Prognostic Relevance of Multi-Antigenic Myeloma-Specific T-Cell Assay in Patients with Monoclonal Gammopathies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Multi-Antigenic MM-Specific Antigen Stimulation
2.3. Enzyme-Linked Immunospot (ELISpot) Assay
2.4. Cytokine Secretion Assay (CSA)
2.5. 51Chromium-Release Assay
2.6. Statistical Analysis
3. Results
3.1. Screening and Quantitation of MM-Specific T-Cell Responses in MGUS/SMM Patients
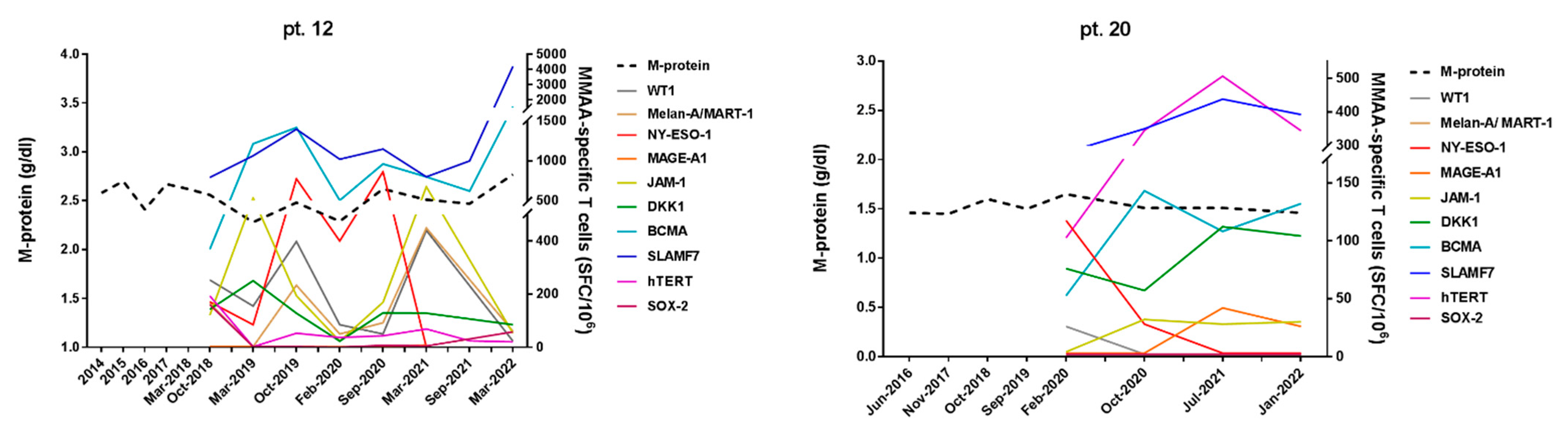
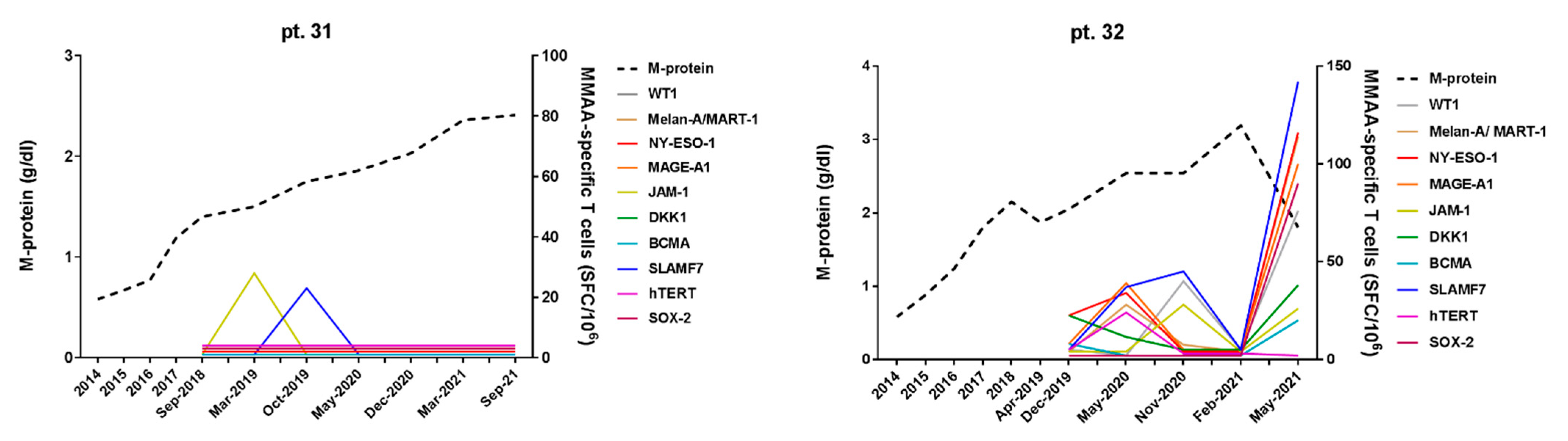
3.2. Correlation of MM-Specific T-Cell Immunity (MaMs Test) with Clinical Course in MGUS/SMM Patients
3.3. Phenotypic and Functional Characterization of Myeloma–Specific T Cells
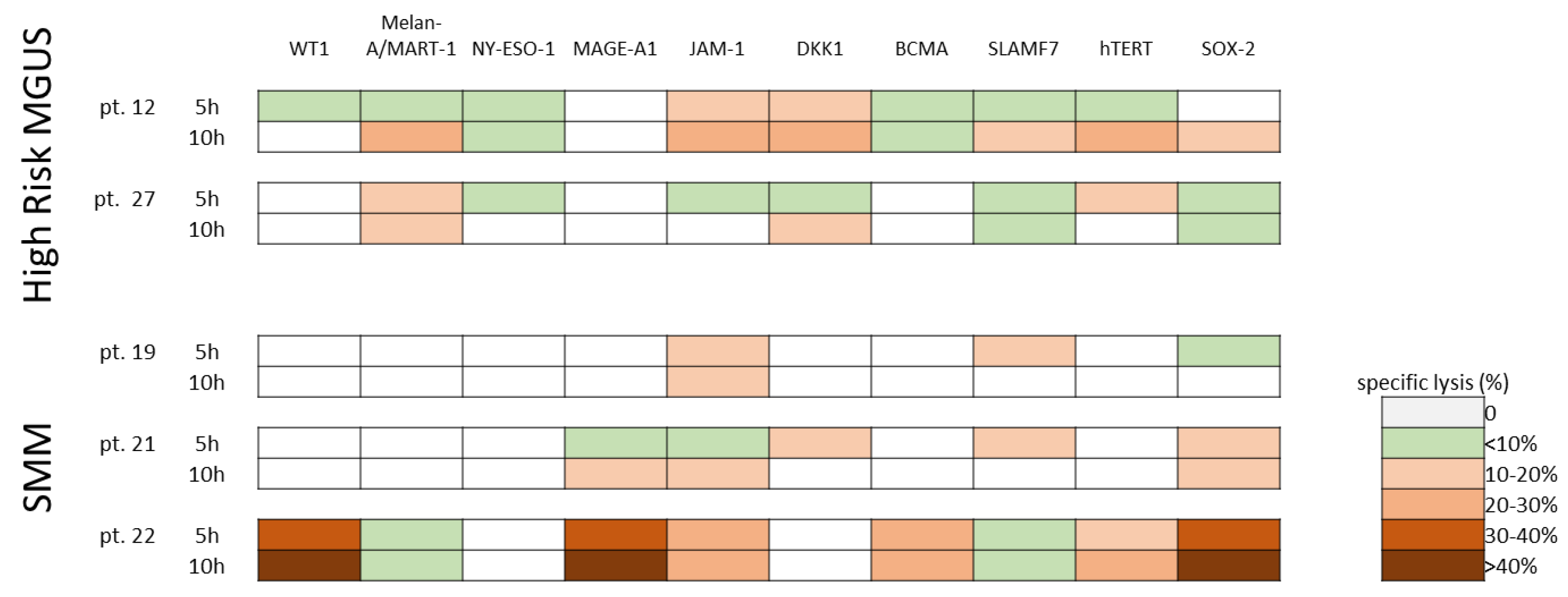
3.4. Cytotoxic Activity of Ex-Vivo Expanded Myeloma-Specific CTLs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van de Donk, N.W.C.J.; Pawlyn, C.; Yong, K.L. Multiple Myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Mouhieddine, T.H.; Weeks, L.D.; Ghobrial, I.M. Monoclonal Gammopathy of Undetermined Significance. Blood 2019, 133, 2484–2494. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Landgren, O.; Mateos, M.V. Smoldering Multiple Myeloma. Blood 2015, 125, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Engelhardt, M.; Caers, J.; Bolli, N.; Kaiser, M.; van de Donk, N.; Terpos, E.; Broijl, A.; de Larrea, C.F.; Gay, F.; et al. 2021 European Myeloma Network Review and Consensus Statement on Smoldering Multiple Myeloma: How to Distinguish (and Manage) Dr. Jekyll and Mr. Hyde. Haematologica 2021, 106, 2799–2812. [Google Scholar] [CrossRef] [PubMed]
- Van de Donk, N.W.J.C.; Mutis, T.; Poddighe, P.J.; Lokhorst, H.M.; Zweegman, S. Diagnosis, risk stratification and management of monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Int. J. Lab. Hematol. 2016, 38, 110–122. [Google Scholar] [CrossRef]
- Dutta, A.K.; Alberge, J.-B.; Sklavenitis-Pistofidis, R.; Lightbody, E.D.; Getz, G.; Ghobrial, I.M. Single-Cell Profiling of Tumour Evolution in Multiple Myeloma—Opportunities for Precision Medicine. Nat. Rev. Clin. Oncol. 2022, 19, 223–236. [Google Scholar] [CrossRef]
- Hagen, P.; Zhang, J.; Barton, K. High-Risk Disease in Newly Diagnosed Multiple Myeloma: Beyond the R-ISS and IMWG Definitions. Blood Cancer J. 2022, 12, 83. [Google Scholar] [CrossRef]
- Lannes, R.; Samur, M.; Perrot, A.; Mazzotti, C.; Divoux, M.; Cazaubiel, T.; Leleu, X.; Schavgoulidze, A.; Chretien, M.-L.; Manier, S.; et al. In Multiple Myeloma, High-Risk Secondary Genetic Events Observed at Relapse Are Present From Diagnosis in Tiny, Undetectable Subclonal Populations. J. Clin. Oncol. 2022. [Google Scholar] [CrossRef]
- Pawlyn, C.; Morgan, G.J. Evolutionary Biology of High-Risk Multiple Myeloma. Nat. Rev. 2017, 17, 543–556. [Google Scholar] [CrossRef]
- Ho, M.; Goh, C.Y.; Patel, A.; Staunton, S.; O’Connor, R.; Godeau, M.; Bianchi, G. Role of the Bone Marrow Milieu in Multiple Myeloma Progression and Therapeutic Resistance. Clin. Lymphoma Myeloma Leuk. 2020, 20, e752–e768. [Google Scholar] [CrossRef]
- Dufva, O.; Pölönen, P.; Brück, O.; Keränen, M.A.I.; Klievink, J.; Mehtonen, J.; Huuhtanen, J.; Kumar, A.; Malani, D.; Siitonen, S.; et al. Immunogenomic Landscape of Hematological Malignancies. Cancer Cell 2020, 38, 380–399.e13. [Google Scholar] [CrossRef] [PubMed]
- Bedognetti, D. A Multi-Layer Molecular Fresco of the Immune Diversity across Hematologic Malignancies. Cancer Cell 2020, 38, 313–316. [Google Scholar] [CrossRef]
- Weng, J.; Neelapu, S.S.; Woo, A.F.; Kwak, L.W. Identification of Human Idiotype-Specific T Cells in Lymphoma and Myeloma. In Cancer Immunology and Immunotherapy; Dranoff, G., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 193–210. ISBN 978-3-642-14136-2. [Google Scholar]
- Bogen, B.; Ruffini, P.A.; Corthay, A.; Fredriksen, A.B.; Frøyland, M.; Lundin, K.; Røsjø, E.; Thompson, K.; Massaia, M. Idiotype-Specific Immunotherapy in Multiple Myeloma: Suggestions for Future Directions of Research. Haematologica 2006, 91, 941–948. [Google Scholar] [PubMed]
- Wen, Y.-J.; Barlogie, B.; Yi, Q. Idiotype-Specific Cytotoxic T Lymphocytes in Multiple Myeloma: Evidence for Their Capacity to Lyse Autologous Primary Tumor Cells. Blood 2001, 97, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Lagreca, I.; Riva, G.; Nasillo, V.; Barozzi, P.; Castelli, I.; Basso, S.; Bettelli, F.; Giusti, D.; Cuoghi, A.; Bresciani, P.; et al. The Role of T Cell Immunity in Monoclonal Gammopathy and Multiple Myeloma: From Immunopathogenesis to Novel Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 5242. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Krasovsky, J.; Osman, K.; Geller, M.D. Vigorous Premalignancy-Specific Effector T Cell Response in Bone Marrow of Patients with Monoclonal Gammopathy. J. Exp. Med. 2003, 198, 1753–1757. [Google Scholar] [CrossRef]
- Goodyear, O.C.; Pratt, G.; McLarnon, A.; Cook, M.; Piper, K.; Moss, P. Differential Pattern of CD4+ and CD8+ T-Cell Immunity to MAGE-A1/A2/A3 in Patients with MGUS and Multiple Myeloma. Blood 2008, 112, 3362–3372. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Sexton, R.; Das, R. Prospective Analysis of Antigen-Specific Immunity, Stem-Cell Antigens, and Immune Checkpoints in Monoclonal Gammopathy. Blood 2015, 126, 2475–2478. [Google Scholar] [CrossRef]
- Tyler, E.M.; Jungbluth, A.A.; O’Reilly, R.J.; Koehne, G. WT1-Specific T-Cell Responses in High-Risk Multiple Myeloma Patients Undergoing Allogeneic T Cell-Depleted Hematopoietic Stem Cell Transplantation and Donor Lymphocyte Infusions. Blood 2013, 121, 308–317. [Google Scholar] [CrossRef]
- Bellucci, R.; Alyea, E.P.; Chiaretti, S.; Wu, C.J.; Zorn, E.; Weller, E.; Wu, B.; Canning, C.; Schlossman, R.; Munshi, N.C.; et al. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood 2005, 105, 3945–3950. [Google Scholar] [CrossRef]
- Minnie, S.A.; Hill, G.R. Immunotherapy of Multiple Myeloma. J. Clin. Investig. 2020, 130, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Ghobrial, I.M. Autologous Graft versus Myeloma: It’s Not a Myth. J. Clin. Investig. 2019, 129, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, S.; Minnie, S.A.; Smith, D.; Gartlan, K.H.; Watkins, T.S.; Markey, K.A.; Mukhopadhyay, P.; Guillerey, C.; Kuns, R.D.; Locke, K.R.; et al. Bone Marrow Transplantation Generates T Cell-Dependent Control of Myeloma in Mice. J. Clin. Investig. 2019, 129, 106–121. [Google Scholar] [CrossRef]
- Christensen, O.; Lupu, A.; Schmidt, S.; Condomines, M.; Belle, S.; Maier, A.; Hose, D.; Neuber, B.; Moos, M.; Kleist, C.; et al. Melan-A/MART1 Analog Peptide Triggers Anti-Myeloma T-Cells through Crossreactivity with HM1.24. J. Immunother. 2009, 32, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Leone, P.; Frassanito, M.A.; Brunetti, C.; Perosa, F.; Ferrone, S.; Dammacco, F. Alterations in the Antigen Processing-Presenting Machinery of Transformed Plasma Cells Are Associated with Reduced Recognition by CD8+ T Cells and Characterize the Progression of MGUS to Multiple Myeloma. Blood 2010, 115, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- van Rhee, F.; Szmania, S.M.; Zhan, F.; Gupta, S.K.; Pomtree, M.; Lin, P.; Batchu, R.B.; Moreno, A.; Spagnoli, G.; Shaughnessy, J.; et al. NY-ESO-1 Is Highly Expressed in Poor-Prognosis Multiple Myeloma and Induces Spontaneous Humoral and Cellular Immune Responses. Blood 2005, 105, 3939–3944. [Google Scholar] [CrossRef]
- Ocadlikova, D.; Kryukov, F.; Mollova, K.; Kovarova, L.; Buresdova, I.; Matejkova, E.; Penka, M.; Buchler, T.; Hajek, R.; Michalek, J. Generation of Myeloma-Specific T Cells Using Dendritic Cells Loaded with MUC1- and HTERT- Drived Nonapeptides or Myeloma Cell Apoptotic Bodies. Neoplasma 2010, 57, 455–464. [Google Scholar] [CrossRef]
- Spisek, R.; Kukreja, A.; Chen, L.-C.; Matthews, P.; Mazumder, A.; Vesole, D.; Jagannath, S.; Zebroski, H.A.; Simpson, A.J.G.; Ritter, G.; et al. Frequent and Specific Immunity to the Embryonal Stem Cell-Associated Antigen SOX2 in Patients with Monoclonal Gammopathy. J. Exp. Med. 2007, 204, 831–840. [Google Scholar] [CrossRef]
- Qian, J.; Xie, J.; Hong, S.; Yang, J.; Zhang, L.; Han, X.; Wang, M.; Zhan, F.; Shaughnessy, J.D.; Epstein, J.; et al. Dickkopf-1 (DKK1) Is a Widely Expressed and Potent Tumor-Associated Antigen in Multiple Myeloma. Blood 2007, 110, 1587–1594. [Google Scholar] [CrossRef]
- Solimando, A.G.; Brandl, A.; Mattenheimer, K.; Graf, C.; Ritz, M.; Ruckdeschel, A.; Stühmer, T.; Mokhtari, Z.; Rudelius, M.; Dotterweich, J.; et al. JAM-A as a Prognostic Factor and New Therapeutic Target in Multiple Myeloma. Leukemia 2018, 32, 736–743. [Google Scholar] [CrossRef]
- Bae, J.; Prabhala, R.; Voskertchian, A.; Brown, A.; Maguire, C.; Richardson, P.; Dranoff, G.; Anderson, K.C.; Munshi, N.C. A Multiepitope of XBP1, CD138 and CS1 Peptides Induces Myeloma-Specific Cytotoxic T Lymphocytes in T Cells of Smoldering Myeloma Patients. Leukemia 2015, 29, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Samur, M.; Richardson, P.; Munshi, N.C.; Anderson, K.C. Selective Targeting of Multiple Myeloma by B Cell Maturation Antigen (BCMA)-Specific Central Memory CD8+ Cytotoxic T Lymphocytes: Immunotherapeutic Application in Vaccination and Adoptive Immunotherapy. Leukemia 2019, 33, 2208–2226. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Luppi, M.; Barozzi, P.; Quadrelli, C.; Basso, S.; Vallerini, D.; Zanetti, E.; Morselli, M.; Forghieri, F.; Maccaferri, M.; et al. Emergence of BCR-ABL–Specific Cytotoxic T Cells in the Bone Marrow of Patients with Ph+ Acute Lymphoblastic Leukemia during Long-Term Imatinib Mesylate Treatment. Blood 2010, 115, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Riva, G.; Lagreca, I.; Barozzi, P.; Vallerini, D.; Morselli, M.; Paolini, A.; Bresciani, P.; Colaci, E.; Maccaferri, M.; et al. Characterization and Dynamics of Specific T Cells against Nucleophosmin-1 (NPM1)-Mutated Peptides in Patients with NPM1-Mutated Acute Myeloid Leukemia. Oncotarget 2019, 10, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Janetzki, S.; Price, L.; Schroeder, H.; Britten, C.M.; Welters, M.J.P.; Hoos, A. Guidelines for the Automated Evaluation of Elispot Assays. Nat. Protoc. 2015, 10, 1098–1115. [Google Scholar] [CrossRef]
- Ruiz-Heredia, Y.; Ortiz-Ruiz, A.; Samur, M.K.; Garrido, V.; Rufian, L.; Sanchez, R.; Aguilar-Garrido, P.; Barrio, S.; Martín, M.A.; Bolli, N.; et al. Pathogenetic and Prognostic Implications of Increased Mitochondrial Content in Multiple Myeloma. Cancers 2021, 13, 3189. [Google Scholar] [CrossRef]
- Dhodapkar, M.V. The Immune System in Multiple Myeloma and Precursor States: Lessons and Implications for Immunotherapy and Interception. Am. J. Hematol. 2022. early view. [Google Scholar] [CrossRef]
- Riva, G.; Nasillo, V.; Ottomano, A.M.; Bergonzini, G.; Paolini, A.; Forghieri, F.; Lusenti, B.; Barozzi, P.; Lagreca, I.; Fiorcari, S.; et al. Multiparametric Flow Cytometry for MRD Monitoring in Hematologic Malignancies: Clinical Applications and New Challenges. Cancers 2021, 13, 4582. [Google Scholar] [CrossRef]
- Manier, S.; Ingegnere, T.; Escure, G.; Prodhomme, C.; Nudel, M.; Mitra, S.; Facon, T. Current State and Next-Generation CAR-T Cells in Multiple Myeloma. Blood Rev. 2022, 54, 100929. [Google Scholar] [CrossRef]
- Lakshman, A.; Kumar, S.K. Chimeric Antigen Receptor T-Cells, Bispecific Antibodies, and Antibody-Drug Conjugates for Multiple Myeloma: An Update. Am. J. Hematol. 2022, 97, 99–118. [Google Scholar] [CrossRef]
- Landgren, O.; Kyle, R.A.; Rajkumar, S.V. From Myeloma Precursor Disease to Multiple Myeloma: New Diagnostic Concepts and Opportunities for Early Intervention. Clin. Cancer Res. 2011, 17, 1243–1252. [Google Scholar] [CrossRef]
- Ho, M.; Patel, A.; Go, C.Y.; Moscvin, M.; Zhang, L.; Bianchi, G. Changing Paradigms in Diagnosis and Treatment of Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering Multiple Myeloma (SMM). Leukemia 2020, 34, 3111–3125. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Hernandez, M.T.; Giraldo, P.; de la Rubia, J.; de Arriba, F.; López Corral, L.; Rosiñol, L.; Paiva, B.; Palomera, L.; Bargay, J.; et al. Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma. N. Engl. J. Med. 2013, 369, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Hernandez, M.T.; Giraldo, P.; de la Rubia, J.; de Arriba, F.; Corral, L.L.; Rosiñol, L.; Paiva, B.; Palomera, L.; Bargay, J.; et al. Lenalidomide plus Dexamethasone versus Observation in Patients with High-Risk Smouldering Multiple Myeloma (QuiRedex): Long-Term Follow-up of a Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2016, 17, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Hernandez, M.T.; Salvador, C.; de la Rubia, J.; de Arriba, F.; López Corral, L.; Rosiñol, L.; Paiva, B.; Palomera, L.; Bargay, J.; et al. Over Ten Years Of F/U For Phase 3 Trial In Smoldering Myeloma At High Risk Of Progression To Myeloma: Sustained Ttp And Os Benefit With Rd Versus No Treatment. In Proceedings of the 25th EHA Congress, European Hematology Association, Virtual, The Hague, The Netherlands, 11–21 June 2020. [Google Scholar]
- Lonial, S.; Jacobus, S.; Fonseca, R.; Weiss, M.; Kumar, S.; Orlowski, R.Z.; Kaufman, J.L.; Yacoub, A.M.; Buadi, F.K.; O’Brien, T.; et al. Randomized Trial of Lenalidomide Versus Observation in Smoldering Multiple Myeloma. J. Clin. Oncol. 2020, 38, 1126–1137. [Google Scholar] [CrossRef]
- Paiva, B.; Mateos, M.V.; Sanchez-Abarca, L.I.; Puig, N.; Vidriales, M.B.; López Corral, L.; Corchete, R.A.; Hernandez, M.T.; Bargay, J.; de Arriba, F.; et al. Immune Status of High-Risk Smoldering Multiple Myeloma Patients and Its Therapeutic Modulation under LenDex: A Longitudinal Analysis. Blood 2016, 127, 1151–1162. [Google Scholar] [CrossRef]
- Comoli, P.; Basso, S.; Riva, G.; Barozzi, P.; Guido, I.; Gurrado, A.; Quartuccio, G.; Rubert, L.; Lagreca, I.; Vallerini, D.; et al. BCR-ABL–Specific T-Cell Therapy in Ph1 ALL Patients on Tyrosine-Kinase Inhibitors. Blood 2017, 129, 582–586. [Google Scholar] [CrossRef]
- Zitvogel, L.; Rusakiewicz, S.; Routy, B.; Ayyoub, M.; Kroemer, G. Immunological Off-Target Effects of Imatinib. Nat. Rev. Clin. Oncol. 2016, 13, 431–446. [Google Scholar] [CrossRef]
- Nasillo, V.; Riva, G.; Paolini, A.; Forghieri, F.; Roncati, L.; Lusenti, B.; Maccaferri, M.; Messerotti, A.; Pioli, V.; Gilioli, A.; et al. Inflammatory Microenvironment and Specific T Cells in Myeloproliferative Neoplasms: Immunopathogenesis and Novel Immunotherapies. Int. J. Mol. Sci. 2021, 22, 1906. [Google Scholar] [CrossRef]
- Forghieri, F.; Riva, G.; Lagreca, I.; Barozzi, P.; Bettelli, F.; Paolini, A.; Nasillo, V.; Lusenti, B.; Pioli, V.; Giusti, D.; et al. Neoantigen-Specific T-Cell Immune Responses: The Paradigm of NPM1-Mutated Acute Myeloid Leukemia. Int. J. Mol. Sci. 2021, 22, 9159. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Number of patients (Sex) | 33 (15 F, 18 M) |
| Age at enrollment (years), median (range) | 65 (26–85) |
| MGUS patients, total number | 22 |
| Low Risk | 4 |
| Intermediate-Low Risk | 10 |
| Intermediate-High Risk | 8 |
| SMM patients, total number | 11 |
| Low Risk | 4 |
| Intermediate Risk | 7 |
| Follow-up (months), median (range) | 28 (6–44) |
| Number of time-points/patient, mean (range) | 4, 6 (2–8) |
| Tested samples, total number | 152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagreca, I.; Nasillo, V.; Barozzi, P.; Castelli, I.; Basso, S.; Castellano, S.; Paolini, A.; Maccaferri, M.; Colaci, E.; Vallerini, D.; et al. Prognostic Relevance of Multi-Antigenic Myeloma-Specific T-Cell Assay in Patients with Monoclonal Gammopathies. Cancers 2023, 15, 972. https://doi.org/10.3390/cancers15030972
Lagreca I, Nasillo V, Barozzi P, Castelli I, Basso S, Castellano S, Paolini A, Maccaferri M, Colaci E, Vallerini D, et al. Prognostic Relevance of Multi-Antigenic Myeloma-Specific T-Cell Assay in Patients with Monoclonal Gammopathies. Cancers. 2023; 15(3):972. https://doi.org/10.3390/cancers15030972
Chicago/Turabian StyleLagreca, Ivana, Vincenzo Nasillo, Patrizia Barozzi, Ilaria Castelli, Sabrina Basso, Sara Castellano, Ambra Paolini, Monica Maccaferri, Elisabetta Colaci, Daniela Vallerini, and et al. 2023. "Prognostic Relevance of Multi-Antigenic Myeloma-Specific T-Cell Assay in Patients with Monoclonal Gammopathies" Cancers 15, no. 3: 972. https://doi.org/10.3390/cancers15030972
APA StyleLagreca, I., Nasillo, V., Barozzi, P., Castelli, I., Basso, S., Castellano, S., Paolini, A., Maccaferri, M., Colaci, E., Vallerini, D., Natali, P., Debbia, D., Pirotti, T., Ottomano, A. M., Maffei, R., Bettelli, F., Giusti, D., Messerotti, A., Gilioli, A., ... Riva, G. (2023). Prognostic Relevance of Multi-Antigenic Myeloma-Specific T-Cell Assay in Patients with Monoclonal Gammopathies. Cancers, 15(3), 972. https://doi.org/10.3390/cancers15030972









