Amino Acid-Metabolizing Enzymes in Advanced High-Grade Serous Ovarian Cancer Patients: Value of Ascites as Biomarker Source and Role for IL4I1 and IDO1
Abstract
Simple Summary
Abstract
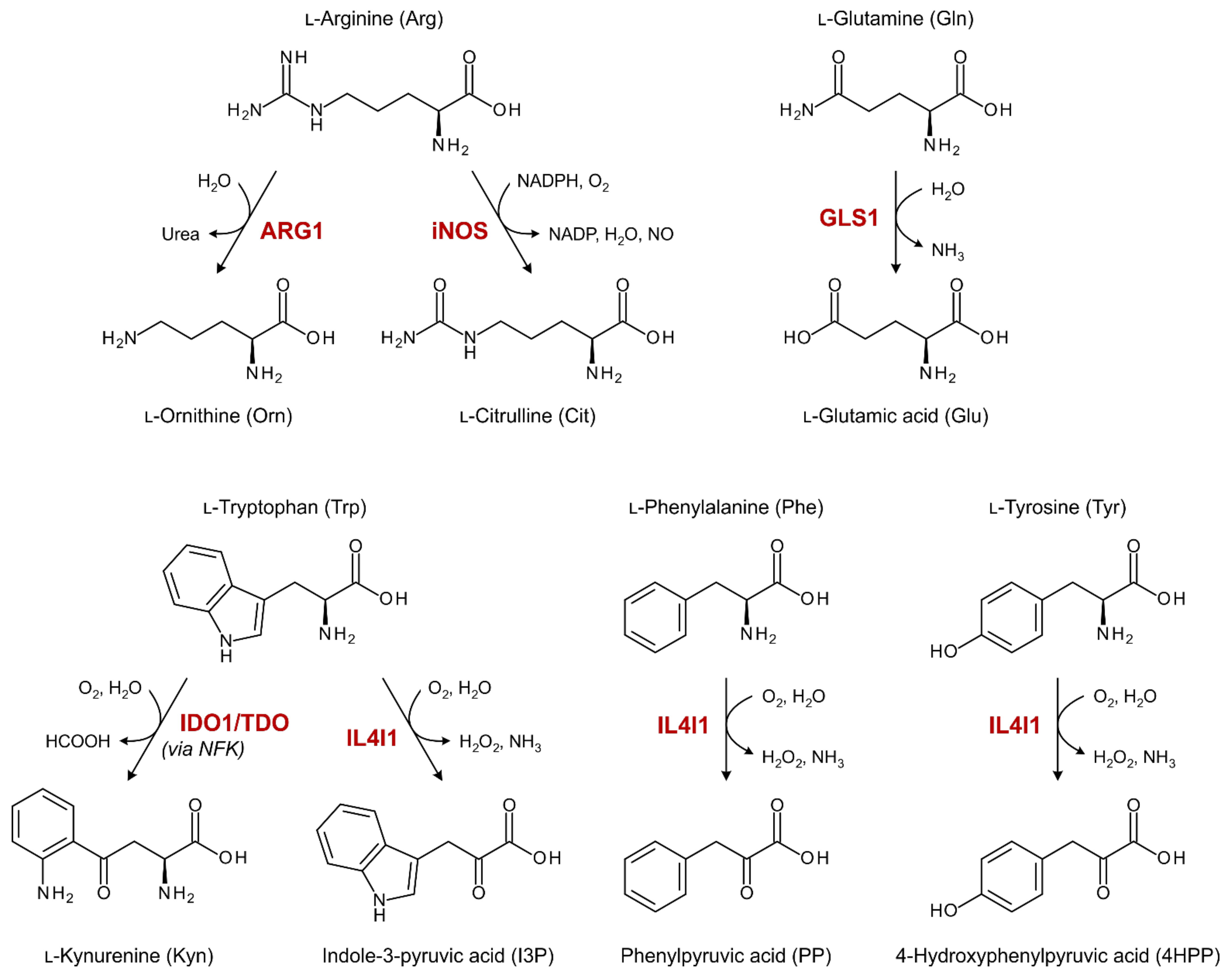
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Patient and Healthy Control Samples
2.3. Standard and Sample Preparation for LC-MS/MS Analysis
2.4. Liquid Chromatography
2.5. Mass Spectrometry
2.6. ELISA
2.7. Statistical Analyses
3. Results
3.1. Patient Characteristics
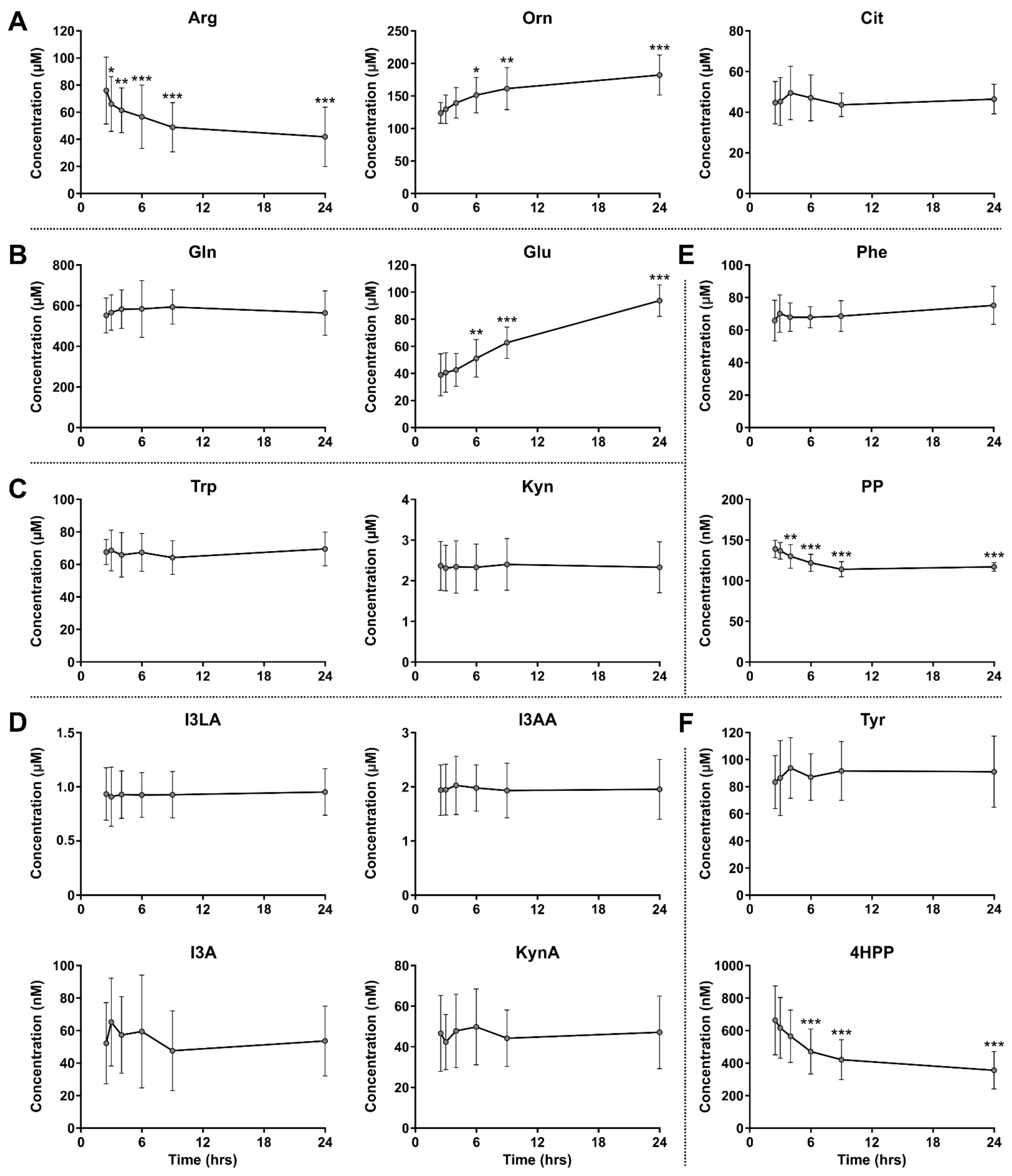
3.2. Stability of Amino Acids and Metabolites in Blood Samples
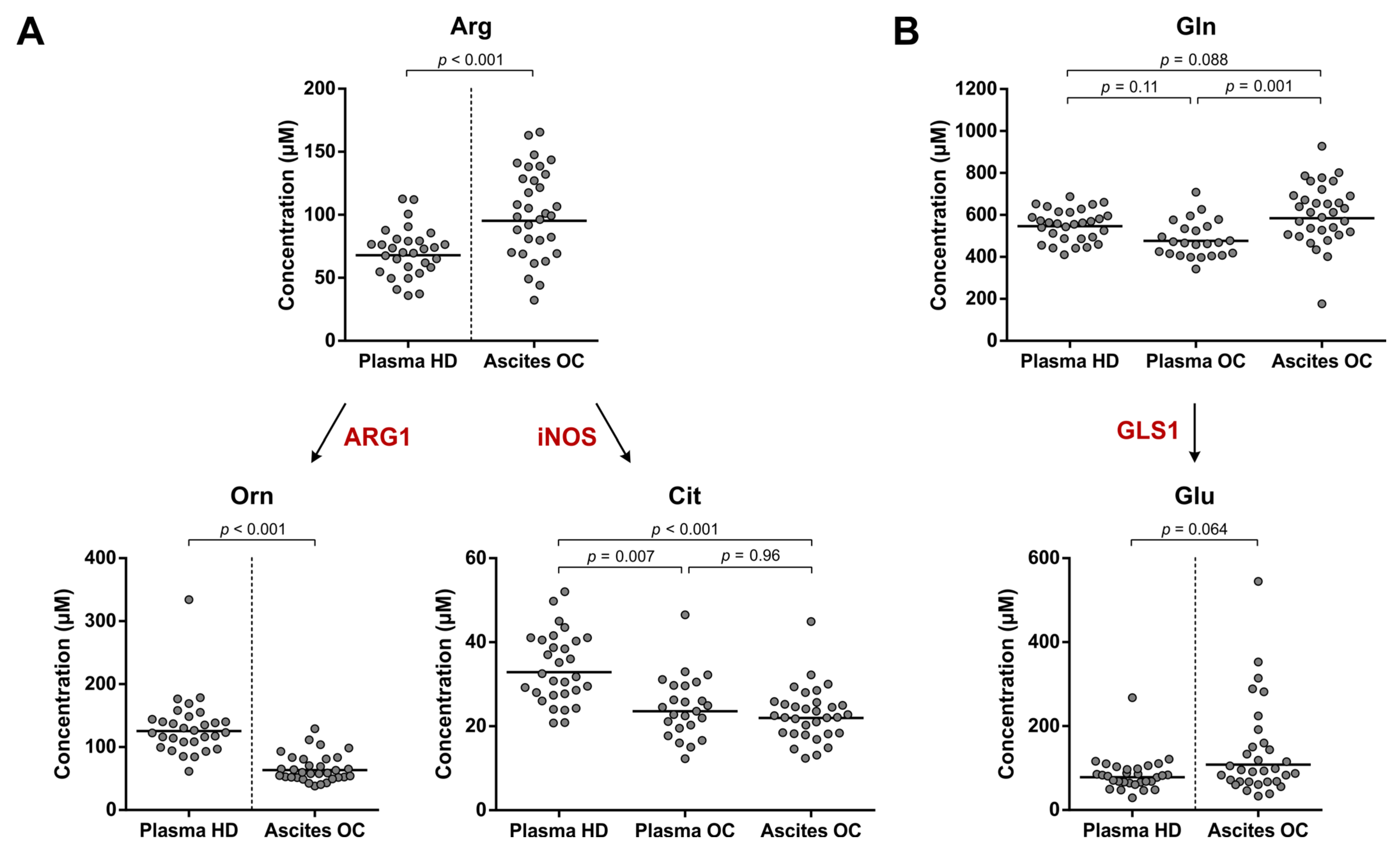
3.3. No Indication for Enhanced ARG1, iNOS or GLS1 Activity in Ovarian Cancer Patients
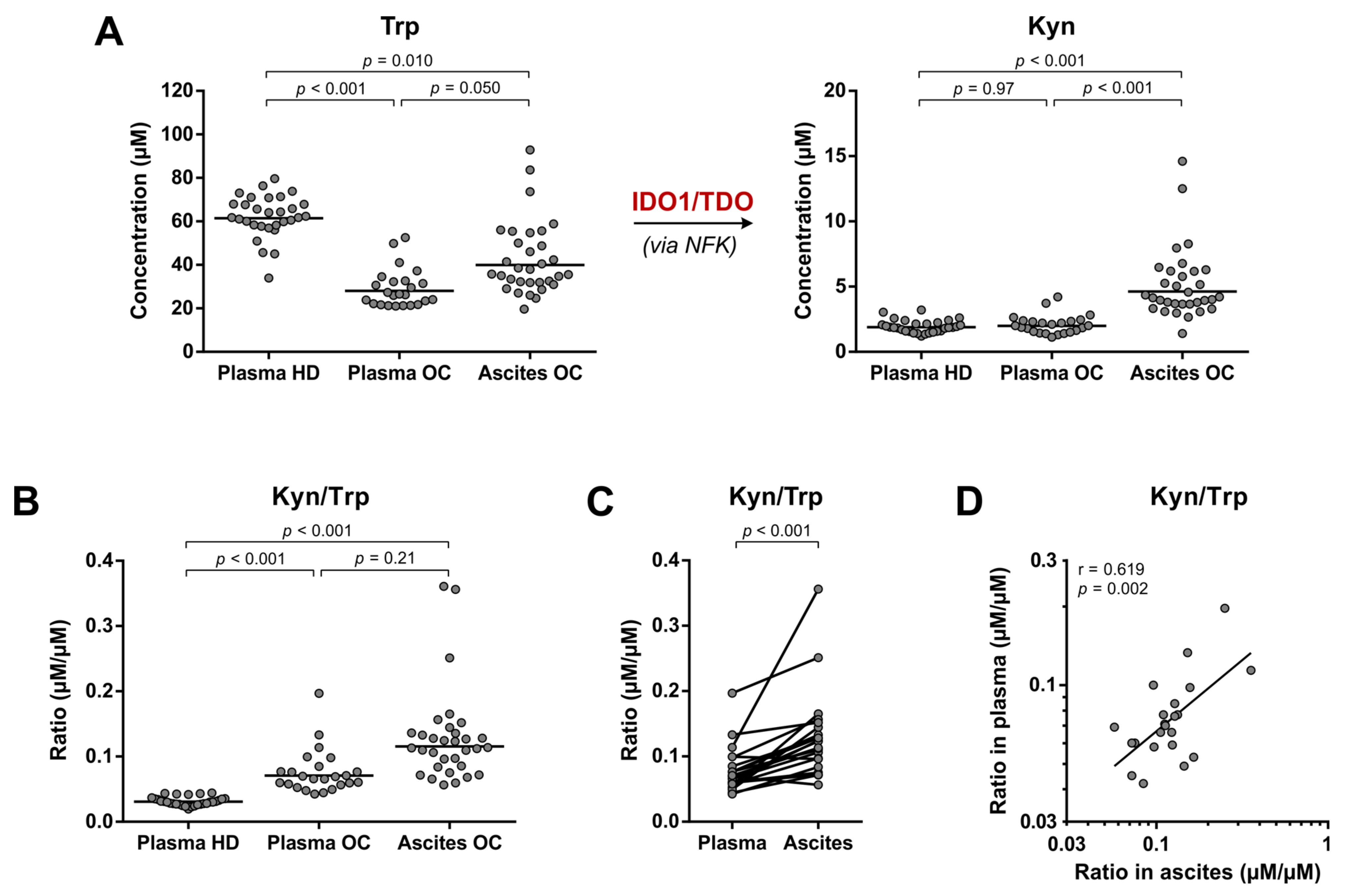
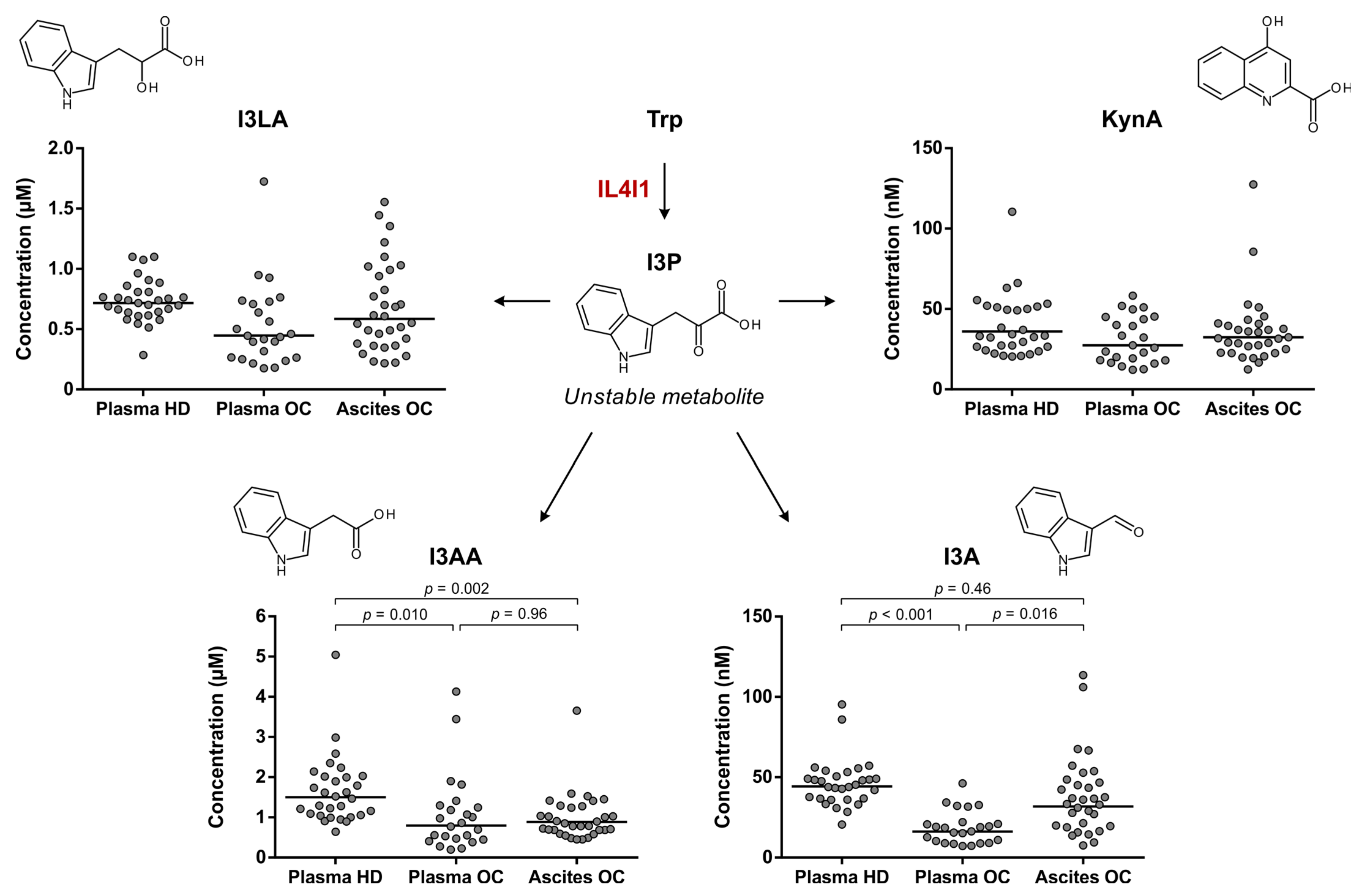
3.4. Elevated Trp Metabolism Is Dominated by IDO1/TDO—Rather Than IL4I1—Activity
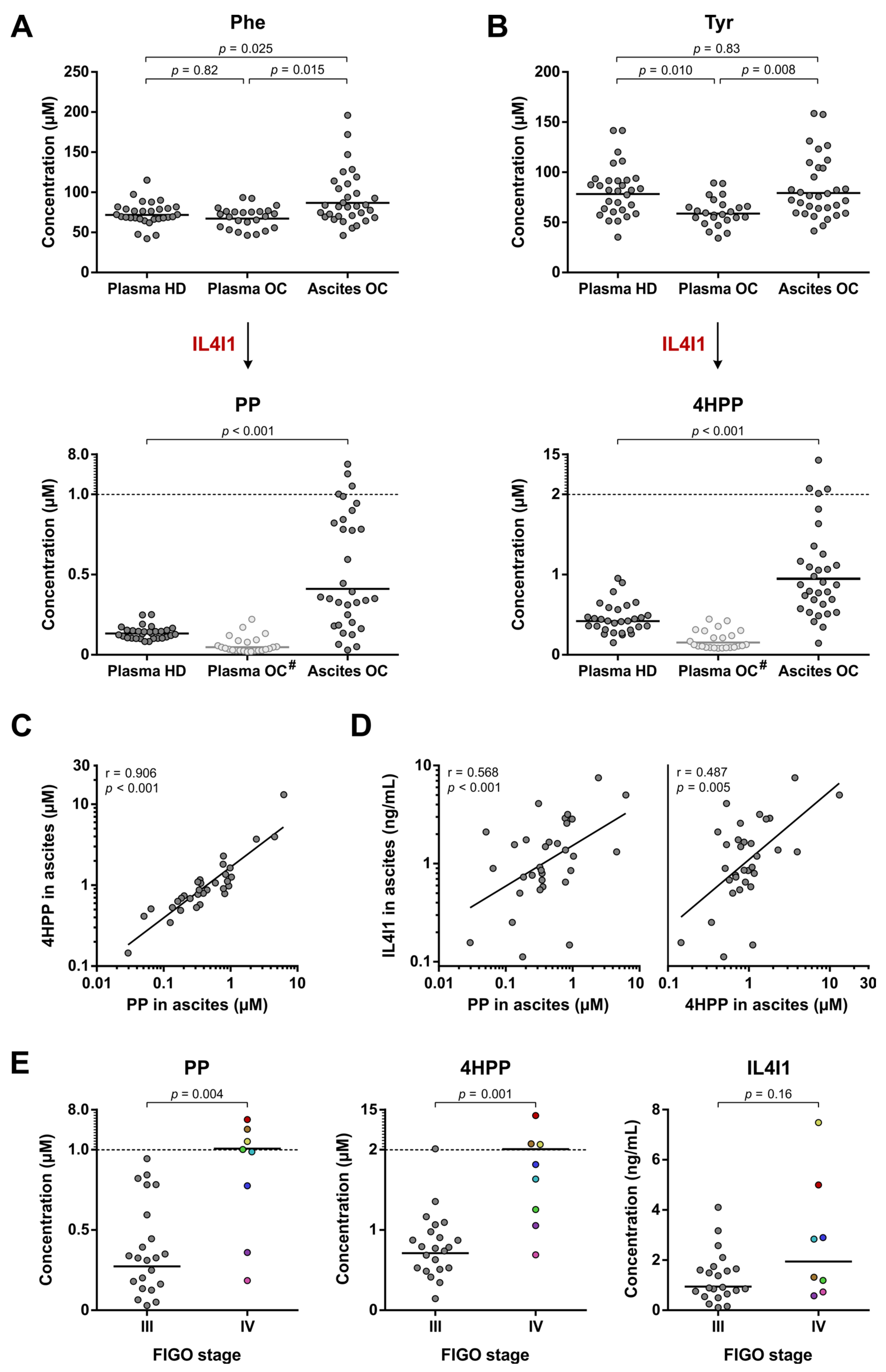
3.5. Enhanced IL4I1-Mediated Phe and Tyr Metabolism Correlates with Disease Stage
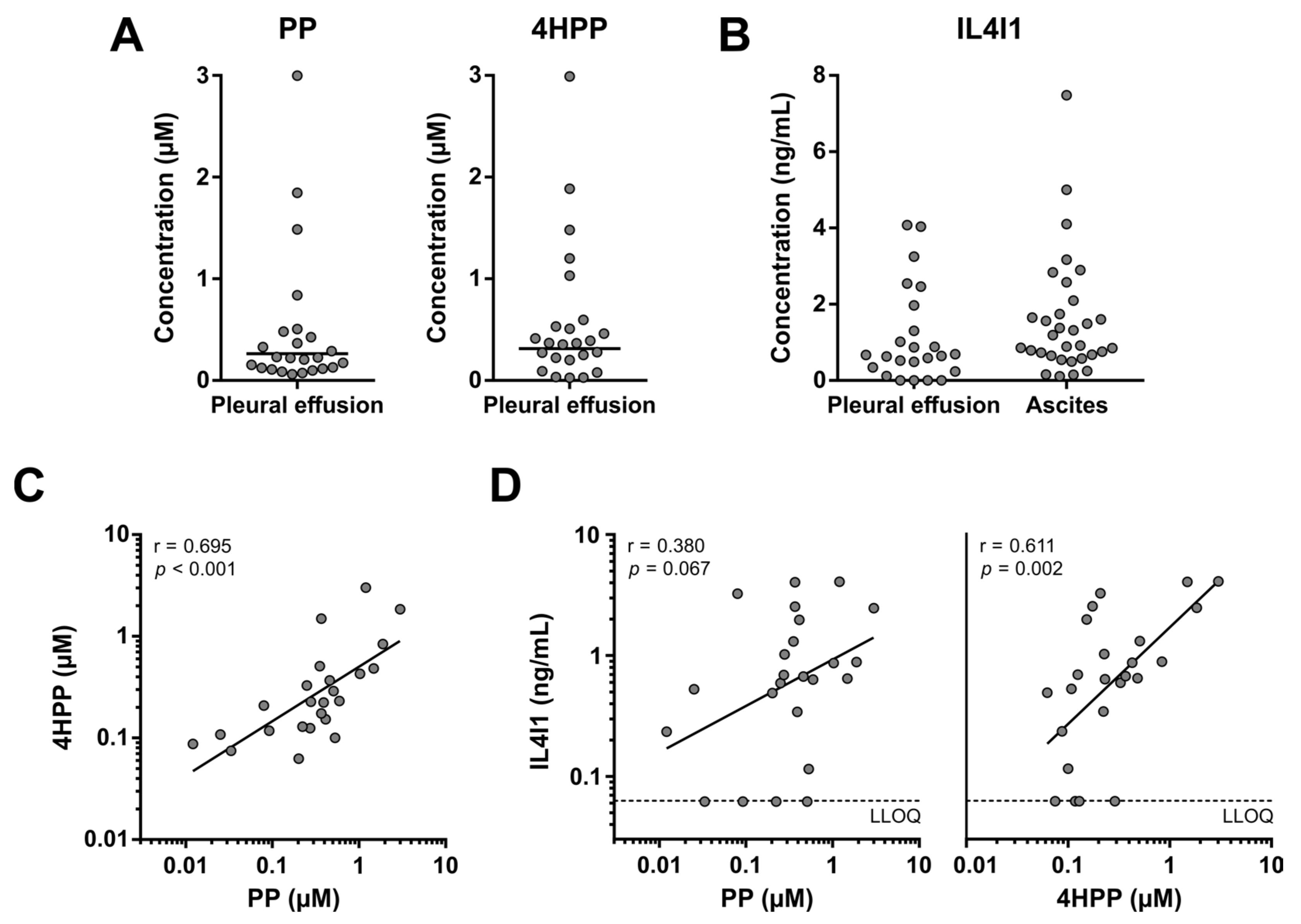
3.6. Enhanced Phe and Tyr Metabolism by IL4I1 in Pleural Effusions of Lung Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Karakasis, K.; Madariaga, A.; Oza, A.M. Overcoming Platinum and PARP-Inhibitor Resistance in Ovarian Cancer. Cancers 2020, 12, 1607. [Google Scholar] [CrossRef]
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.L.; Secord, A.A.; Monk, B. The Role of Immune Checkpoint Inhibition in the Treatment of Ovarian Cancer. Gynecol. Oncol. Res. Pract. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Galpin, K.J.C.; McCloskey, C.W.; Vanderhyden, B.C. The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers 2018, 10, 242. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Fan, N.; Zhou, C.; Li, D.; Macvicar, T.; Dong, Q.; Bruns, C.J.; Zhao, Y. Targeting Glutaminolysis: New Perspectives to Understand Cancer Development and Novel Strategies for Potential Target Therapies. Front. Oncol. 2020, 10, 589508. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Sosnowska, A.; Matryba, P.; Rydzynska, Z.; Jasinski, M.; Nowis, D.; Golab, J. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front. Immunol. 2020, 11, 938. [Google Scholar] [CrossRef]
- Peñarando, J.; Aranda, E.; Rodríguez-Ariza, A. Immunomodulatory Roles of Nitric Oxide in Cancer: Tumor Microenvironment Says “NO” to Antitumor Immune Response. Transl. Res. 2019, 210, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Somarribas Patterson, L.F.; Mohapatra, S.R.; Dewi, D.L.; Sadik, A.; Platten, M.; Trump, S. The Therapeutic Potential of Targeting Tryptophan Catabolism in Cancer. Br. J. Cancer 2020, 122, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An Endogenous Tumour-Promoting Ligand of the Human Aryl Hydrocarbon Receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Dvorakova, T.; Stroobant, V.; Bouzin, C.; Daumerie, A.; Solvay, M.; Klaessens, S.; Letellier, M.-C.; Renauld, J.-C.; van Baren, N.; et al. Tryptophan 2,3-Dioxygenase Expression Identified in Human Hepatocellular Carcinoma Cells and in Intratumoral Pericytes of Most Cancers. Cancer Immunol. Res. 2020, 8, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Carbonnelle-Puscian, A.; Copie-Bergman, C.; Baia, M.; Martin-Garcia, N.; Allory, Y.; Haioun, C.; Crémades, A.; Abd-Alsamad, I.; Farcet, J.-P.; Gaulard, P.; et al. The Novel Immunosuppressive Enzyme IL4I1 Is Expressed by Neoplastic Cells of Several B-Cell Lymphomas and by Tumor-Associated Macrophages. Leukemia 2009, 23, 952–960. [Google Scholar] [CrossRef]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint That Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [CrossRef]
- Boulland, M.-L.; Marquet, J.; Molinier-Frenkel, V.; Möller, P.; Guiter, C.; Lasoudris, F.; Copie-Bergman, C.; Baia, M.; Gaulard, P.; Leroy, K.; et al. Human IL4I1 Is a Secreted L-Phenylalanine Oxidase Expressed by Mature Dendritic Cells That Inhibits T-Lymphocyte Proliferation. Blood 2007, 110, 220–227. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a Tumoral Immune Resistance Mechanism Based on Tryptophan Degradation by Indoleamine 2,3-Dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Steggerda, S.M.; Bennett, M.K.; Chen, J.; Emberley, E.; Huang, T.; Janes, J.R.; Li, W.; MacKinnon, A.L.; Makkouk, A.; Marguier, G.; et al. Inhibition of Arginase by CB-1158 Blocks Myeloid Cell-Mediated Immune Suppression in the Tumor Microenvironment. J. Immunother. Cancer 2017, 5, 101. [Google Scholar] [CrossRef]
- Polat, M.F.; Taysi, S.; Polat, S.; Böyük, A.; Bakan, E. Elevated Serum Arginase Activity Levels in Patients with Breast Cancer. Surg. Today 2003, 33, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B.; Guillemin, G. The Plasma [Kynurenine]/[Tryptophan] Ratio and Indoleamine 2,3-Dioxygenase: Time for Appraisal. Int. J. Tryptophan Res. 2019, 12, 1178646919868978. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Louie, A.; Yang, Q.; Massenkoff, N.; Xu, C.; Hunt, P.W.; Gee, W. A Simple LC-MS/MS Method for Determination of Kynurenine and Tryptophan Concentrations in Human Plasma from HIV-Infected Patients. Bioanalysis 2013, 5, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.R.; Schultz, G.A.; Eckstein, J.A.; Ackermann, B.L. Surrogate Matrix and Surrogate Analyte Approaches for Definitive Quantitation of Endogenous Biomolecules. Bioanalysis 2012, 4, 2343–2356. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Conrad, C.; Sorrin, A.J.; Ruhi, M.K.; Reader, J.C.; Huang, S.A.; Franco, W.; Scarcelli, G.; Polacheck, W.J.; Roque, D.M.; et al. Malignant Ascites in Ovarian Cancer: Cellular, Acellular, and Biophysical Determinants of Molecular Characteristics and Therapy Response. Cancers 2021, 13, 4318. [Google Scholar] [CrossRef]
- Sardar, P.; Kempken, F. Characterization of Indole-3-Pyruvic Acid Pathway-Mediated Biosynthesis of Auxin in Neurospora Crassa. PLoS ONE 2018, 13, e0192293. [Google Scholar] [CrossRef] [PubMed]
- Bittinger, M.A.; Nguyen, L.P.; Bradfield, C.A. Aspartate Aminotransferase Generates Proagonists of the Aryl Hydrocarbon Receptor. Mol. Pharmacol. 2003, 64, 550–556. [Google Scholar] [CrossRef]
- Mason, J.M.; Naidu, M.D.; Barcia, M.; Porti, D.; Chavan, S.S.; Chu, C.C. IL-4-Induced Gene-1 Is a Leukocyte L-Amino Acid Oxidase with an Unusual Acidic PH Preference and Lysosomal Localization. J. Immunol. 2004, 173, 4561–4567. [Google Scholar] [CrossRef]
- Zeitler, L.; Fiore, A.; Meyer, C.; Russier, M.; Zanella, G.; Suppmann, S.; Gargaro, M.; Sidhu, S.S.; Seshagiri, S.; Ohnmacht, C.; et al. Anti-Ferroptotic Mechanism of IL4i1-Mediated Amino Acid Metabolism. eLife 2021, 10, e64806. [Google Scholar] [CrossRef]
- Psallidas, I.; Kalomenidis, I.; Porcel, J.M.; Robinson, B.W.; Stathopoulos, G.T. Malignant Pleural Effusion: From Bench to Bedside. Eur. Respir. Rev. 2016, 25, 189–198. [Google Scholar] [CrossRef]
- Okamoto, A.; Nikaido, T.; Ochiai, K.; Takakura, S.; Saito, M.; Aoki, Y.; Ishii, N.; Yanaihara, N.; Yamada, K.; Takikawa, O.; et al. Indoleamine 2,3-Dioxygenase Serves as a Marker of Poor Prognosis in Gene Expression Profiles of Serous Ovarian Cancer Cells. Clin. Cancer Res. 2005, 11, 6030–6039. [Google Scholar] [CrossRef] [PubMed]
- Takao, M.; Okamoto, A.; Nikaido, T.; Urashima, M.; Takakura, S.; Saito, M.; Saito, M.; Okamoto, S.; Takikawa, O.; Sasaki, H.; et al. Increased Synthesis of Indoleamine-2,3-Dioxygenase Protein Is Positively Associated with Impaired Survival in Patients with Serous-Type, but Not with Other Types of, Ovarian Cancer. Oncol. Rep. 2007, 17, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Ino, K.; Kajiyama, H.; Yamamoto, E.; Shibata, K.; Nawa, A.; Nagasaka, T.; Akimoto, H.; Takikawa, O.; Kikkawa, F. Role of the Immunosuppressive Enzyme Indoleamine 2,3-Dioxygenase in the Progression of Ovarian Carcinoma. Gynecol. Oncol. 2009, 115, 185–192. [Google Scholar] [CrossRef]
- De Jong, R.A.; Nijman, H.W.; Boezen, H.M.; Volmer, M.; Ten Hoor, K.A.; Krijnen, J.; van der Zee, A.G.J.; Hollema, H.; Kema, I.P. Serum Tryptophan and Kynurenine Concentrations as Parameters for Indoleamine 2,3-Dioxygenase Activity in Patients with Endometrial, Ovarian, and Vulvar Cancer. Int. J. Gynecol. Cancer 2011, 21, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Sperner-Unterweger, B.; Neurauter, G.; Klieber, M.; Kurz, K.; Meraner, V.; Zeimet, A.; Fuchs, D. Enhanced Tryptophan Degradation in Patients with Ovarian Carcinoma Correlates with Several Serum Soluble Immune Activation Markers. Immunobiology 2011, 216, 296–301. [Google Scholar] [CrossRef]
- Gostner, J.M.; Obermayr, E.; Braicu, I.E.; Concin, N.; Mahner, S.; Vanderstichele, A.; Sehouli, J.; Vergote, I.; Fuchs, D.; Zeillinger, R. Immunobiochemical Pathways of Neopterin Formation and Tryptophan Breakdown via Indoleamine 2,3-Dioxygenase Correlate with Circulating Tumor Cells in Ovarian Cancer Patients- A Study of the OVCAD Consortium. Gynecol. Oncol. 2018, 149, 371–380. [Google Scholar] [CrossRef]
- Smith, L.P.; Bitler, B.G.; Richer, J.K.; Christenson, J.L. Tryptophan Catabolism in Epithelial Ovarian Carcinoma. Trends Cancer Res. 2019, 14, 1–9. [Google Scholar]
- Grobben, Y.; de Man, J.; van Doornmalen, A.M.; Muller, M.; Willemsen-Seegers, N.; Vu-Pham, D.; Mulder, W.R.; Prinsen, M.B.W.; de Wit, J.; Sterrenburg, J.G.; et al. Targeting Indoleamine 2,3-Dioxygenase in Cancer Models Using the Novel Small Molecule Inhibitor NTRC 3883-0. Front. Immunol. 2020, 11, 609490. [Google Scholar] [CrossRef]
- Seegers, N.; van Doornmalen, A.M.; Uitdehaag, J.C.M.; de Man, J.; Buijsman, R.C.; Zaman, G.J.R. High-Throughput Fluorescence-Based Screening Assays for Tryptophan-Catabolizing Enzymes. J. Biomol. Screen. 2014, 19, 1266–1274. [Google Scholar] [CrossRef]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.-J.; Kim, T.M.; et al. Epacadostat plus Pembrolizumab versus Placebo plus Pembrolizumab in Patients with Unresectable or Metastatic Melanoma (ECHO-301/KEYNOTE-252): A Phase 3, Randomised, Double-Blind Study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef]
- Van den Eynde, B.J.; van Baren, N.; Baurain, J.-F. Is There a Clinical Future for IDO1 Inhibitors after the Failure of Epacadostat in Melanoma? Annu. Rev. Cancer Biol. 2020, 4, 241–256. [Google Scholar] [CrossRef]
- Muller, A.J.; Manfredi, M.G.; Zakharia, Y.; Prendergast, G.C. Inhibiting IDO Pathways to Treat Cancer: Lessons from the ECHO-301 Trial and Beyond. Semin. Immunopathol. 2019, 41, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Heath-Pagliuso, S.; Rogers, W.J.; Tullis, K.; Seidel, S.D.; Cenijn, P.H.; Brouwer, A.; Denison, M.S. Activation of the Ah Receptor by Tryptophan and Tryptophan Metabolites. Biochemistry 1998, 37, 11508–11515. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand That Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-Lactic Acid Associated with Bifidobacterium-Dominated Microbiota Significantly Decreases Inflammation in Intestinal Epithelial Cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- Castellano, F.; Prevost-Blondel, A.; Cohen, J.L.; Molinier-Frenkel, V. What Role for AHR Activation in IL4I1-Mediated Immunosuppression ? Oncoimmunology 2021, 10, 1924500. [Google Scholar] [CrossRef]
- Rao, D.; Yu, C.; Wang, T.; Sheng, J.; Lv, E.; Liang, H.; Huang, W.; Dong, H. Pan-Cancer Analysis Combined with Experimental Validation Revealed IL4I1 as an Immunological and Prognostic Biomarker. Int. Immunopharmacol. 2022, 111, 109091. [Google Scholar] [CrossRef]
- Zhao, H.; Teng, Y.; Hao, W.; Li, J.; Li, Z.; Chen, Q.; Yin, C.; Yue, W. Single-Cell Analysis Revealed That IL4I1 Promoted Ovarian Cancer Progression. J. Transl. Med. 2021, 19, 454. [Google Scholar] [CrossRef]
- Villatoro, S.; Mayo-de-Las-Casas, C.; Jordana-Ariza, N.; Viteri-Ramírez, S.; Garzón-Ibañez, M.; Moya-Horno, I.; García-Peláez, B.; González-Cao, M.; Malapelle, U.; Balada-Bel, A.; et al. Prospective Detection of Mutations in Cerebrospinal Fluid, Pleural Effusion, and Ascites of Advanced Cancer Patients to Guide Treatment Decisions. Mol. Oncol. 2019, 13, 2633–2645. [Google Scholar] [CrossRef]
- Kuk, C.; Kulasingam, V.; Gunawardana, C.G.; Smith, C.R.; Batruch, I.; Diamandis, E.P. Mining the Ovarian Cancer Ascites Proteome for Potential Ovarian Cancer Biomarkers. Mol. Cell. Proteom. 2009, 8, 661–669. [Google Scholar] [CrossRef]
- Fong, M.Y.; McDunn, J.; Kakar, S.S. Identification of Metabolites in the Normal Ovary and Their Transformation in Primary and Metastatic Ovarian Cancer. PLoS ONE 2011, 6, e19963. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.P.; Alonso, A.; Turk, M.J.; Berwin, B. Murine Ovarian Cancer Vascular Leukocytes Require Arginase-1 Activity for T Cell Suppression. Mol. Immunol. 2008, 46, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Czystowska-Kuzmicz, M.; Sosnowska, A.; Nowis, D.; Ramji, K.; Szajnik, M.; Chlebowska-Tuz, J.; Wolinska, E.; Gaj, P.; Grazul, M.; Pilch, Z.; et al. Small Extracellular Vesicles Containing Arginase-1 Suppress T-Cell Responses and Promote Tumor Growth in Ovarian Carcinoma. Nat. Commun. 2019, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The Role of Nitric Oxide in Tumour Progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Nomelini, R.S.; de Abreu Ribeiro, L.C.; Tavares-Murta, B.M.; Adad, S.J.; Murta, E.F.C. Production of Nitric Oxide and Expression of Inducible Nitric Oxide Synthase in Ovarian Cystic Tumors. Mediators Inflamm. 2008, 2008, 186584. [Google Scholar] [CrossRef]
- Shen, Y.-A.; Hong, J.; Asaka, R.; Asaka, S.; Hsu, F.-C.; Suryo Rahmanto, Y.; Jung, J.-G.; Chen, Y.-W.; Yen, T.-T.; Tomaszewski, A.; et al. Inhibition of the MYC-Regulated Glutaminase Metabolic Axis Is an Effective Synthetic Lethal Approach for Treating Chemoresistant Ovarian Cancers. Cancer Res. 2020, 80, 4514–4526. [Google Scholar] [CrossRef]
- Plewa, S.; Horała, A.; Dereziński, P.; Klupczynska, A.; Nowak-Markwitz, E.; Matysiak, J.; Kokot, Z.J. Usefulness of Amino Acid Profiling in Ovarian Cancer Screening with Special Emphasis on Their Role in Cancerogenesis. Int. J. Mol. Sci. 2017, 18, 2727. [Google Scholar] [CrossRef]
- D’Amora, P.; Silva, I.D.C.G.; Tewari, K.S.; Bristow, R.E.; Cappuccini, F.; Evans, S.S.; Salzgeber, M.B.; Addis-Bernard, P.J.; Palma, A.M.; Marchioni, D.M.L.; et al. Platinum Resistance in Gynecologic Malignancies: Response, Disease Free and Overall Survival Are Predicted by Biochemical Signature: A Metabolomic Analysis. Gynecol. Oncol. 2021, 163, 162–170. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Grimshaw, M.J.; Wilbanks, G.D.; Hagemann, T.; Wilson, J.L.; Burke, F.; Stamp, G.; Balkwill, F.R. Aberrant Regulation of Argininosuccinate Synthetase by TNF-Alpha in Human Epithelial Ovarian Cancer. Int. J. Cancer 2007, 121, 6–11. [Google Scholar] [CrossRef]
- Cheon, D.-J.; Walts, A.E.; Beach, J.A.; Lester, J.; Bomalaski, J.S.; Walsh, C.S.; Ruprecht Wiedemeyer, W.; Karlan, B.Y.; Orsulic, S. Differential Expression of Argininosuccinate Synthetase in Serous and Non-Serous Ovarian Carcinomas. J. Pathol. Clin. Res. 2015, 1, 41–53. [Google Scholar] [CrossRef]
- Huang, H.-L.; Chen, W.-C.; Hsu, H.-P.; Cho, C.-Y.; Hung, Y.-H.; Wang, C.-Y.; Lai, M.-D. Argininosuccinate Lyase Is a Potential Therapeutic Target in Breast Cancer. Oncol. Rep. 2015, 34, 3131–3139. [Google Scholar] [CrossRef]
- Gong, R.; He, L.; Zhou, H.; Cheng, S.; Ren, F.; Chen, J.; Ren, J. Down-Regulation of Argininosuccinate Lyase Induces Hepatoma Cell Apoptosis through Activating Bax Signaling Pathway. Genes Dis. 2019, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Jerram, A.; Guy, T.V.; Beutler, L.; Gunasegaran, B.; Sluyter, R.; Fazekas de St Groth, B.; McGuire, H.M. Effects of Storage Time and Temperature on Highly Multiparametric Flow Analysis of Peripheral Blood Samples; Implications for Clinical Trial Samples. Biosci. Rep. 2021, 41, BSR20203827. [Google Scholar] [CrossRef]
- Davis, J.S.; Darcy, C.J.; Piera, K.; McNeil, Y.R.; Woodberry, T.; Anstey, N.M. Ex-Vivo Changes in Amino Acid Concentrations from Blood Stored at Room Temperature or on Ice: Implications for Arginine and Taurine Measurements. BMC Clin. Pathol. 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.L.; Hoover, E.; Wang, Y.; Zanetti, K.A. Pre-Analytical Factors That Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
| Subgroups Based on Collected Samples * | ||||
|---|---|---|---|---|
| All Patients (n = 34) | Patients with Plasma Collected (n = 24) | Patients with Ascites Collected (n = 32) | Patients with Both Plasma and Ascites Collected (n = 22) † | |
| Age (years) | ||||
| Median (IQR) | 62.5 (58–68) | 62.5 (56–69) | 63 (59–69) | 63 (58–70) |
| BMI (kg/m2) | ||||
| Median (IQR) | 24 (22–27) | 24 (22–27) | 25 (22–28) | 24 (22–27) |
| FIGO stage | ||||
| II | 2 (5.9%) | 1 (4.2%) | 2 (6.3%) | 1 (4.5%) |
| III | 24 (70.6%) | 16 (66.7%) | 22 (68.8%) | 14 (63.6%) |
| IV | 8 (23.5%) | 7 (29.2%) | 8 (25.0%) | 7 (31.8%) |
| Primary treatment completed | ||||
| Yes | 26 (76.5%) | 20 (83.3%) | 24 (75.0%) | 18 (81.8%) |
| No | 8 (23.5%) | 4 (16.7%) | 8 (25.0%) | 4 (18.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grobben, Y.; den Ouden, J.E.; Aguado, C.; van Altena, A.M.; Kraneveld, A.D.; Zaman, G.J.R. Amino Acid-Metabolizing Enzymes in Advanced High-Grade Serous Ovarian Cancer Patients: Value of Ascites as Biomarker Source and Role for IL4I1 and IDO1. Cancers 2023, 15, 893. https://doi.org/10.3390/cancers15030893
Grobben Y, den Ouden JE, Aguado C, van Altena AM, Kraneveld AD, Zaman GJR. Amino Acid-Metabolizing Enzymes in Advanced High-Grade Serous Ovarian Cancer Patients: Value of Ascites as Biomarker Source and Role for IL4I1 and IDO1. Cancers. 2023; 15(3):893. https://doi.org/10.3390/cancers15030893
Chicago/Turabian StyleGrobben, Yvonne, Judith E. den Ouden, Cristina Aguado, Anne M. van Altena, Aletta D. Kraneveld, and Guido J. R. Zaman. 2023. "Amino Acid-Metabolizing Enzymes in Advanced High-Grade Serous Ovarian Cancer Patients: Value of Ascites as Biomarker Source and Role for IL4I1 and IDO1" Cancers 15, no. 3: 893. https://doi.org/10.3390/cancers15030893
APA StyleGrobben, Y., den Ouden, J. E., Aguado, C., van Altena, A. M., Kraneveld, A. D., & Zaman, G. J. R. (2023). Amino Acid-Metabolizing Enzymes in Advanced High-Grade Serous Ovarian Cancer Patients: Value of Ascites as Biomarker Source and Role for IL4I1 and IDO1. Cancers, 15(3), 893. https://doi.org/10.3390/cancers15030893








