Development of a Machine Learning-Based Prediction Model for Chemotherapy-Induced Myelosuppression in Children with Wilms’ Tumor
Abstract
Simple Summary
Abstract
1. Introduction
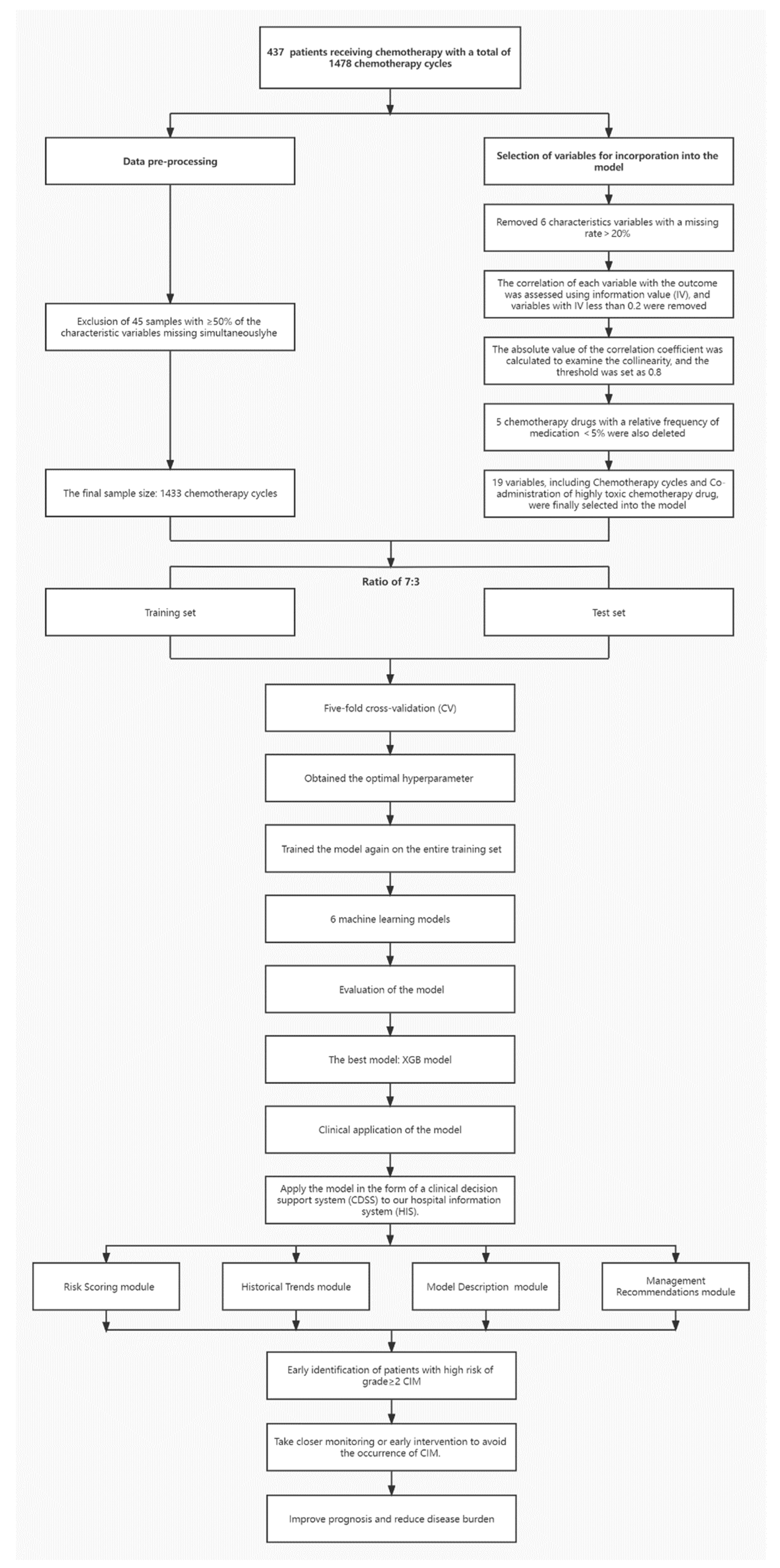
2. Methods
2.1. Patients
2.2. Collection and Definition of Variables
2.2.1. General Variables
2.2.2. Outcome Indicators
2.2.3. Calculation of Composite Variables
- (1)
- Systemic immune-inflammation index (SII) = PLT × ANC/absolute lymphocyte count (ALC) [28]
- (2)
- Neutrophil to lymphocyte ratio (NLR) = ANC/ALC
- (3)
- Platelet to lymphocyte ratio (PLR) = PLT/ALC
- (4)
- Body surface area (BSA) = 0.035 × body weight + 0.1 (body weight ≤ 30 kg)
2.2.4. Derived Variables
2.3. Data Preprocessing
2.3.1. Quality Control of Samples
2.3.2. Imputation Methods of Missing Data
2.4. Model Building
2.4.1. Datasets and Algorithms
2.4.2. Original Variables and Variable Selection
2.4.3. Modelling Procedure
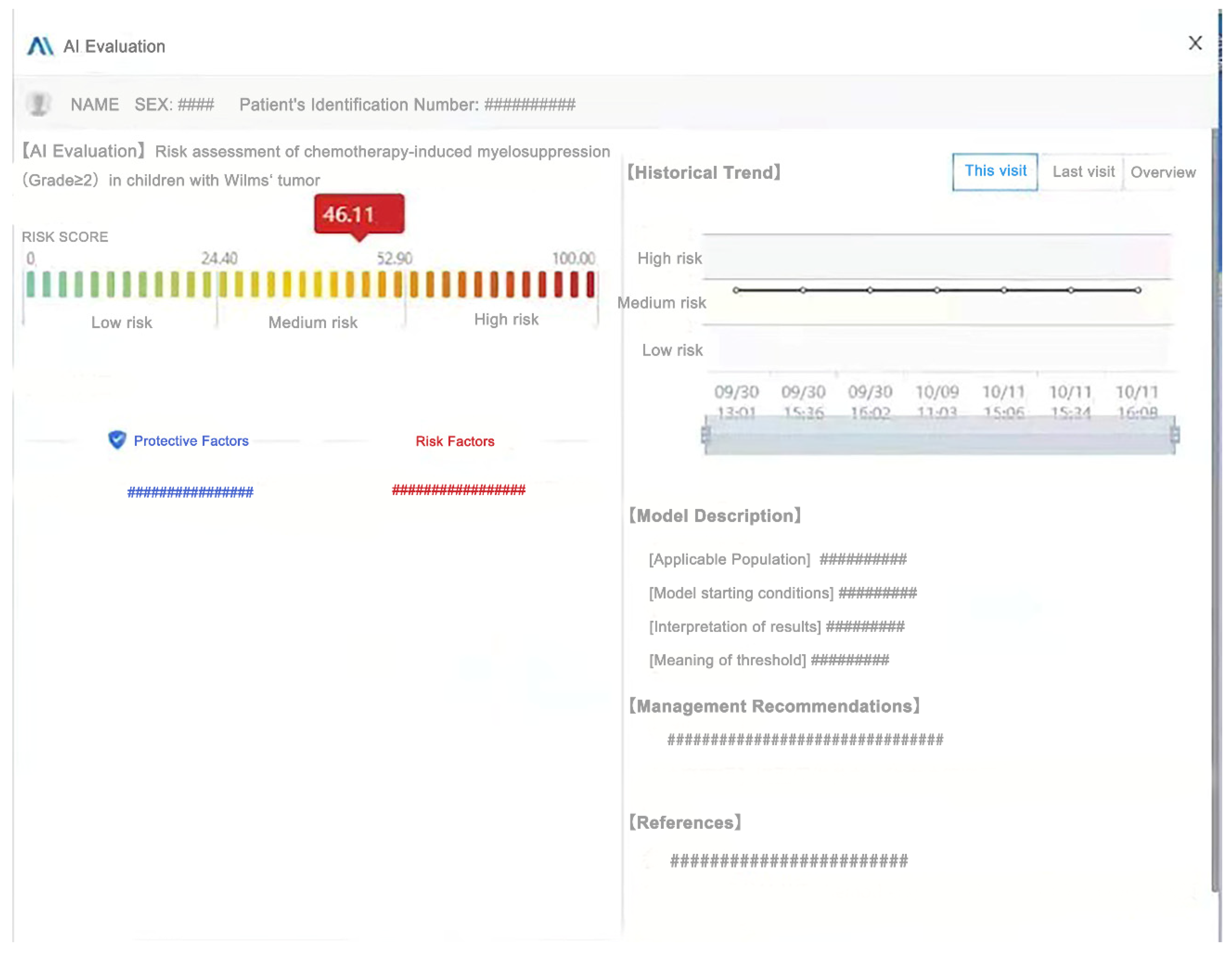
2.4.4. Clinical Application of the Model
2.5. Statistical Analysis Methods
3. Result
3.1. Description of Baseline Characteristics
3.2. Selection of Variables during Modeling
3.3. Selection of Chemotherapy Drugs
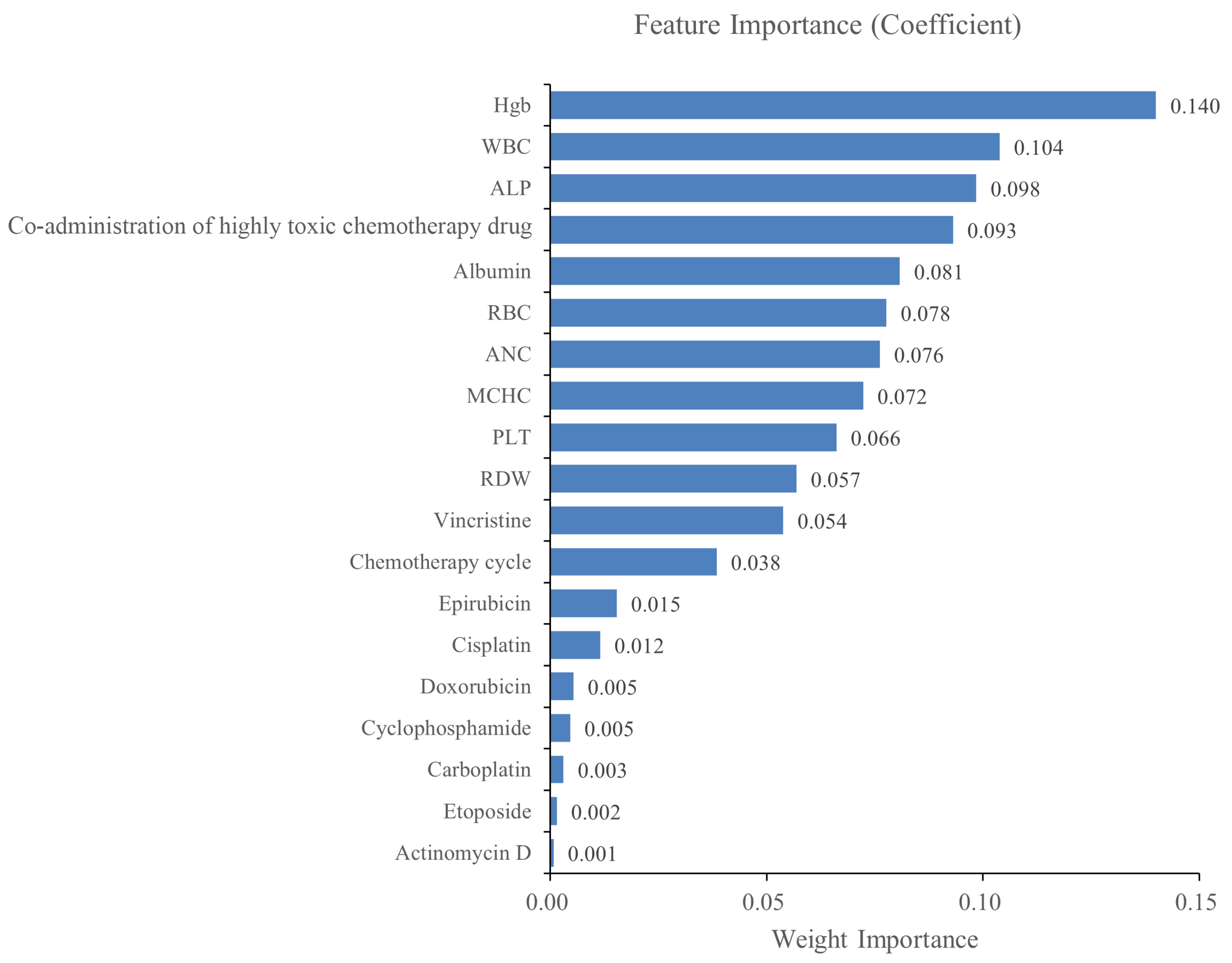
3.4. Variables Finally Selected for the Model
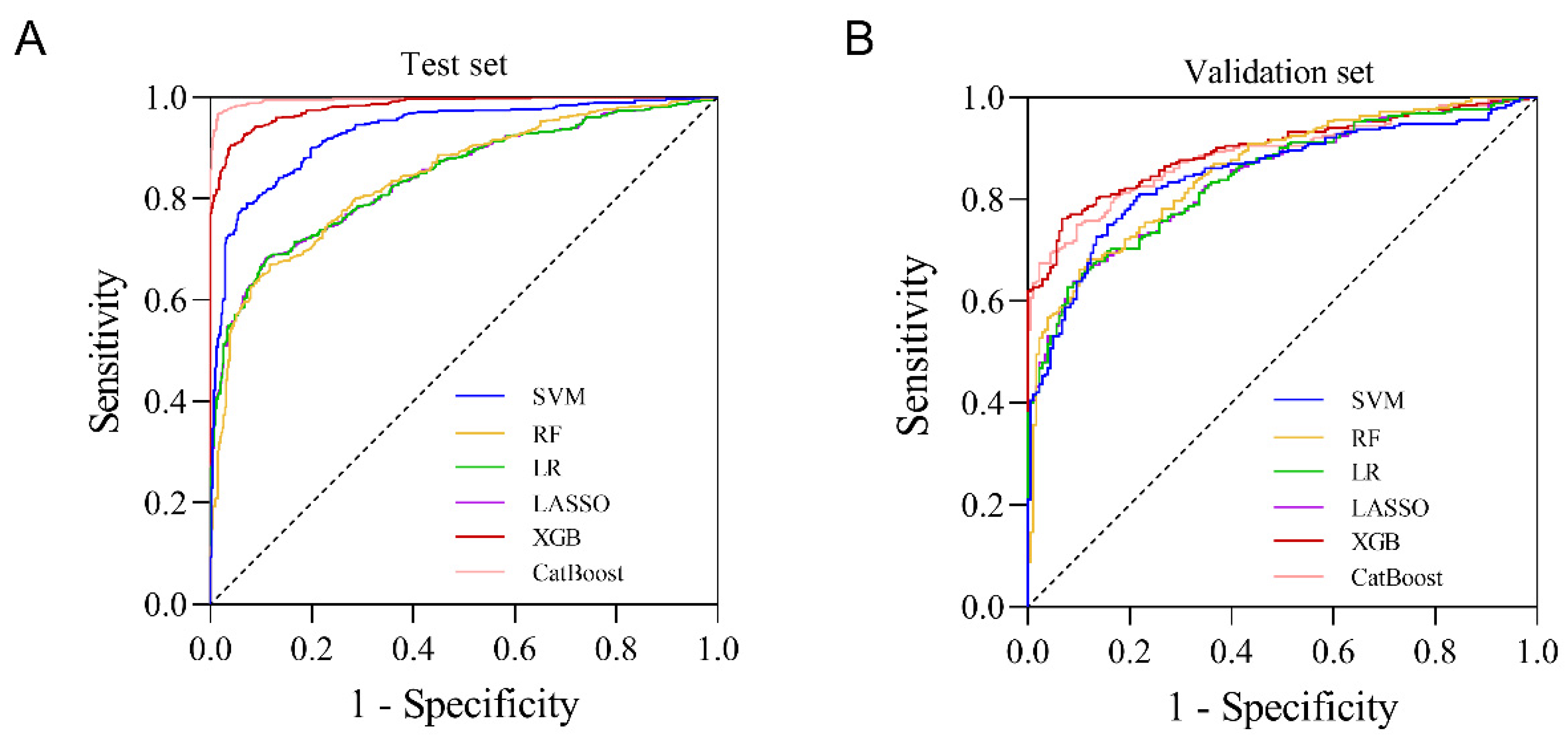
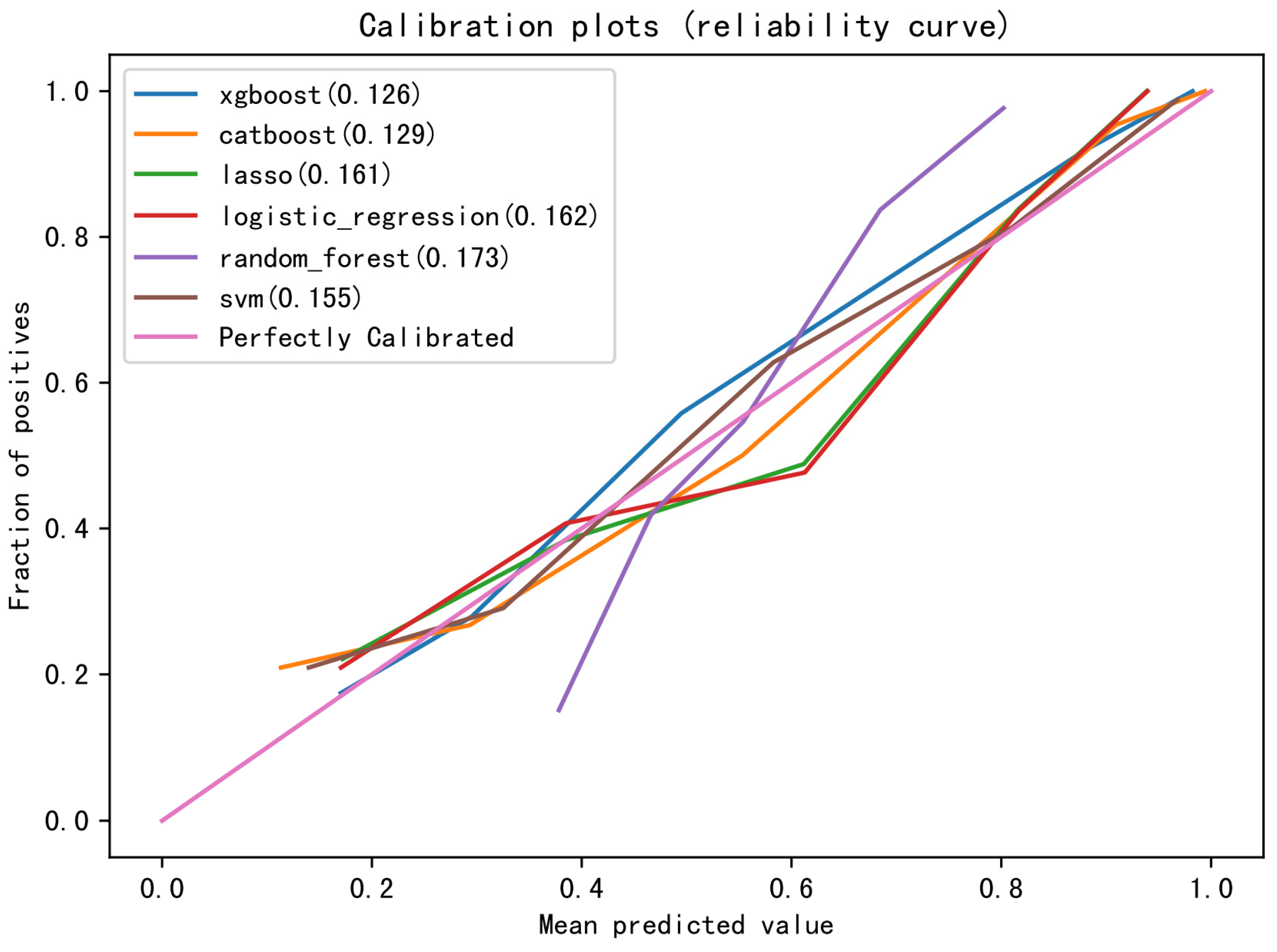
3.5. Evaluation of the Model
3.6. Clinical Application of the Model
4. Discussion
4.1. CIM Is Not Rare during the Treatment of Children with WT
4.2. Contribution of Variables to Model Prediction Results
4.3. XGB Model Has Good Predictive Performance for Grade ≥ 2 CIM
4.4. Application of CIM Prediction Model in Clinical Practice
4.5. Limitations and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalapurakal, J.A.; Dome, J.S.; Perlman, E.; Malogolowkin, M.; Haase, G.M.; Grundy, P.; Coppes, M.J. Management of Wilms’ tumour: Current practice and future goals. Lancet Oncol. 2004, 5, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Brok, J.; Treger, T.D.; Gooskens, S.L.; van den Heuvel-Eibrink, M.M.; Pritchard-Jones, K. Biology and treatment of renal tumours in childhood. Eur. J. Cancer 2016, 68, 179–195. [Google Scholar] [CrossRef]
- Malogolowkin, M.; Spreafico, F.; Dome, J.S.; van Tinteren, H.; Pritchard-Jones, K.; van den Heuvel-Eibrink, M.; Bergeron, C.; de Kraker, J.; Graf, N.; On behalf of the COG Renal Tumors Committee and the SIOP Renal Tumor Study Group. Incidence and outcomes of patients with late recurrence of Wilms’ tumor. Pediatr. Blood Cancer 2013, 60, 1612–1615. [Google Scholar] [CrossRef]
- Barreto, J.N.; McCullough, K.; Ice, L.L.; Smith, J.A. Antineoplastic Agents and the Associated Myelosuppressive Effects. J. Pharm. Pr. 2014, 27, 440–446. [Google Scholar] [CrossRef]
- Bryer, E.; Henry, D. Chemotherapy-induced anemia: Etiology, pathophysiology, and implications for contemporary practice. Int. J. Clin. Transfus. Med. 2018, 6, 21–31. [Google Scholar] [CrossRef]
- Kuter, D.J. Managing thrombocytopenia associated with cancer chemotherapy. Oncology 2015, 29, 282–294. [Google Scholar]
- Taylor, S.J.; Duyvestyn, J.M.; Dagger, S.A.; Dishington, E.J.; Rinaldi, C.A.; Dovey, O.M.; Vassiliou, G.S.; Grove, C.S.; Langdon, W.Y. Preventing chemotherapy-induced myelosuppression by repurposing the FLT3 inhibitor quizartinib. Sci. Transl. Med. 2017, 9, eaam8060. [Google Scholar] [CrossRef]
- Lennan, E.; Roe, H. Role of nurses in the assessment and management of chemotherapy-related side effects in cancer patients. Nurs. Res. Rev. 2014, 4, 103. [Google Scholar] [CrossRef]
- Epstein, R.S.; Aapro, M.S.; Roy, U.K.B.; Salimi, T.; Krenitsky, J.; Leone-Perkins, M.L.; Girman, C.; Schlusser, C.; Crawford, J. Patient Burden and Real-World Management of Chemotherapy-Induced Myelosuppression: Results from an Online Survey of Patients with Solid Tumors. Adv. Ther. 2020, 37, 3606–3618. [Google Scholar] [CrossRef]
- Talcott, J.A.; Siegel, R.D.; Finberg, R.; Goldman, L. Risk assessment in cancer patients with fever and neutropenia: A prospective, two-center validation of a prediction rule. J. Clin. Oncol. 1992, 10, 316–322. [Google Scholar] [CrossRef]
- Epstein, R.S.; Weerasinghe, R.K.; Parrish, A.S.; Krenitsky, J.; Sanborn, R.E.; Salimi, T. Real-world burden of chemotherapy-induced myelosuppression in patients with small cell lung cancer: A retrospective analysis of electronic medical data from community cancer care providers. J. Med. Econ. 2022, 25, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Morrison, V.A.; Dale, D.C.; Crawford, J.; Delgado, D.J.; Fridman, M. Risk of Febrile Neutropenia among Patients with Intermediate-grade non-Hodgkin’s Lymphoma Receiving CHOP Chemotherapy. Leuk. Lymphoma 2003, 44, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Delgado, D.J. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 2003, 98, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Kuderer, N.M.; Crawford, J.; Wolff, D.A.; Culakova, E.; Poniewierski, M.S.; Dale, D.C. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 2010, 117, 1917–1927. [Google Scholar] [CrossRef]
- Lyman, G.H. Impact of Chemotherapy Dose Intensity on Cancer Patient Outcomes. J. Natl. Compr. Cancer Netw. 2009, 7, 99–108. [Google Scholar] [CrossRef]
- Friberg, L.E.; Henningsson, A.; Maas, H.; Nguyen, L.; Karlsson, M.O. Model of Chemotherapy-Induced Myelosuppression With Parameter Consistency Across Drugs. J. Clin. Oncol. 2002, 20, 4713–4721. [Google Scholar] [CrossRef]
- Quartino, A.L.; Friberg, L.; Karlsson, M.O. A simultaneous analysis of the time-course of leukocytes and neutrophils following docetaxel administration using a semi-mechanistic myelosuppression model. Investig. New Drugs 2010, 30, 833–845. [Google Scholar] [CrossRef]
- Sanjuan, V.M.; Buil-Bruna, N.; Garrido, M.J.; Soto, E.; Trocóniz, I.F. Semimechanistic Cell-Cycle Type–Based Pharmacokinetic/Pharmacodynamic Model of Chemotherapy-Induced Neutropenic Effects of Diflomotecan under Different Dosing Schedules. Experiment 2015, 354, 55–64. [Google Scholar] [CrossRef]
- Wallin, J.E.; Friberg, L.; Karlsson, M.O. Model-Based Neutrophil-Guided Dose Adaptation in Chemotherapy: Evaluation of Predicted Outcome with Different Types and Amounts of Information. Basic Clin. Pharmacol. Toxicol. 2010, 106, 234–242. [Google Scholar] [CrossRef]
- Netterberg, I.; Nielsen, E.I.; Friberg, L.E.; Karlsson, M.O. Model-based prediction of myelosuppression and recovery based on frequent neutrophil monitoring. Cancer Chemother. Pharmacol. 2017, 80, 343–353. [Google Scholar] [CrossRef]
- Pfeil, A.M.; Vulsteke, C.; Paridaens, R.; Dieudonne, A.-S.; Pettengell, R.; Hatse, S.; Neven, P.; Lambrechts, D.; Szucs, T.D.; Schwenkglenks, M.; et al. Multivariable regression analysis of febrile neutropenia occurrence in early breast cancer patients receiving chemotherapy assessing patient-related, chemotherapy-related and genetic risk factors. BMC Cancer 2014, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, Y.; Son, M.; Chae, D.; Park, K. A Pharmacometric Model to Predict Chemotherapy-Induced Myelosuppression and Associated Risk Factors in Non-Small Cell Lung Cancer. Pharmaceutics 2022, 14, 914. [Google Scholar] [CrossRef] [PubMed]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 2021, 23, 40–55. [Google Scholar] [CrossRef]
- Gould, M.K.; Huang, B.Z.; Tammemagi, M.C.; Kinar, Y.; Shiff, R. Machine Learning for Early Lung Cancer Identification Using Routine Clinical and Laboratory Data. Am. J. Respir. Crit. Care Med. 2021, 204, 445–453. [Google Scholar] [CrossRef]
- Radakovich, N.; Nagy, M.; Nazha, A. Machine learning in haematological malignancies. Lancet Haematol. 2020, 7, e541–e550. [Google Scholar] [CrossRef] [PubMed]
- Chernbumroong, S.; Johnson, J.; Gupta, N.; Miller, S.; McCormack, F.X.; Garibaldi, J.M.; Johnson, S.R. Machine learning can predict disease manifestations and outcomes in lymphangioleiomyomatosis. Eur. Respir. J. 2020, 57, 2003036. [Google Scholar] [CrossRef]
- Shibahara, T.; Ikuta, S.; Muragaki, Y. Machine-Learning Approach for Modeling Myelosuppression Attributed to Nimustine Hydrochloride. JCO Clin. Cancer Inform. 2018, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cheng, Z.; Hu, Y.; Chen, Z.; Zhang, J.; Ke, C.; Yang, Q.; Lin, F.; Chen, Y.; Wang, J. Prognostic Value of the Systemic Immune-Inflammation Index and Prognostic Nutritional Index in Patients With Medulloblastoma Undergoing Surgical Resection. Front. Nutr. 2021, 8, 754958. [Google Scholar] [CrossRef] [PubMed]
- Mac Manus, M.; Lamborn, K.; Khan, W.; Varghese, A.; Graef, L.; Knox, S. Radiotherapy-associated neutropenia and thrombocytopenia: Analysis of risk factors and development of a predictive model. Blood 1997, 89, 2303–2310. [Google Scholar] [CrossRef]
- Carey, P.J. Drug-Induced Myelosuppression. Drug Saf. 2003, 26, 691–706. [Google Scholar] [CrossRef]
- Gong, Y.-F.; Zhu, L.-Q.; Li, Y.-L.; Zhang, L.-J.; Xue, J.-B.; Xia, S.; Lv, S.; Xu, J.; Li, S.-Z. Identification of the high-risk area for schistosomiasis transmission in China based on information value and machine learning: A newly data-driven modeling attempt. Infect. Dis. Poverty 2021, 10, 88. [Google Scholar] [CrossRef]
- Yurdakul, B.; Naranjo, J. Statistical properties of the population stability index. J. Risk Model Valid. 2020. [Google Scholar] [CrossRef]
- Friberg, L.E. Mechanistic Models for Myelosuppression. Investig. New Drugs 2003, 21, 183–194. [Google Scholar] [CrossRef]
- Lyman, G.H.; Kuderer, N.M.; Aapro, M. Improving Outcomes of Chemotherapy: Established and Novel Options for Myeloprotection in the COVID-19 Era. Front. Oncol. 2021, 11, 697908. [Google Scholar] [CrossRef]
- Straka, C.; Oduncu, F.; Hinke, A.; Einsele, H.; Drexler, E.; Schnabel, B.; Arseniev, L.; Walther, J.; König, A.; Emmerich, B. Responsiveness to G-CSF before leukopenia predicts defense to infection in high-dose chemotherapy recipients. Blood 2004, 104, 1989–1994. [Google Scholar] [CrossRef]
- Castagnola, E.; Garrè, M.L.; Bertoluzzo, L.; Pignatelli, S.; Pavanello, M.; Caviglia, I.; Caruso, S.; Bagnasco, F.; Moroni, C.; Tacchella, A.; et al. Epidemiology of Febrile Neutropenia in Children With Central Nervous System Tumor. J. Pediatr. Hematol. 2011, 33, e310–e315. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L.; Aapro, M.; Paesmans, M.; Van Eeden, R.; Smit, T.; Krendyukov, A.; Klastersky, J. Febrile neutropenia (FN) occurrence outside of clinical trials: Occurrence and predictive factors in adult patients treated with chemotherapy and an expected moderate FN risk. Rationale and design of a real-world prospective, observational, multinational study. BMC Cancer 2018, 18, 917. [Google Scholar] [CrossRef]
- Ba, E.A.Y.; Shi, Y.; Jiang, W.; Feng, J.; Cheng, Y.; Xiao, L.; Zhang, Q.; Qiu, W.; Xu, B.; Xu, R.; et al. Current management of chemotherapy-induced neutropenia in adults: Key points and new challenges. Cancer Biol. Med. 2020, 17, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Intragumtornchai, T.; Sutheesophon, J.; Sutcharitchan, P.; Swasdikul, D. A Predictive Model for Life-Threatening Neutropenia and Febrile Neutropenia after the First Course of CHOP Chemotherapy in Patients with Aggressive Non-Hodgkin’s Lymphoma. Leuk. Lymphoma 2000, 37, 351–360. [Google Scholar] [CrossRef]
- Razzaghdoust, A.; Mofid, B.; Moghadam, M. Development of a simplified multivariable model to predict neutropenic complications in cancer patients undergoing chemotherapy. Support. Care Cancer 2018, 26, 3691–3699. [Google Scholar] [CrossRef]
- Lyman, G.H.; Abella, E.; Pettengell, R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit. Rev. Oncol. 2014, 90, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L. Myelosuppression and its consequences in elderly patients with cancer. Oncology 2003, 17, 27–32. [Google Scholar] [PubMed]
- Aagaard, T.; Roen, A.; Reekie, J.; Daugaard, G.; Brown, P.D.N.; Specht, L.; Sengeløv, H.; Mocroft, A.; Lundgren, J.; Helleberg, M. Development and Validation of a Risk Score for Febrile Neutropenia After Chemotherapy in Patients With Cancer: The FENCE Score. JNCI Cancer Spectr. 2018, 2, pky053. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pitarch, A.; Guglieri-López, B.; Nacher, A.; Merino, V.; Merino-Sanjuán, M. Impact of Undernutrition on the Pharmacokinetics and Pharmacodynamics of Anticancer Drugs: A Literature Review. Nutr. Cancer 2017, 69, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.L.; Dome, J.S. Current Therapy for Wilms’ Tumor. Oncologist 2005, 10, 815–826. [Google Scholar] [CrossRef]
- Fernandez, C.V.; Mullen, E.A.; Chi, Y.-Y.; Ehrlich, P.F.; Perlman, E.; Kalapurakal, J.A.; Khanna, G.; Paulino, A.C.; Hamilton, T.E.; Gow, K.W.; et al. Outcome and Prognostic Factors in Stage III Favorable-Histology Wilms Tumor: A Report From the Children’s Oncology Group Study AREN0532. J. Clin. Oncol. 2018, 36, 254–261. [Google Scholar] [CrossRef]
- Dix, D.B.; Seibel, N.L.; Chi, Y.-Y.; Khanna, G.; Gratias, E.; Anderson, J.R.; Mullen, E.A.; Geller, J.I.; Kalapurakal, J.A.; Paulino, A.C.; et al. Treatment of Stage IV Favorable Histology Wilms Tumor With Lung Metastases: A Report From the Children’s Oncology Group AREN0533 Study. J. Clin. Oncol. 2018, 36, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Klastersky, J.; Schwarzbold, A.; Muanza, F.; Georgala, A.; Aoun, M.; Loizidou, A.; Barette, M.; Costantini, S.; Delmelle, M.; et al. A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies. Ann. Oncol. 2009, 20, 513–519. [Google Scholar] [CrossRef]
- Pettengell, R.; Bosly, A.; Szucs, T.D.; Jackisch, C.; Leonard, R.; Paridaens, R.; Constenla, M.; Schwenkglenks, M.; for the Impact of Neutropenia in Chemotherapy—European Study Group (INC-EU). Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: Data from the INC-EU Prospective Observational European Neutropenia Study. Br. J. Haematol. 2009, 144, 677–685. [Google Scholar] [CrossRef]
- Craig, M. Towards Quantitative Systems Pharmacology Models of Chemotherapy-Induced Neutropenia. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 293–304. [Google Scholar] [CrossRef]
- Fornari, C.; O’Connor, L.O.; Yates, J.W.; Cheung, S.A.; Jodrell, D.I.; Mettetal, J.T.; Collins, T.A. Understanding Hematological Toxicities Using Mathematical Modeling. Clin. Pharmacol. Ther. 2018, 104, 644–654. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Zhu, Y.-H.; Zhou, L.-Y.; Shi, W.-Q.; Qin, Z.; Wu, B.; Yan, Y.; Pei, Y.-W.; Chao, N.-N.; Zhang, R.; et al. Association Between Genetic Polymorphisms of Metabolic Enzymes and Azathioprine-Induced Myelosuppression in 1419 Chinese Patients: A Retrospective Study. Front. Pharmacol. 2021, 12, 672769. [Google Scholar] [CrossRef] [PubMed]
- Björn, N.; Badam, T.V.S.; Spalinskas, R.; Brandén, E.; Koyi, H.; Lewensohn, R.; De Petris, L.; Lubovac-Pilav, Z.; Sahlén, P.; Lundeberg, J.; et al. Whole-genome sequencing and gene network modules predict gemcitabine/carboplatin-induced myelosuppression in non-small cell lung cancer patients. NPJ Syst. Biol. Appl. 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhou, C.; Liu, Y.; Su, K.; Jia, L.; Chen, L.; Li, M.; Ma, J.; Zhou, W.; Zhang, S.; et al. Genetic associations of docetaxel-based chemotherapy-induced myelosuppression in Chinese Han population. J. Clin. Pharm. Ther. 2019, 45, 354–364. [Google Scholar] [CrossRef]
- Belard, A.; Buchman, T.; Forsberg, J.; Potter, B.K.; Dente, C.J.; Kirk, A.; Elster, E. Precision diagnosis: A view of the clinical decision support systems (CDSS) landscape through the lens of critical care. J. Clin. Monit. Comput. 2016, 31, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, V.; Haga, Y.; Takei, H.; Mansell, E.; Okamatsu-Haga, C.; Suzuki, M.; Radulovic, V.; van der Garde, M.; Koide, S.; Soboleva, S.; et al. Induction of blood-circulating bile acids supports recovery from myelosuppressive chemotherapy. Blood Adv. 2020, 4, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
| Variable | ALL (N = 1433) | Training Set (N = 1003) | Test Set (N = 430) | Statistic (Z/χ2) | p Value |
|---|---|---|---|---|---|
| Age (days), M (Q1–Q3) | 1388 (807–2221) | 1391 (810–2245) | 1376 (803–2163) | −0.814 | 0.416 |
| Sex | 2.707 | 0.100 | |||
| Female | 674 (47.0%) | 486 (48.5%) | 188 (43.7%) | ||
| Male | 759 (53.0%) | 517 (51.5%) | 242 (56.3%) | ||
| Weight (kg), M (Q1–Q3) | 14.5 (11.5–19.0) | 14.5 (11.5–19.0) | 14.0 (11.5–18.0) | −0.844 | 0.398 |
| BSA (m2), M (Q1–Q3) | 0.61 (0.50–0.77) | 0.61 (0.50–0.77) | 0.59 (0.50–0.73) | −0.823 | 0.410 |
| Tumor stage | 6.561 | 0.161 | |||
| I | 116 (8.1%) | 84 (8.4%) | 32 (7.4%) | ||
| II | 224 (15.6%) | 149 (14.9%) | 75 (17.4%) | ||
| III | 495 (34.5%) | 360 (35.9%) | 135 (31.4%) | ||
| IV | 516 (36.0%) | 360 (35.9%) | 156 (36.3%) | ||
| V | 82 (5.7%) | 50 (5.0%) | 32 (7.4%) | ||
| Risk classification (COG) | 0.045 | 0.831 | |||
| FH | 1022 (71.3%) | 717 (71.5%) | 305 (70.9%) | ||
| uFH | 411 (28.7%) | 286 (28.5%) | 125 (29.1%) | ||
| Chemotherapy cycles, M (Q1–Q3) | 4.0 (2.0–9.0) | 4.0 (2.0–8.0) | 5.0 (2.0–9.0) | 1.408 | 0.159 |
| Hematologic index, M (Q1–Q3) | |||||
| Neutrophil percentage | 0.59 (0.48–0.70) | 0.59 (0.48–0.70) | 0.58 (0.47–0.69) | −1.087 | 0.277 |
| ANC (×109/L) | 3.50 (2.36–4.91) | 3.53 (2.35–4.99) | 3.49 (2.39–4.83) | −0.031 | 0.975 |
| Monocyte percentage | 0.04 (0.03–0.07) | 0.04 (0.03–0.06) | 0.04 (0.03–0.07) | −0.659 | 0.510 |
| AMC (×109/L) | 0.28 (0.19–0.39) | 0.28 (0.19–0.38) | 0.30 (0.19–0.41) | −1.212 | 0.225 |
| P–LCR (%) | 24.2 (19.0–29.8) | 24.2 (19.0–29.9) | 24.4 (18.9–29.7) | −0.381 | 0.703 |
| MCV (fL) | 82.9 (78.7–87.6) | 83.0 (78.6–87.6) | 82.9 (78.8–87.5) | −0.283 | 0.777 |
| MCHC (g/L) | 325.0 (315.0–333.0) | 325.0 (316.0–333.0) | 325.0(315.0–333.0) | −0.100 | 0.920 |
| MCH (pg) | 27.1 (25.4–28.8) | 27.0 (25.4–28.9) | 27.1 (25.3–28.7) | −0.423 | 0.673 |
| Lymphocyte percentage (%) | 0.30 (0.20–0.43) | 0.30 (0.20–0.43) | 0.32 (0.21–0.43) | −0.842 | 0.400 |
| ALC (×109/L) | 1.75 (0.95–3.11) | 1.71 (0.97–2.99) | 1.94 (0.93–3.36) | −1.220 | 0.223 |
| WBC (×109/L) | 6.10 (4.32–8.73) | 6.01 (4.27–8.73) | 6.28 (4.45–8.74) | −0.809 | 0.419 |
| RBC (×109/L) | 3.96 (3.52–4.36) | 3.94 (3.51–4.33) | 4.02 (3.53–4.41) | −1.427 | 0.154 |
| RDW (%) | 15.5 (14.0–17.3) | 15.5 (14.0–17.3) | 15.5 (14.1–17.5) | −0.551 | 0.582 |
| ARD (fL) | 47.0 (41.0–52.0) | 47.0 (42.0–52.0) | 47.0 (41.0–52.0) | −0.248 | 0.804 |
| Hematocrit (%) | 32.9 (29.9–35.5) | 32.8 (29.9–35.3) | 33.2 (29.8–35.9) | −1.257 | 0.209 |
| PDW (fL) | 11.0 (9.8–12.4) | 11.0 (9.8–12.4) | 11.1 (9.8–12.3) | −0.329 | 0.742 |
| Thrombocytocrit (%) | 0.31 (0.24–0.38) | 0.31 (0.24–0.38) | 0.32 (0.25–0.38) | −0.114 | 0.909 |
| MPV (fL) | 10.0 (9.3–10.7) | 9.9 (9.3–10.7) | 10.0 (9.3–10.7) | −0.274 | 0.784 |
| PLT (×109/L) | 297.0 (227.0–387.0) | 295.0 (223.0–390.0) | 304.0 (238.0–378.0) | −0.903 | 0.366 |
| Hgb (g/L) | 107.0 (95.0–116.0) | 107.0 (95.0–116.0) | 107.0 (96.0–118.0) | −1.100 | 0.271 |
| SII | 575.2 (334.7–951.1) | 579.8 (336.0–967.5) | 569.4 (333.4–917.1) | −0.398 | 0.691 |
| NLR | 1.97 (1.12–3.37) | 2.00 (1.13–3.44) | 1.85 (1.09–3.24) | −0.996 | 0.319 |
| PLR | 162.8 (101.4–274.4) | 169.7 (102.5–276.5) | 149.6 (96.8–268.8) | −1.180 | 0.238 |
| Urinalysis index | |||||
| pH | 6.52 (6.00–7.00) | 6.52 (6.00–7.00) | 6.52 (6.00–7.00) | −0.433 | 0.665 |
| Biochemical index | |||||
| LDH (U/L) | 286.8 (227.0–418.4) | 286.5 (228.0–418.4) | 287.0 (225.0–418.4) | −0.246 | 0.806 |
| UA (μmol/L) | 284.8 (242.0–325.0) | 284.8 (237.0–325.0) | 284.8 (249.5–325.0) | −1.598 | 0.110 |
| TBIL (μmol/L) | 6.80 (4.00–8.10) | 6.80 (4.00–8.20) | 6.50 (4.00–7.80) | −0.970 | 0.332 |
| TP (g/L) | 63.8 (60.7–67.4) | 63.8 (60.9–67.3) | 63.8 (60.2–67.8) | −0.313 | 0.755 |
| Globulin (g/L) | 22.2 (19.3–24.4) | 22.2 (19.1–24.4) | 22.2 (19.6–24.2) | −0.202 | 0.840 |
| Albumin (g/L) | 41.7 (39.6–44.8) | 41.7 (39.7–44.9) | 41.7 (39.3–44.7) | −0.592 | 0.554 |
| ALP (U/L) | 175.8(133.1–197.3) | 175.8 (134.0–199.0) | 175.8(132.0–193.5) | −0.637 | 0.524 |
| Scr (μmol/L) | 34.3 (28.0–38.0) | 34.3 (28.0–38.0) | 34.3 (28.0–38.5) | −1.139 | 0.255 |
| ALT (U/L) | 21.7 (14.4–26.0) | 21.1 (14.3–25.6) | 22.5 (14.6–27.1) | −1.303 | 0.192 |
| AST (U/L) | 35.9 (28.1–40.0) | 35.4 (28.0–39.4) | 37.2 (29.0–41.3) | −2.270 | 0.023 |
| Grade ≥ 2 CIM | 0.001 | 0.977 | |||
| With | 594 (41.5%) | 416 (41.5%) | 178 (41.4%) | ||
| Without | 839 (58.5%) | 587 (58.5%) | 252 (58.6%) |
| Variable | Grade ≥ 2 CIM | Statistic | p Value | |
|---|---|---|---|---|
| Without (N = 594) | With (N = 839) | |||
| Age (days), M (Q1–Q3) | 1554 (905–2478) | 1294 (726–2022) | −4.432 | <0.001 |
| Sex | 11.368 | 0.001 | ||
| Female | 248 (41.7%) | 426 (50.8%) | ||
| Male | 346 (58.3%) | 413 (49.2%) | ||
| Weight (kg), M (Q1–Q3) | 16.0 (12.0–20.0) | 14.0 (11.0–18.0) | −5.388 | <0.001 |
| BSA (m2), M (Q1–Q3) | 0.66 (0.52–0.80) | 0.59 (0.49–0.73) | −5.385 | <0.001 |
| Tumor stage | 1.915 | 0.751 | ||
| I | 51 (8.6%) | 65 (7.8%) | ||
| II | 92 (15.5%) | 132 (15.7%) | ||
| III | 211 (35.5%) | 284 (33.9%) | ||
| IV | 211 (35.5%) | 305 (36.4%) | ||
| V | 29 (4.9%) | 53 (6.3%) | ||
| Risk classification (COG) | 3.011 | 0.083 | ||
| FH | 409 (68.9%) | 613 (73.1%) | ||
| uFH | 185 (31.1%) | 226 (26.9%) | ||
| Chemotherapy cycles | 5 (2.0–10.0) | 4 (1.0–8.0) | 5.574 | <0.001 |
| Hematologic index, M (Q1–Q3) | ||||
| Neutrophil percentage (%) | 0.59 (0.48–0.71) | 0.59 (0.47–0.69) | −1.398 | 0.162 |
| ANC (×109/L) | 3.68 (2.65–4.74) | 3.38 (2.06–5.10) | −2.631 | 0.009 |
| Monocyte percentage (%) | 0.04 (0.03–0.06) | 0.05 (0.03–0.07) | −4.919 | <0.001 |
| AMC (×109/L) | 0.27 (0.19–0.37) | 0.29 (0.20–0.40) | −2.650 | 0.008 |
| P–LCR (%) | 25.2 (20.1–31.3) | 23.7 (18.3–28.4) | −3.726 | <0.001 |
| MCV (fL) | 83.1 (79.4–87.3) | 82.9 (78.1–87.7) | −0.853 | 0.393 |
| MCHC (g/L) | 328.0 (319.0–334.0) | 322.0 (312.0–332.0) | −7.379 | <0.001 |
| MCH (pg) | 27.3 (26.0–28.8) | 26.9 (24.8–28.8) | −3.545 | <0.001 |
| Lymphocyte percentage (%) | 0.31 (0.19–0.43) | 0.30 (0.21–0.43) | −0.562 | 0.574 |
| ALC(×109/L) | 1.85 (1.01–3.01) | 1.70 (0.92–3.21) | −1.272 | 0.203 |
| WBC (×109/L) | 6.35 (4.80–8.23) | 5.98 (3.78–9.05) | −2.253 | 0.024 |
| RBC (×109/L) | 4.21 (3.88–4.54) | 3.73 (3.29–4.18) | −13.946 | <0.001 |
| RDW (%) | 14.8 (13.7–16.1) | 16.1 (14.4–18.3) | −9.918 | <0.001 |
| ARD (fL) | 45.0 (41.0–49.0) | 47.7 (42.0–54.0) | −6.889 | <0.001 |
| Hematocrit (%) | 34.9 (33.0–36.8) | 30.8 (27.9–33.6) | −18.756 | <0.001 |
| PDW (fL) | 11.2 (10.0–12.6) | 10.8 (9.7–12.1) | −4.376 | <0.001 |
| Thrombocytocrit (%) | 0.29 (0.24–0.37) | 0.34 (0.25–0.40) | −5.935 | <0.001 |
| MPV (fL) | 10.0 (9.4–10.9) | 9.9 (9.2–10.5) | −4.009 | <0.001 |
| PLT (×109/L) | 278.0 (218.0–345.0) | 316.0 (237.0–413.0) | −5.989 | <0.001 |
| Hgb (g/L) | 114.0 (107.0–121.0) | 98.0 (89.0–110.0) | −19.054 | <0.001 |
| SII | 516.2 (322.5–909.0) | 616.0 (346.0–977.5) | −2.349 | 0.019 |
| NLR | 1.90 (1.13–3.65) | 2.00 (1.11–3.22) | −0.650 | 0.516 |
| PLR | 140.0 (94.9–243.2) | 180.1 (109.0–301.0) | −4.873 | <0.001 |
| Urinalysis index | ||||
| pH | 6.52 (6.00–7.00) | 6.52 (6.00–7.00) | −0.535 | 0.593 |
| Biochemical index | ||||
| LDH (U/L) | 275.0 (227.8–418.4) | 297.1 (226.8–418.4) | −2.666 | 0.008 |
| UA (μmol/L) | 284.8 (242.0–305.1) | 284.8 (241.0–335.0) | −3.076 | 0.002 |
| TBIL (μmol/L) | 6.86 (4.20–8.30) | 6.10 (3.80–8.00) | −2.992 | 0.003 |
| TP (g/L) | 63.8 (62.0–68.1) | 63.8 (59.9–67.0) | −4.537 | <0.001 |
| Globulin (g/L) | 22.2 (19.2–23.9) | 22.2 (19.5–24.8) | −1.555 | 0.120 |
| Albumin (g/L) | 42.5 (41.6–45.5) | 41.7 (38.1–44.1) | −7.927 | <0.001 |
| ALP (U/L) | 175.8 (160.0–204.8) | 159.5 (118.9–188.0) | −8.870 | <0.001 |
| Scr (μmol/L) | 34.3 (28.0–37.0) | 34.3 (27.5–39.0) | −0.525 | 0.600 |
| ALT(U/L) | 21.0 (14.4–24.0) | 22.0 (14.2–27.6) | −1.216 | 0.224 |
| AST (U/L) | 35.9 (28.8–38.1) | 35.9 (28.0–42.0) | −1.416 | 0.157 |
| Variable | Missing Sample | Miss Rate (%) |
|---|---|---|
| Age | 0 | 0.00 |
| Sex | 0 | 0.00 |
| Weight | 49 | 3.42 |
| BSA | 49 | 3.42 |
| Tumor stage | 1 | 0.07 |
| Risk classification (COG) | 48 | 3.35 |
| Chemotherapy cycle | 18 | 1.26 |
| Neutrophil percentage | 5 | 0.35 |
| ANC | 18 | 1.26 |
| Monocyte percentage | 8 | 0.56 |
| AMC | 45 | 3.14 |
| P–LCR | 94 | 6.56 |
| MCV | 3 | 0.21 |
| MCHC | 3 | 0.21 |
| MCH | 2 | 0.14 |
| Lymphocyte percentage | 6 | 0.42 |
| ALC | 16 | 1.12 |
| WBC | 2 | 0.14 |
| RBC | 1 | 0.07 |
| RDW | 4 | 0.28 |
| ARD | 102 | 7.12 |
| Hematocrit | 3 | 0.21 |
| PDW | 86 | 6.00 |
| Thrombocytocrit | 114 | 7.96 |
| MPV | 81 | 5.65 |
| PLT | 2 | 0.14 |
| Hgb | 1 | 0.07 |
| SII | 16 | 1.12 |
| NLR | 16 | 1.12 |
| PLR | 16 | 1.12 |
| LDH | 219 | 15.28 |
| UA | 204 | 14.24 |
| TBIL | 220 | 15.35 |
| TP | 219 | 15.28 |
| Globulin | 220 | 15.35 |
| Albumin | 219 | 15.28 |
| ALP | 220 | 15.35 |
| Scr | 205 | 14.31 |
| ALT | 221 | 15.42 |
| AST | 220 | 15.35 |
| Drug | Relative Frequency | Frequency |
|---|---|---|
| Bleomycin | 0.001 | 1 |
| Fluorouracil | 0.006 | 9 |
| Topotecan | 0.011 | 16 |
| Vindesine | 0.012 | 17 |
| Ifosfamide | 0.017 | 23 |
| Cisplatin | 0.124 | 172 |
| Doxorubicin | 0.126 | 176 |
| Epirubicin | 0.175 | 243 |
| Carboplatin | 0.254 | 354 |
| Etoposide | 0.342 | 476 |
| Actinomycin D | 0.348 | 485 |
| Cyclophosphamide | 0.504 | 701 |
| Vincristine | 0.703 | 978 |
| Variable (n = 19) | IV |
|---|---|
| Hgb | 1.770 |
| RBC | 0.708 |
| ALP | 0.422 |
| RDW | 0.392 |
| WBC | 0.372 |
| ANC | 0.369 |
| Albumin | 0.328 |
| MCHC | 0.243 |
| PLT | 0.213 |
| Chemotherapy cycles | 0.082 |
| Coadministration of highly toxic chemotherapy drug | 0.061 |
| Cisplatin | 0.028 |
| Vincristine | 0.022 |
| Epirubicin | 0.013 |
| Carboplatin | 0.007 |
| Actinomycin D | 0.005 |
| Etoposide | 0.001 |
| Cyclophosphamide | 0.000 |
| Doxorubicin | 0.000 |
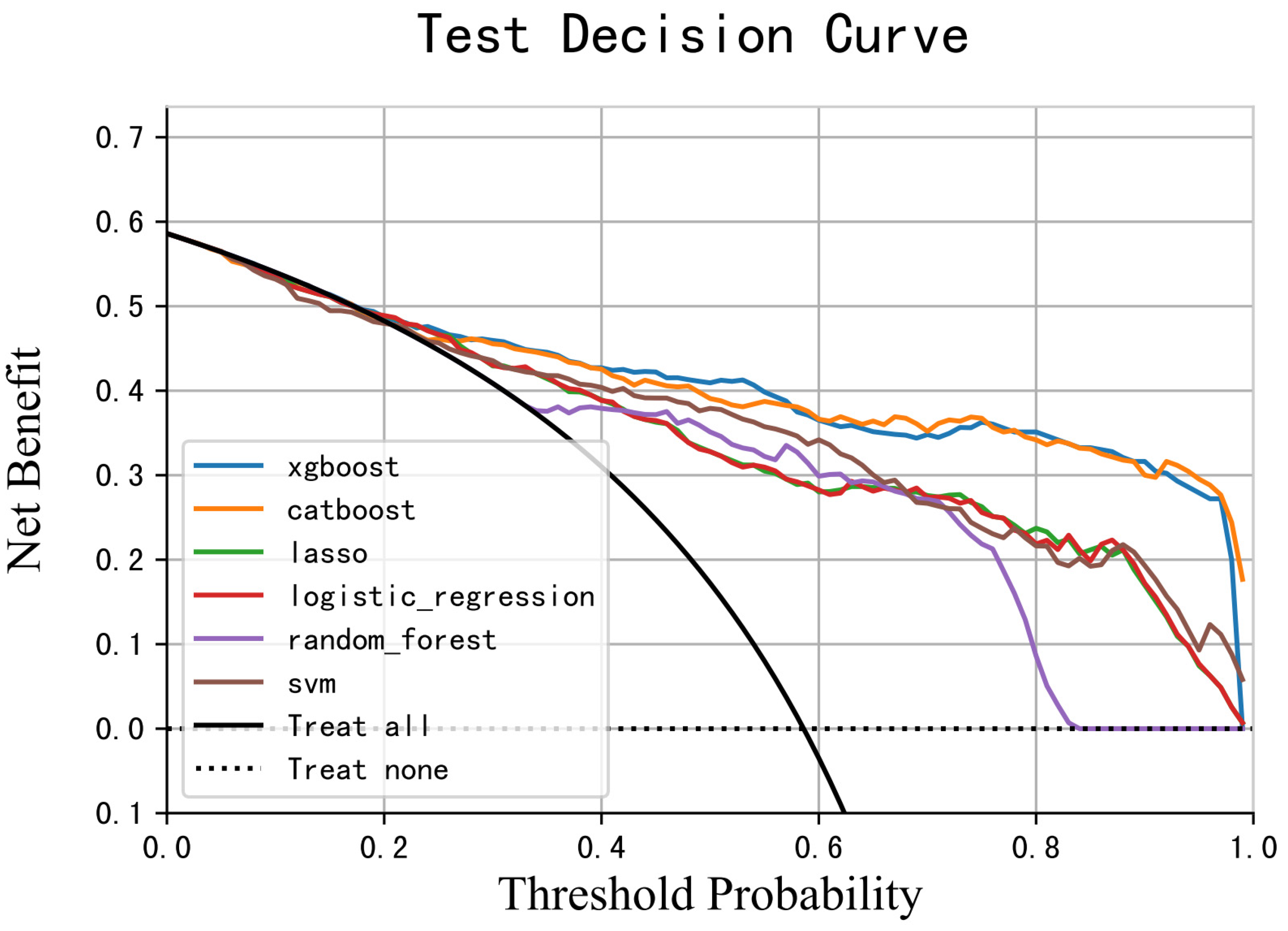
| Model | AUC | PSI | |
|---|---|---|---|
| Training Set | Test Set | ||
| XGB | 0.981 | 0.896 | 0.033 |
| CatBoost | 0.996 | 0.888 | 0.086 |
| RF | 0.842 | 0.856 | 0.015 |
| SVM | 0.930 | 0.849 | 0.066 |
| LR | 0.843 | 0.842 | 0.007 |
| LASSO | 0.843 | 0.842 | 0.007 |
| Model | Best Cutoff | TPR | TNR | ACC | PPV |
|---|---|---|---|---|---|
| XGB | 0.529 | 76.2% | 93.3% | 83.3% | 94.1% |
| RF | 0.569 | 68.3% | 88.2% | 76.5% | 89.1% |
| CatBoost | 0.585 | 75.0% | 90.4% | 81.4% | 91.7% |
| SVM | 0.581 | 75.0% | 84.3% | 78.8% | 87.1% |
| LR | 0.687 | 66.3% | 88.8% | 75.6% | 89.3% |
| LASSO | 0.685 | 66.3% | 88.8% | 75.6% | 89.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, Q.; Lu, P.; Zhang, D.; Hua, Y.; Liu, F.; Liu, X.; Lin, T.; Wei, G.; He, D. Development of a Machine Learning-Based Prediction Model for Chemotherapy-Induced Myelosuppression in Children with Wilms’ Tumor. Cancers 2023, 15, 1078. https://doi.org/10.3390/cancers15041078
Li M, Wang Q, Lu P, Zhang D, Hua Y, Liu F, Liu X, Lin T, Wei G, He D. Development of a Machine Learning-Based Prediction Model for Chemotherapy-Induced Myelosuppression in Children with Wilms’ Tumor. Cancers. 2023; 15(4):1078. https://doi.org/10.3390/cancers15041078
Chicago/Turabian StyleLi, Mujie, Quan Wang, Peng Lu, Deying Zhang, Yi Hua, Feng Liu, Xing Liu, Tao Lin, Guanghui Wei, and Dawei He. 2023. "Development of a Machine Learning-Based Prediction Model for Chemotherapy-Induced Myelosuppression in Children with Wilms’ Tumor" Cancers 15, no. 4: 1078. https://doi.org/10.3390/cancers15041078
APA StyleLi, M., Wang, Q., Lu, P., Zhang, D., Hua, Y., Liu, F., Liu, X., Lin, T., Wei, G., & He, D. (2023). Development of a Machine Learning-Based Prediction Model for Chemotherapy-Induced Myelosuppression in Children with Wilms’ Tumor. Cancers, 15(4), 1078. https://doi.org/10.3390/cancers15041078







