Analysis of the Incidence of Type 2 Diabetes, Requirement of Insulin Treatment, and Diabetes-Related Complications among Patients with Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
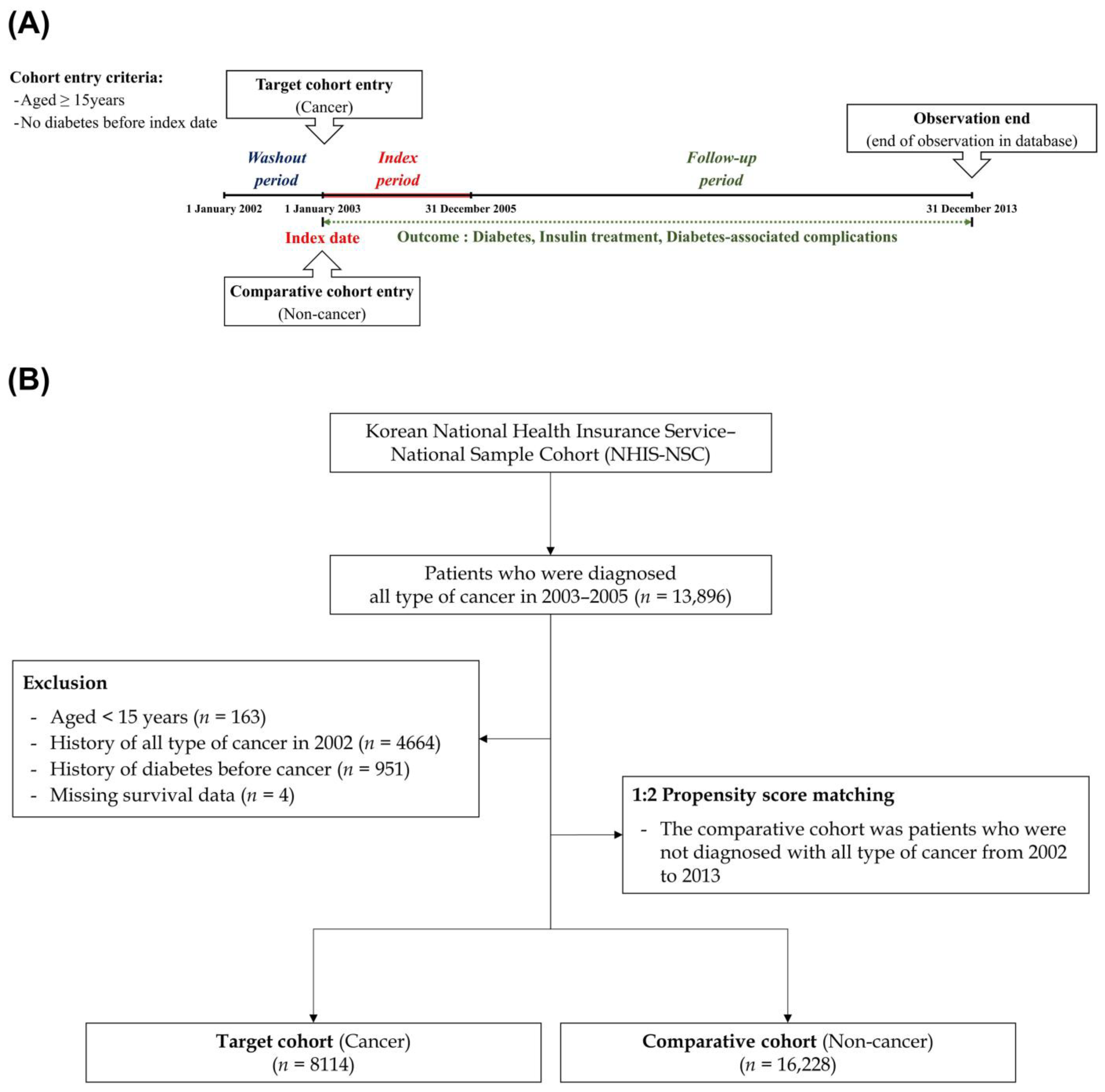
2.1. Study Sample
2.2. Study Design
2.3. Independent Variables
2.4. Statistical Analysis
3. Results
3.1. Cohort Sample Characteristics and Incidence of Diabetes
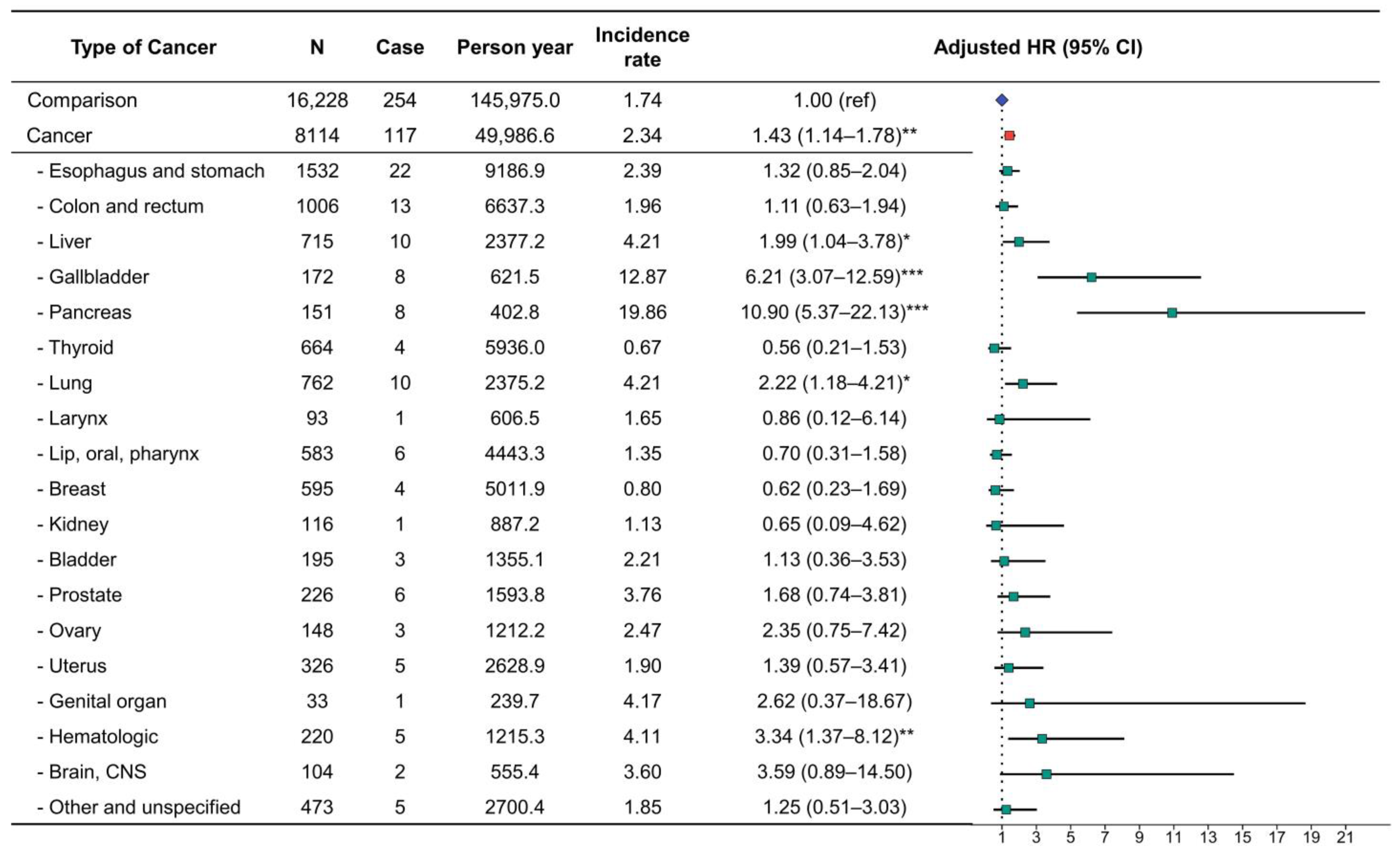
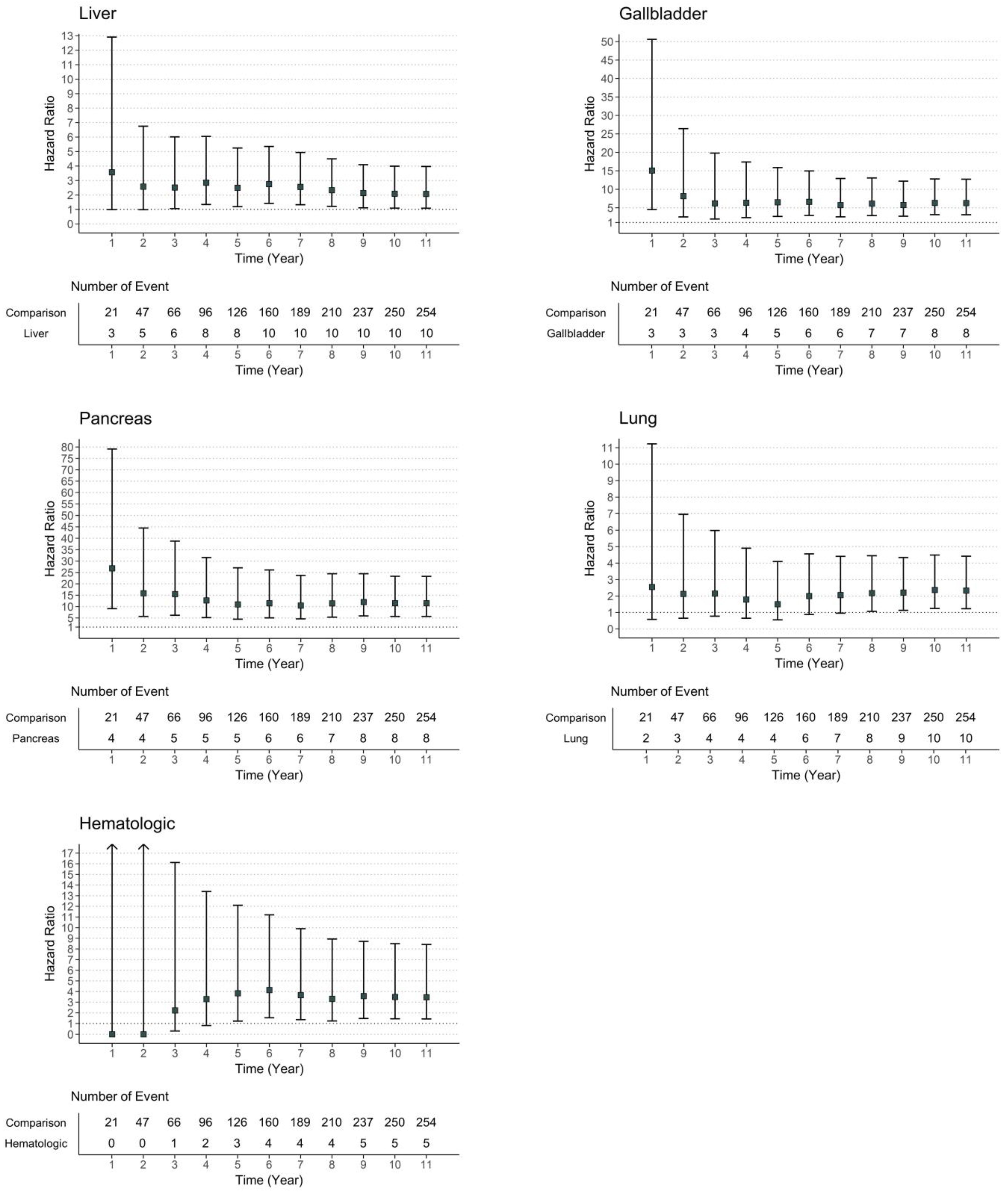
3.2. Risk of Diabetes Development in Patients with Cancer
3.3. Risk of Diabetes Treated with Insulin Therapy in Patients with Cancer
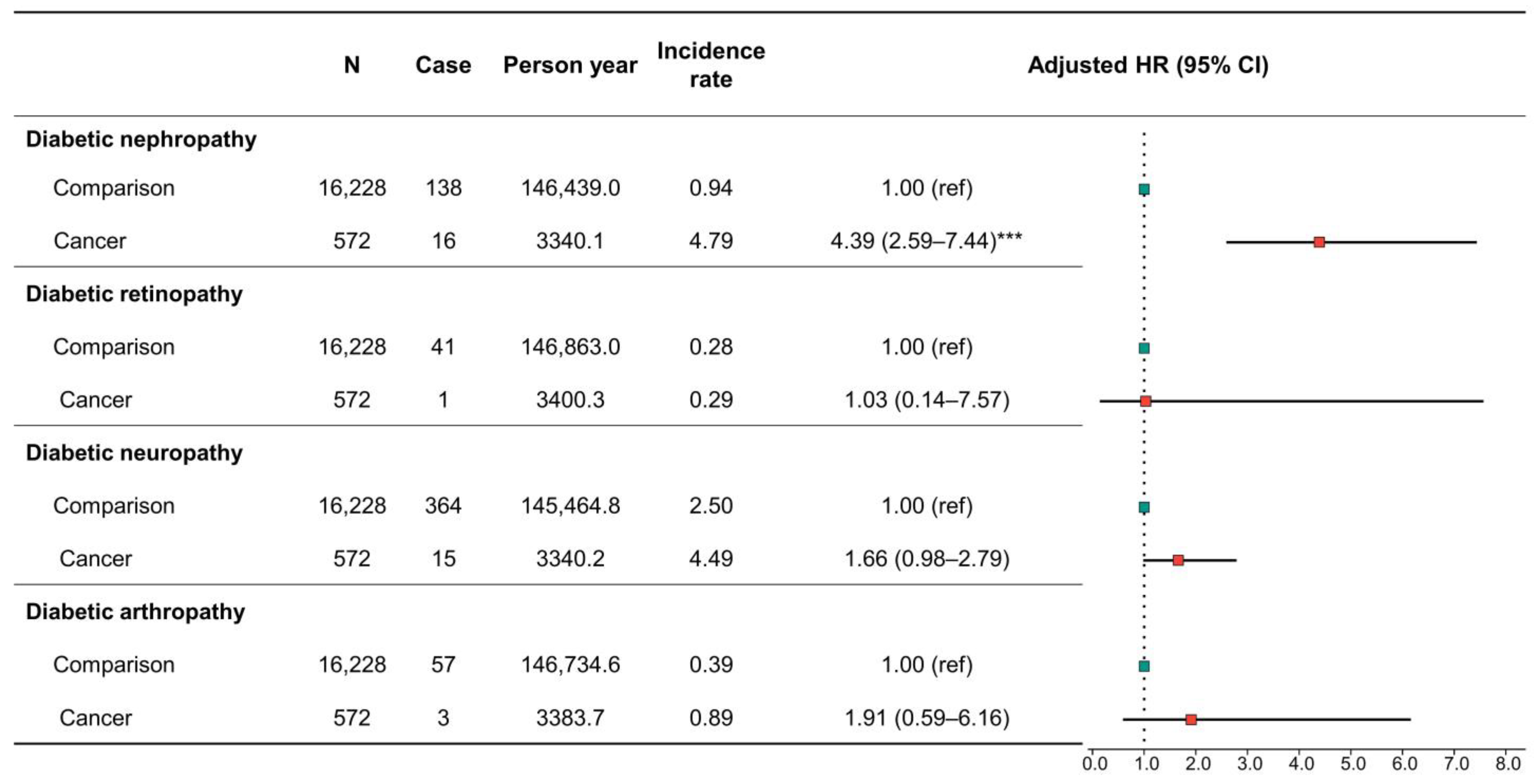
3.4. Risk of Diabetes-Associated Complications in Patients with Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Sarfati, D.; Koczwara, B.; Jackson, C. The impact of comorbidity on cancer and its treatment. CA Cancer J. Clin. 2016, 66, 337–350. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Liao, Z. Diabetes and cancer: Epidemiological and biological links. World J. Diabetes. 2020, 11, 227–238. [Google Scholar] [CrossRef]
- Tai, Y.S.; Chen, C.H.; Huang, C.Y.; Tai, H.C.; Wang, S.M.; Pu, Y.S. Diabetes mellitus with poor glycemic control increases bladder cancer recurrence risk in patients with upper urinary tract urothelial carcinoma. Diabetes Metab. Res. Rev. 2015, 31, 307–314. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, J.; Zhang, H.; Sun, L.; Tang, F.; Deng, Q.; Yu, J. Associations between diabetes and quality of life among breast cancer survivors. PLoS ONE 2016, 11, e0157791. [Google Scholar] [CrossRef]
- Hur, K.Y.; Moon, M.K.; Park, J.S.; Kim, S.K.; Lee, S.H.; Yun, J.S.; Baek, J.H.; Noh, J.; Lee, B.W.; Oh, T.J.; et al. 2021 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association. Diabetes Metab. J. 2021, 45, 461–481. [Google Scholar] [CrossRef]
- Lipscombe, L.L.; Chan, W.W.; Yun, L.; Austin, P.C.; Anderson, G.M.; Rochon, P.A. Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia 2013, 56, 476–483. [Google Scholar] [CrossRef]
- Yu, J.; Sun, R.; Han, X.; Liu, Z. New-onset diabetes mellitus after distal pancreatectomy: A systematic review and meta-analysis. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 1215–1222. [Google Scholar] [CrossRef]
- Singh, S.; Earle, C.C.; Bae, S.J.; Fischer, H.D.; Yun, L.; Austin, P.C.; Rochon, P.A.; Anderson, G.M.; Lipscombe, L. Incidence of diabetes in colorectal cancer survivors. J. Natl. Cancer Inst. 2016, 108, djv402. [Google Scholar] [CrossRef]
- Dong, L.; Ying, X.; Tao, S.; Guang-Peng, Z.; Tai-Ming, S. Diabetes is a risk factor for the prognosis of patients with bladder cancer: A meta-analysis. J. Oncol. 2022, 2022, 1997507. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Jeon, I.; Lee, J.M.; Yoon, J.M.; Park, S.M. Diabetes mellitus as an independent risk factor for lung cancer: A meta-analysis of observational studies. Eur. J. Cancer 2013, 49, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Grand, M.K.; Heymann, A.; Persson, F.; Siersma, V.; Kriegbaum, M.; Lykkegaard Andersen, C.; Johansen, C. Incidence of new-onset type 2 diabetes after cancer: A Danish cohort study. Diabetes Care 2022, 2, e105–e106. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, Y.; Kang, D.; Kang, M.; Kim, S.; Lee, E.K.; Kim, Y.A.; Chang, Y.J.; Choi, K.S.; Jung, S.Y.; Woo, S.M.; et al. Incidence of diabetes after cancer development: A Korean national cohort study. JAMA Oncol. 2018, 4, 1099–1105. [Google Scholar] [CrossRef]
- Ose, D.J.; Viskochil, R.; Holowatyj, A.N.; Larson, M.; Wilson, D.; Dunson, W.A.; Deshmukh, V.G.; Butcher, J.R.; Taylor, B.R.; Svoboda, K.; et al. Understanding the prevalence of prediabetes and diabetes in patients with cancer in clinical practice: A real-world cohort study. J. Natl. Compr. Canc. Netw. 2021, 19, 709–718. [Google Scholar] [CrossRef]
- Hershey, D.S.; Tipton, J.; Given, B.; Davis, E. Perceived impact of cancer treatment on diabetes self-management. Diabetes Educ. 2012, 38, 779–790. [Google Scholar] [CrossRef]
- Calip, G.S.; Hubbard, R.A.; Stergachis, A.; Malone, K.E.; Gralow, J.R.; Boudreau, D.M. Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment. Pharmacoepidemiol. Drug. Saf. 2015, 24, 75–85. [Google Scholar] [CrossRef]
- Calip, G.S.; Elmore, J.G.; Boudreau, D.M. Characteristics associated with nonadherence to medications for hypertension, diabetes, and dyslipidemia among breast cancer survivors. Breast Cancer Res. Treat. 2017, 161, 161–172. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Chen, P.; Yang, J.; Lin, S.; Liu, T.; Chen, S.; Lu, S.; Chen, J.; Chen, W.; et al. An elevated HbA1c level is associated with short-term adverse outcomes in patients with gastrointestinal cancer and type 2 diabetes mellitus. J. Clin. Med. Res. 2017, 9, 303–309. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Kaur, H.; Nilo, D.; Safford, M.M.; DeRosa, A.P.; Kern, L.M. Determining the impact of a cancer diagnosis on diabetes management: A systematic literature review. Am. J. Clin. Oncol. 2019, 42, 870–883. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort profile: The National Health Insurance Service-national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Kim, Y.Y.; Yoon, J.L.; Won, C.W.; Ha, S.; Cho, K.D.; Park, B.R.; Bae, S.; Lee, E.J.; Park, S.Y.; et al. Cohort Profile: National Health Insurance Service-senior (NHIS-senior) cohort in Korea. BMJ 2019, 9, e024344. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, H.; Tang, Y.; Yan, J.; Cao, L.; Chen, Z.; Shao, Z.; Mei, Z.; Jiang, Z. Increased risk of diabetes in cancer survivors: A pooled analysis of 13 population-based cohort studies. ESMO Open 2021, 6, 100218. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Barts, A.; Connors, J.; Dahl, M.; Elliott, T.; Kong, J.; Keane, T.; Thompson, D.; Stafford, S.; Ur, E.; et al. Glucocorticoid-induced hyperglycemia is prevalent and unpredictable for patients undergoing cancer therapy: An observational cohort study. Curr. Oncol. 2013, 20, e532–e538. [Google Scholar] [CrossRef]
- Feng, J.P.; Yuan, X.L.; Li, M.; Fang, J.; Xie, T.; Zhou, Y.; Zhu, Y.M.; Luo, M.; Lin, M.; Ye, D.W. Secondary diabetes associated with 5-fluorouracil-based chemotherapy regimens in non-diabetic patients with colorectal cancer: Results from a single-centre cohort study. Colorectal. Dis. 2013, 15, 27–33. [Google Scholar] [CrossRef]
- Lipscombe, L.L.; Fischer, H.D.; Yun, L.; Gruneir, A.; Austin, P.; Paszat, L.; Anderson, G.M.; Rochon, P.A. Association between tamoxifen treatment and diabetes: A population-based study. Cancer 2012, 118, 2615–2622. [Google Scholar] [CrossRef]
- Meacham, L.R.; Sklar, C.A.; Li, S.; Liu, Q.; Gimpel, N.; Yasui, Y.; Whitton, J.A.; Stovall, M.; Robison, L.L.; Oeffinger, K.C. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: A report for the childhood cancer survivor study. Arch. Intern. Med. 2009, 169, 1381–1388. [Google Scholar] [CrossRef]
- Raghavan, S.; Vassy, J.L.; Ho, Y.L.; Song, R.J.; Gagnon, D.R.; Cho, K.; Wilson, P.W.F.; Phillips, L.S. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J. Am. Heart Assoc. 2019, 8, e011295. [Google Scholar] [CrossRef]
- Koczwara, B.; Meng, R.; Miller, M.D.; Clark, R.A.; Kaambwa, B.; Marin, T.; Damarell, R.A.; Roder, D.M. Late mortality in people with cancer: A population-based Australian study. Med. J. Aust. 2021, 214, 318–323. [Google Scholar] [CrossRef]
- Suh, E.; Stratton, K.L.; Leisenring, W.M.; Nathan, P.C.; Ford, J.S.; Freyer, D.R.; McNeer, J.L.; Stock, W.; Stovall, M.; Krull, K.R.; et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: A retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020, 21, 421–435. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Z.; Thong, M.S.Y.; Doege, D.; Arndt, V. Higher incidence of diabetes in cancer patients compared to cancer-free population controls: A systematic review and meta-analysis. Cancers 2022, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Lega, I.C.; Pole, J.D.; Austin, P.C.; Lau, C.; Nathan, P.C.; Baxter, N.N. Diabetes risk in childhood cancer survivors: A population-based study. Can. J. Diabetes 2018, 42, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, Y.; Lee, E.K. Acute hyperglycemia associated with anti-cancer medication. Endocrinol. Metab. 2017, 32, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Mohn, A.; Di Marzio, A.; Capanna, R.; Fioritoni, G.; Chiarelli, F. Persistence of impaired pancreatic beta-cell function in children treated for acute lymphoblastic leukaemia. Lancet 2004, 363, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Ariaans, G.; de Jong, S.; Gietema, J.A.; Lefrandt, J.D.; de Vries, E.G.; Jalving, M. Cancer-drug induced insulin resistance: Innocent bystander or unusual suspect. Cancer Treat Rev. 2015, 41, 376–384. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J. Natl. Cancer Inst. 2010, 102, 39–46. [Google Scholar] [CrossRef]
- Crawley, D.; Garmo, H.; Rudman, S.; Stattin, P.; Häggström, C.; Zethelius, B.; Holmberg, L.; Adolfsson, J.; Van Hemelrijck, M. Association between duration and type of androgen deprivation therapy and risk of diabetes in men with prostate cancer. Int. J. Cancer. 2016, 139, 2698–2704. [Google Scholar] [CrossRef]
- Litwak, L.; Goh, S.Y.; Hussein, Z.; Malek, R.; Prusty, V.; Khamseh, M.E. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational A1chieve study. Diabetol. Metab. Syndr. 2013, 5, 57. [Google Scholar] [CrossRef]
- Nanayakkara, N.; Ranasinha, S.; Gadowski, A.; Heritier, S.; Flack, J.R.; Wischer, N.; Wong, J.; Zoungas, S. Age, age at diagnosis and diabetes duration are all associated with vascular complications in type 2 diabetes. J. Diabetes Complicat. 2018, 32, 279–290. [Google Scholar] [CrossRef]
- Worndl, E.; Fung, K.; Fischer, H.D.; Austin, P.C.; Krzyzanowska, M.K.; Lipscombe, L.L. Preventable diabetic complications after a cancer diagnosis in patients with diabetes: A population-based cohort study. JNCI Cancer Spectr. 2018, 2, pky008. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Valderas, J.M.; McFadden, E.C.; Bankhead, C.R.; Lavery, B.A.; Khan, N.F.; Stevens, R.J.; Keating, N.L. Outcomes of preexisting diabetes mellitus in breast, colorectal, and prostate cancer. J. Cancer Surviv. 2017, 11, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Airy, M.; Raghavan, R.; Truong, L.D.; Eknoyan, G. Tubulointerstitial nephritis and cancer chemotherapy: Update on a neglected clinical entity. Nephrol. Dial. Transplant. 2013, 28, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Launay-Vacher, V.; Oudard, S.; Janus, N.; Gligorov, J.; Pourrat, X.; Rixe, O.; Morere, J.F.; Beuzeboc, P.; Deray, G.; Renal Insufficiency and Cancer Medications (IRMA) Study Group. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: The renal insufficiency and anticancer medications (IRMA) study. Cancer 2007, 110, 1376–1384. [Google Scholar] [CrossRef]
- Shin, H.Y.; Linton, J.A.; Shim, J.Y.; Kang, H.T. Cancer survivors aged 40 years or elder are associated with high risk of chronic kidney disease: The 2010–2012 Korean National Health and Nutrition Examination Survey. Asian Pac. J. Cancer Prev. 2015, 16, 1355–1360. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lee, S.H.; Kim, C.; Yu, H.; Sohn, J.H.; Lee, J.J.; Kim, D.K. Risk of dementia according to surgery type: A nationwide cohort study. J. Pers. Med. 2022, 12, 468. [Google Scholar] [CrossRef]
- Lee, I.H.; Ha, S.S.; Son, G.M.; Yang, H.G.; Kim, D.K. Could chronic rhinosinusitis increase the risk of ulcerative colitis? A nationwide cohort study. Diagnostics 2022, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Son, D.S.; Cho, M.S.; Kim, D.K. Chronic rhinosinusitis could increase the risk of cholesteatoma of middle ear. Int. Forum Allergy Rhinol. 2022, 13, 168–171. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, H.J.; Lee, I.H.; Lee, J.J. Risk of burning mouth syndrome in patients with migraine: A nationwide cohort study. J. Pers. Med. 2022, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ko, I.; Kim, D.K. Sudden sensorineural hearing loss may increase the risk of retinal vein occlusion: A nationwide cohort study. Healthcare 2022, 10, 408. [Google Scholar] [CrossRef]



| Variables | N | Case | Person Year | Incidence Rate | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| Comparison | 16,228 | 2319 | 135,352.5 | 17.13 | 1.00 (ref) | 1.00 (ref) |
| Overall cancer | 8114 | 975 | 46,032.1 | 21.18 | 1.21 (1.12–1.30) *** | 1.29 (1.20–1.39) *** |
| Cancer without pancreatic cancer | 7963 | 959 | 45,669.9 | 21.00 | 1.20 (1.11–1.29) *** | 1.28 (1.19–1.38) *** |
| Variables | N | Case | Person Year | Incidence Rate | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| Comparison | 16,228 | 254 | 145,975.0 | 1.74 | 1.00 (ref) | 1.00 (ref) |
| Overall cancer | 8114 | 117 | 49,986.6 | 2.34 | 1.33 (1.06–1.65) * | 1.43 (1.14–1.78) ** |
| Cancer without pancreatic cancer | 7963 | 109 | 49,583.8 | 2.20 | 1.25 (1.00–1.56) | 1.35 (1.07–1.69) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Kim, C.; Yu, H.; Kim, D.-K. Analysis of the Incidence of Type 2 Diabetes, Requirement of Insulin Treatment, and Diabetes-Related Complications among Patients with Cancer. Cancers 2023, 15, 1094. https://doi.org/10.3390/cancers15041094
Lee SJ, Kim C, Yu H, Kim D-K. Analysis of the Incidence of Type 2 Diabetes, Requirement of Insulin Treatment, and Diabetes-Related Complications among Patients with Cancer. Cancers. 2023; 15(4):1094. https://doi.org/10.3390/cancers15041094
Chicago/Turabian StyleLee, Su Jung, Chulho Kim, Hyunjae Yu, and Dong-Kyu Kim. 2023. "Analysis of the Incidence of Type 2 Diabetes, Requirement of Insulin Treatment, and Diabetes-Related Complications among Patients with Cancer" Cancers 15, no. 4: 1094. https://doi.org/10.3390/cancers15041094
APA StyleLee, S. J., Kim, C., Yu, H., & Kim, D.-K. (2023). Analysis of the Incidence of Type 2 Diabetes, Requirement of Insulin Treatment, and Diabetes-Related Complications among Patients with Cancer. Cancers, 15(4), 1094. https://doi.org/10.3390/cancers15041094






