 4, 87–92. [Google Scholar] [CrossRef]
- Nelson, J.S.; Liaw, L.H.; Berns, M.W. Mechanism of tumor destruction in photodynamic therapy. Photochem. Photobiol. 1987, 46, 829–836. [Google Scholar]
- Foote, C.S. Photosensitized oxidation and singlet oxygen: Consequences in biological systems. In Radicalsin Biology; Pryor, W.A., Ed.; Academic Press: New York, NY, USA, 1976; Volume 2, pp. 85–102. [Google Scholar]
- Homer, D.W.; Lemeshaw, S. Applied Logistic Regression; John Wiley: New York, NY, USA, 1989. [Google Scholar]
- Berns, M.W.; Hammer-Wilson, M.; Walter, R.J.; Wright, W.; Chow, M.H.; Nahabedian, M.; Wile, A. Uptake and localization of HpD and active fraction in tissue cultureand in serially biopsy human tumors. Prog. Clin. Biol. Res. 1983, 170, 501–520. [Google Scholar]
- Dellinger, M. Apoptosis or necrosis following Photofrin® photosensitization: Influence of the incubation protocol. Photochem. Photobiol. 1996, 64, 182–187. [Google Scholar]
- Gaullier, J.M.; Geze, M.; Santus, R.; Melo, T.S.; Mazière, J.C.; Bazin, M.; Morlière, P.; Dubertret, L. Subcellular localization of and photosensitization by protoporphyrin IX in human keratinocytes and fibroblasts cultivated with 5-aminolevulinicacid. Photochem. Photobiol. 1995, 62, 114–122. [Google Scholar] [PubMed]
- Gibson, S.L.; Hilf, R. Interdependence of fluence, drug dose and oxygen on hematoporphyrin derivative induced photosensitization of tumor mitochondria. Photochem. Photobiol. 1985, 42, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Wu, C.C.; Chang, C.J.; Yu, J.S. Subcellular localization of Photofrin® determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: When plasma membranes are the main targets. J. Cell Physiol. 2003, 194, 363–375. [Google Scholar] [CrossRef]
- Peng, T.I.; Chang, C.J.; Guo, M.J.; Wang, Y.H.; Yu, J.S.; Wu, H.Y.; Jou, M.J. Mitochondrion-targeted photosensitizer enhances the photodynamic effect-induced mitochondrial dysfunction and apoptosis. Ann. N. Y. Acad. Sci. 2005, 1042, 419–428. [Google Scholar]
- Peng, T.I.; Chang, C.J.; Jou, S.B.; Yang, C.M.; Jou, M.J. Photosensitizer targeting: Mitochondrion-Targeted photosensitizer enhances mitochondrial reactive oxygen species and mitochondrial calcium-mediated apoptosis. Opt. Quantum Electron. 2005, 37, 1377–1384. [Google Scholar] [CrossRef]
- Chang, C.J.; Cheng Sally, M.H.; Nelson, J.S. Microvascular effect of Photofrin® -induced photodynamic therapy. Photodiagn. Photodyn. Ther. 2007, 4, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Wilder-Smith, P. Topical application of Photofrin® for photodynamic diagnosis of oral neoplasms. Plast. Reconstr. Surg. 2005, 115, 1877–1886. [Google Scholar] [CrossRef]
- Rovers, J.P.; de Jode, M.L.; Grahn, M.F. Significantly increased lesion size by using the near-infrared photosensitizer 5, 10, 15, 20-tetrakis (m-hydroxyphenyl) bacteriochlorin in interstitial photodynamic therapy of normal rat liver tissue. Laser Surg. Med. 2000, 27, 235–240. [Google Scholar] [CrossRef]
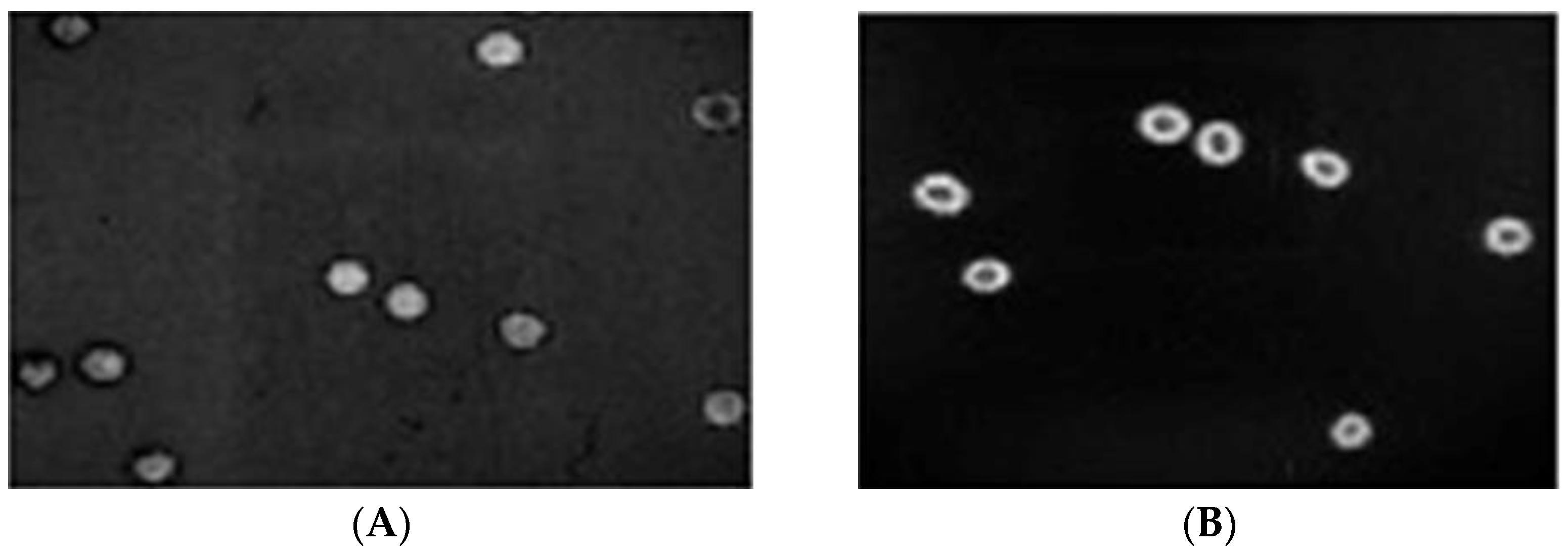
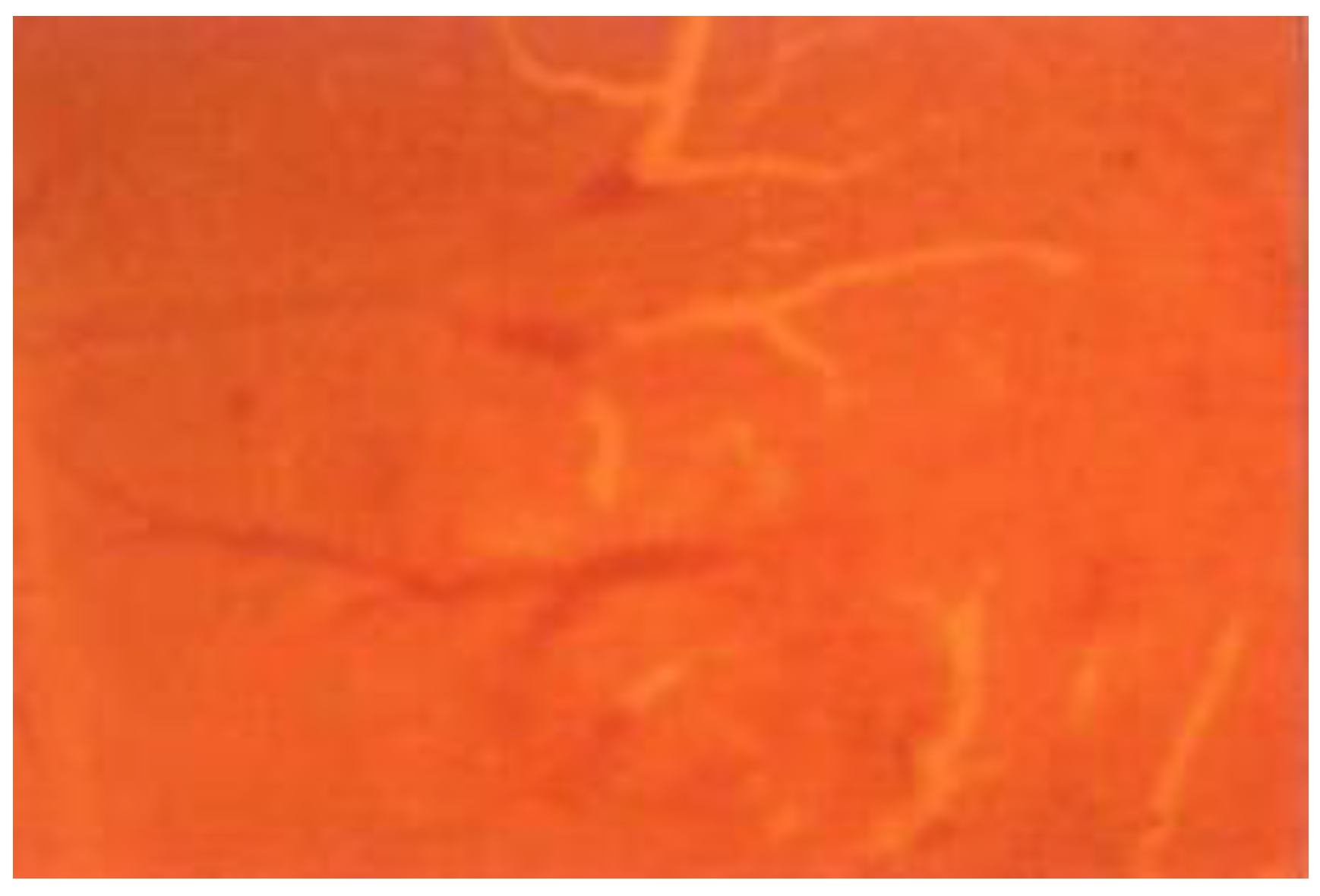
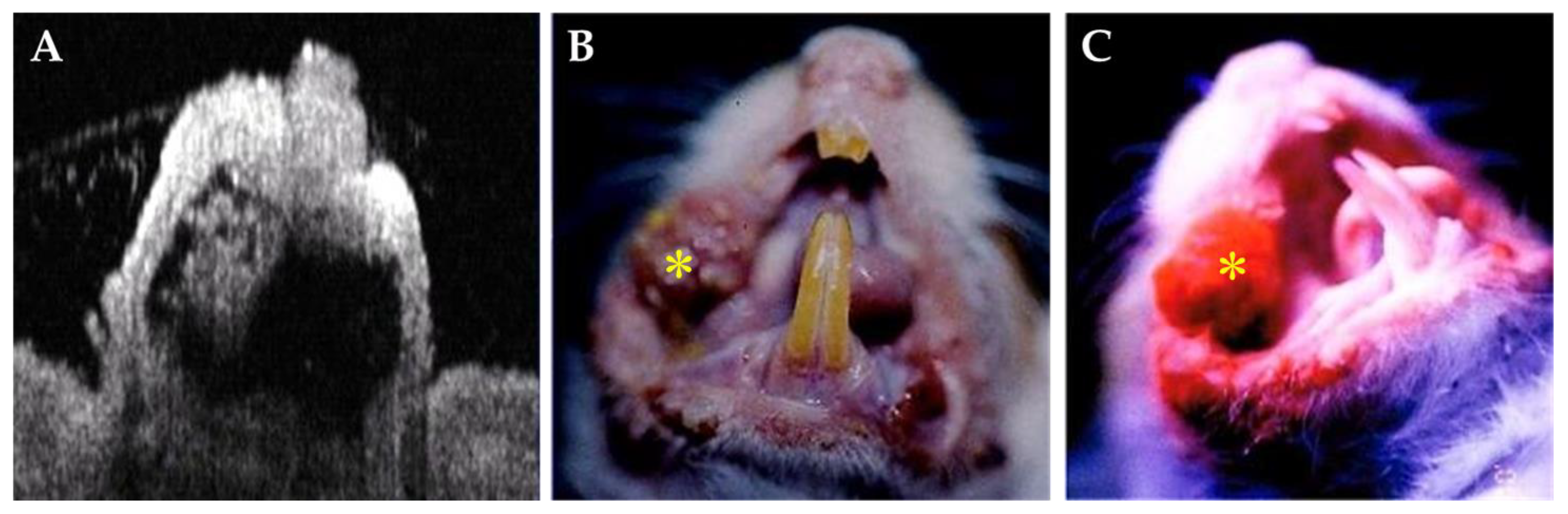

| Day (s) | 0 | 2nd | 4th | 6th | 8th | 10th | 12th |
|---|---|---|---|---|---|---|---|
| HbO2(μM) | 63 | 80 | 109 | 123 | 159 | 210 | 215 |
| Hb (μM) | 57 | 52 | 47 | 40 | 38 | 36 | 35 |
| HbT (μM) | 120 | 132 | 138 | 163 | 197 | 246 | 250 |
| StO2(%) | 38 | 45 | 54 | 62 | 72 | 89 | 92 |
| Day (s) | * 14th | 16th | 18th | 20th | 22nd | 24th | 26th |
|---|---|---|---|---|---|---|---|
| HbO2 (μM) | 216 | 160 | 130 | 117 | 91 | 84 | 78 |
| Hb (μM) | 36 | 45 | 51 | 57 | 65 | 69 | 72 |
| HbT (μM) | 252 | 220 | 200 | 187 | 173 | 168 | 150 |
| StO2 (%) | 95 | 86 | 72 | 65 | 60 | 55 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-S.; Hsiao, Y.-C.; Chiang, Y.-F.; Chang, C.-J. Imaging and Histopathological Analysis of Microvascular Angiogenesis in Photodynamic Therapy for Oral Cancer. Cancers 2023, 15, 1110. https://doi.org/10.3390/cancers15041110
Yang T-S, Hsiao Y-C, Chiang Y-F, Chang C-J. Imaging and Histopathological Analysis of Microvascular Angiogenesis in Photodynamic Therapy for Oral Cancer. Cancers. 2023; 15(4):1110. https://doi.org/10.3390/cancers15041110
Chicago/Turabian StyleYang, Tzu-Sen, Yen-Chang Hsiao, Yu-Fan Chiang, and Cheng-Jen Chang. 2023. "Imaging and Histopathological Analysis of Microvascular Angiogenesis in Photodynamic Therapy for Oral Cancer" Cancers 15, no. 4: 1110. https://doi.org/10.3390/cancers15041110
APA StyleYang, T.-S., Hsiao, Y.-C., Chiang, Y.-F., & Chang, C.-J. (2023). Imaging and Histopathological Analysis of Microvascular Angiogenesis in Photodynamic Therapy for Oral Cancer. Cancers, 15(4), 1110. https://doi.org/10.3390/cancers15041110





